Ventilation System Influence on Hydrogen Explosion Hazards in Industrial Lead-Acid Battery Rooms
Abstract
1. Introduction
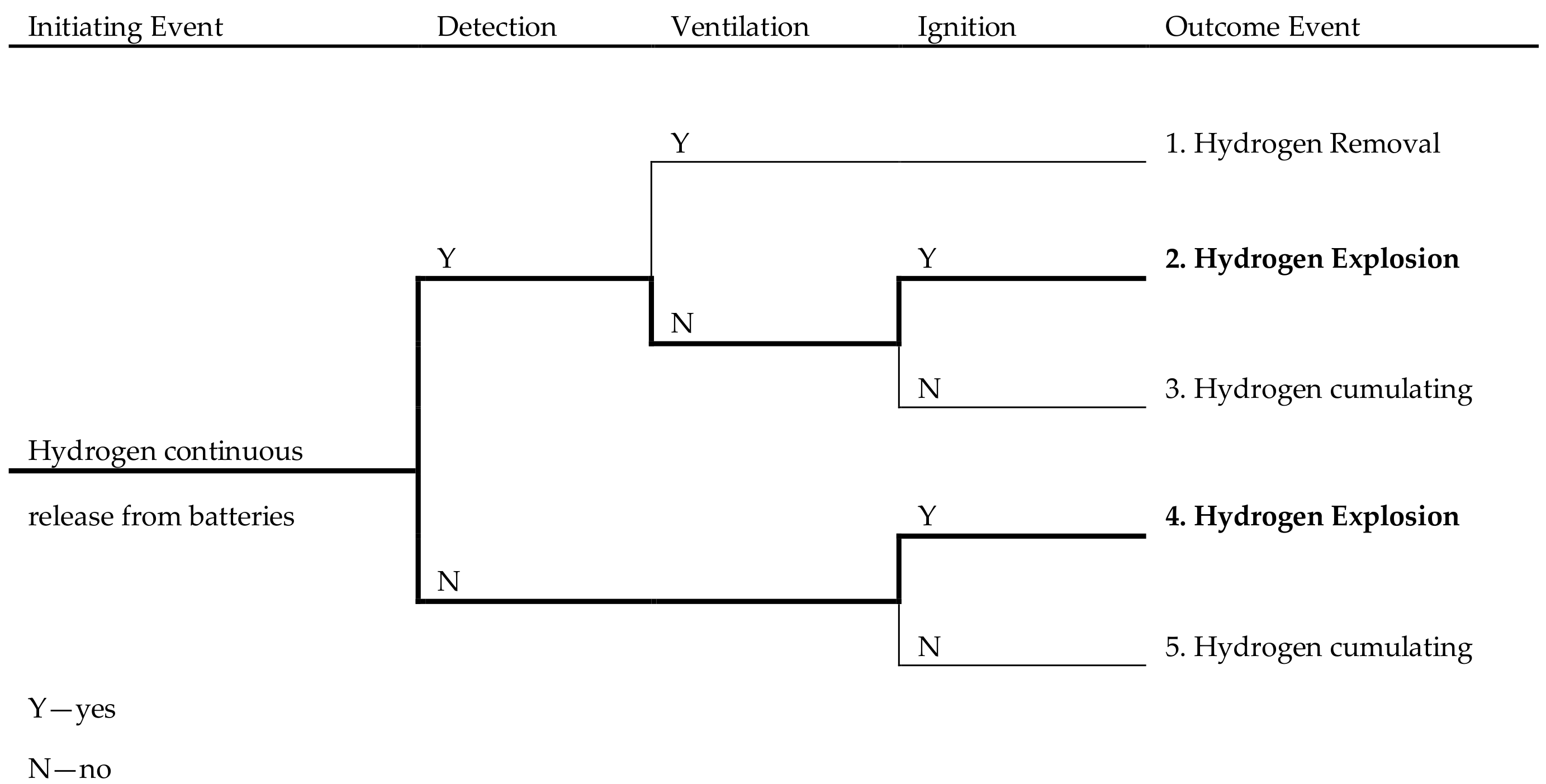
2. Battery Rooms Failure Scenarios
3. Conducted Real Scale Experiment
3.1. Experiment Layout
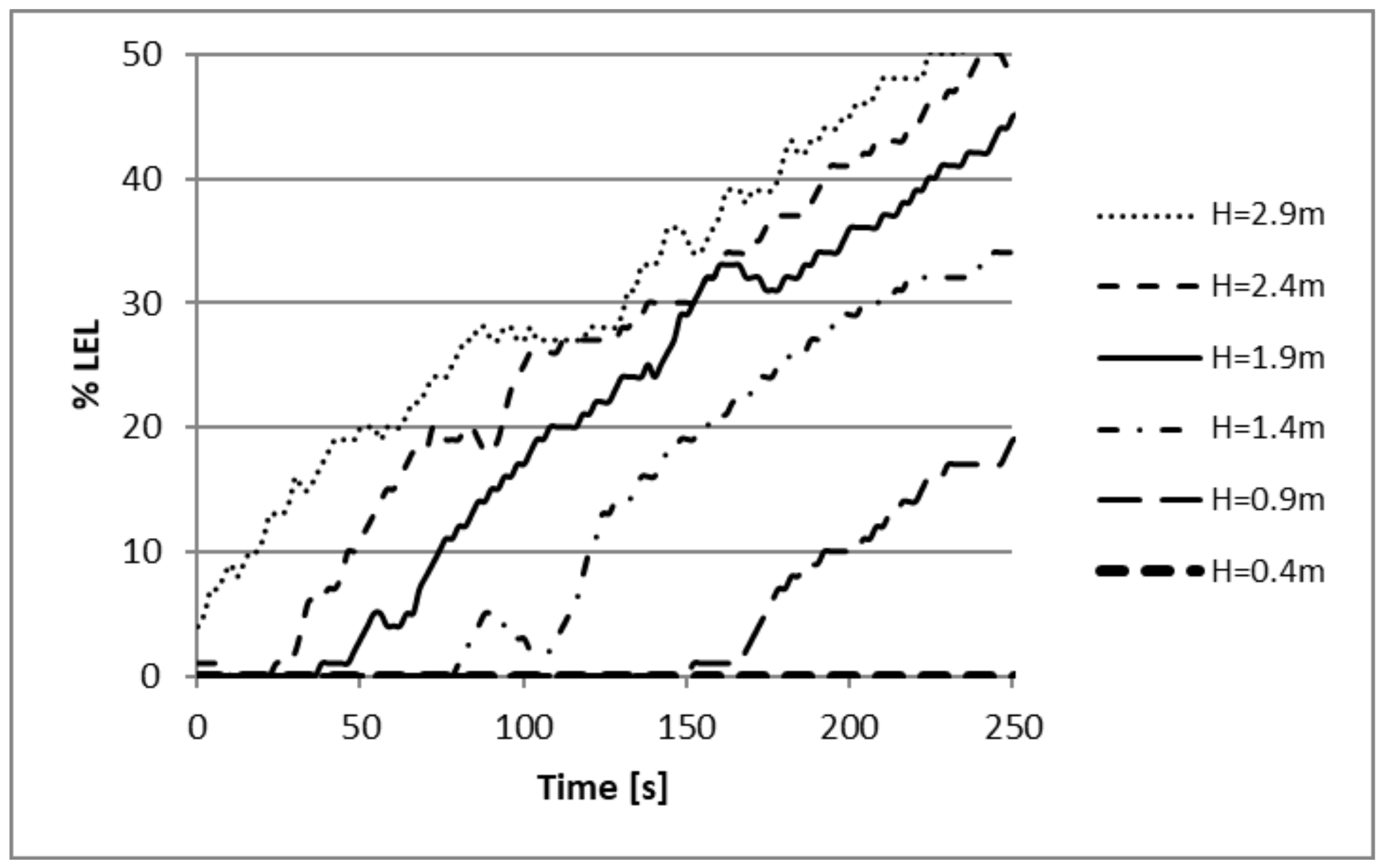
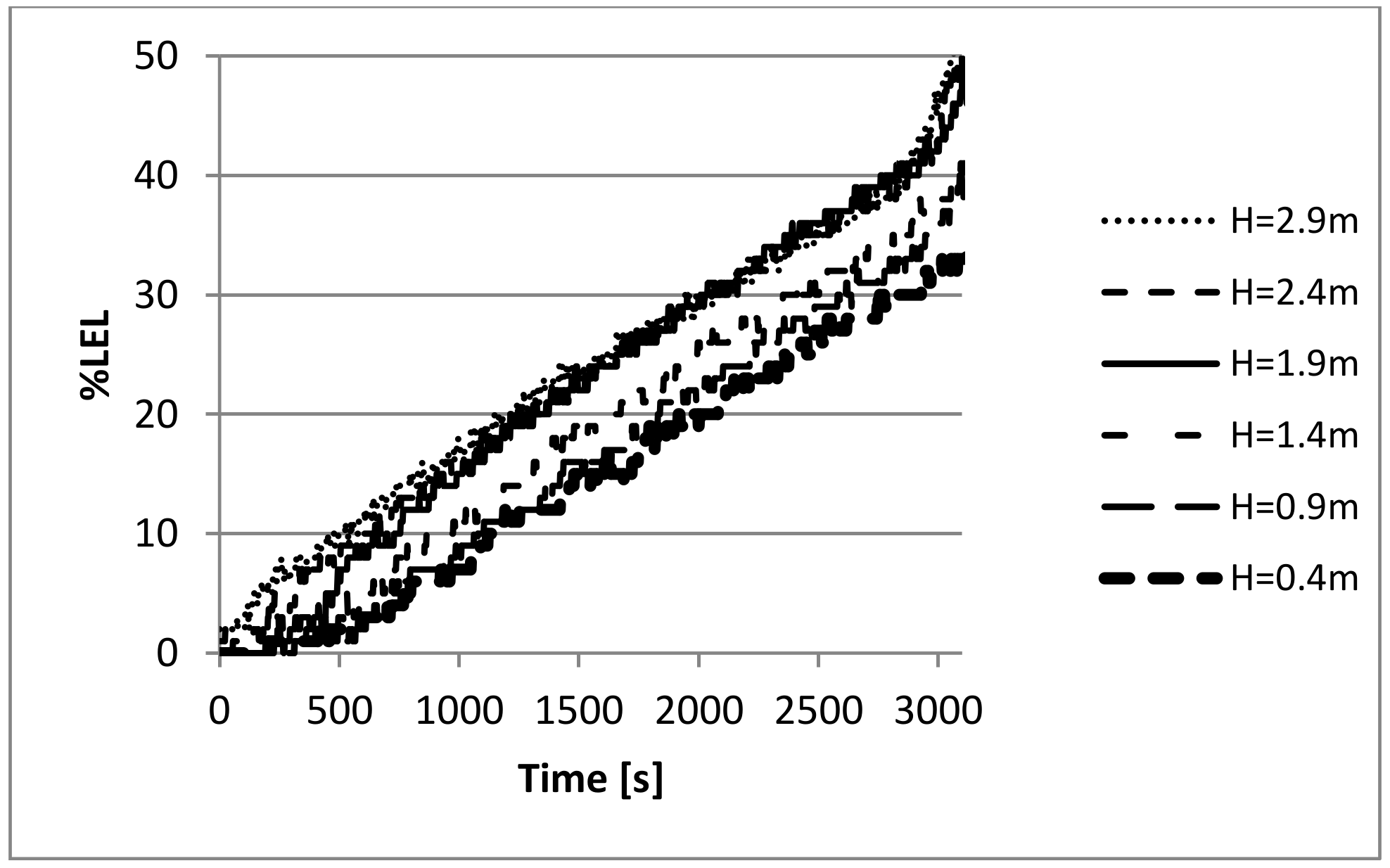
3.2. Experiment Results
4. CFD Analysis of Different Ventilation Systems Effectiveness
4.1. Explosive Hazards in Battery Rooms without Ventilation
4.2. Ventilation Systems in Battery Rooms
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ubertini, S.; Facci, A.L.; Andreassi, L. Hybrid Hydrogen and Mechanical Distributed Energy Storage. Energies 2017, 10, 2035. [Google Scholar] [CrossRef]
- Seo, M.; Goh, T.; Park, M.; Woo, K.S. Detection Method for Soft Internal Short Circuit in Lithium-Ion Battery Pack by Extracting Open Circuit Voltage of Faulted Cell. Energies 2018, 11, 1669. [Google Scholar] [CrossRef]
- Hesse, H.C.; Schimpe, M.; Kucevic, D.; Jossen, A. Lithium-Ion Battery Storage for the Grid—A Review of Stationary Battery Storage System Design Tailored for Applications in Modern Power Grids. Energies 2017, 10, 2107. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). World Energy Investment 2017. Available online: https://www.iea.org/publications/wei2017/ (accessed on 11 July 2017).
- Facci, A.L.; Cigolotti, V.; Jannelli, E.; Ubertini, S. Technical and economic assessment of a SOFC-based energy system for combined cooling, heating and power. Appl. Energy 2017, 192, 563–574. [Google Scholar] [CrossRef]
- Chisholm, G.; Cronin, L. Hydrogen From Water Electrolysis. In Storing Energy With Special Reference to Renewable Energy Sources; Elsevier: Amsterdam, The Netherlands, 2016; pp. 315–343. [Google Scholar]
- Safety Requirements for Secondary Batteries and Battery Installations, Part 1: General safety information. Available online: https://old.tic.ir/Content/media/article/BSI%20EN%2050272-1%20(2011)_321.PDF (accessed on 2 August 2018).
- Hydrogen Material Safety Data Sheet. Available online: http://www.praxair.com/-/media/documents/sds/hydrogen/hydrogen-gas-h2-safety-data-sheet-sds-p4604.pdf?la=en (accessed on 2 August 2018).
- FM Global Property Loss Prevention Data Sheets. Available online: https://www.fmglobal.com/research-and-resources/fm-global-data-sheets (accessed on 2 August 2018).
- Gelain, T.; Prévost, C. Experimental and numerical study of light gas dispersion in a ventilated room. Nucl. Eng. Des. 2015, 293, 476–484. [Google Scholar] [CrossRef]
- Grimsrud, D.T.; Sherman, M.H.; Janssen, J.E.; Pearman, A.; Harrje, D. An intercomparison of tracer gases used for air infiltration measurements. ASHRAE Trans. 1980, 86, 258–267. [Google Scholar]
- Saw, C.Y. The effect of tracer gas on the accuracy of air change measurements in buildings. ASHRAE Trans. 1984, 90, 212–225. [Google Scholar]
- Khan, J.A.; Feigley, C.E.; Lee, E.; Ahmed, M.R.; Tamanna, S. Effects of inlet and exhaust locations and emitted gas density on indoor air contaminant concentrations. Build. Environ. 2006, 41, 851–863. [Google Scholar] [CrossRef]
- Tamura, Y.; Takeuchi, M.; Kenji, S.K. Effectiveness of a blower in reducing the hazard of hydrogen leaking from a hydrogen-fueled vehicle. Int. J. Hydrog. Energy 2014, 39, 20339–20349. [Google Scholar] [CrossRef]
- Palazzi, E.; Currò, F.; Fabiano, B. Accidental Continuous Releases from Coal Processing in Semi-Confined Environment. Energies 2013, 6, 5003–5022. [Google Scholar] [CrossRef]
- Cariteau, B.; Brinster, J.; Tkatschenko, I. Experiments on the distribution of concentration due to buoyant gas low flow rate release in an enclosure. In Proceedings of the International Conference on Hydrogen Safety, Ajaccio, Corsica, France, 16–18 September 2009. [Google Scholar]
- Houf, W.G.; Evans, G.H.; Ekoto, I.W.; Merilo, E.G.; Groethe, M.A. Hydrogen fuel-cell Forklift Vehicle Releases in Enclosed Spaces. In Proceedings of the International Conference on Hydrogen Safety, San Francisco, CA, USA, 17–19 September 2011. [Google Scholar]
- Pitts, W.; Yang, J.; Prasad, K. Experimental characterization of a helium dispersion in a 1/4 scale two-car residential garage. In Proceedings of the IEA Hydrogen Implementing Agreement Task 19 Experts Meeting, Paris, France, 23 April 2009. [Google Scholar]
- Jallais, S.; Ruban, S. Engineering tools and Methods for Dispersion and Explosion Vent Sizing. In Proceedings of the IEA Hydrogen Implementing Agreement Task 19 Experts Meeting, Ajaccio, Corsica, France, 14 September 2009. [Google Scholar]
- Weiner, S.C. Advancing the hydrogen safety knowledge base. Int. J. Hydrog. Energy 2014, 39, 20357–20361. [Google Scholar] [CrossRef]
- McGrattan, K. Fire Dynamics Simulator Technical Reference Guide Volume 1: Mathematical Model. NIST Spec. Publ. 2013, 1018, 175. [Google Scholar]


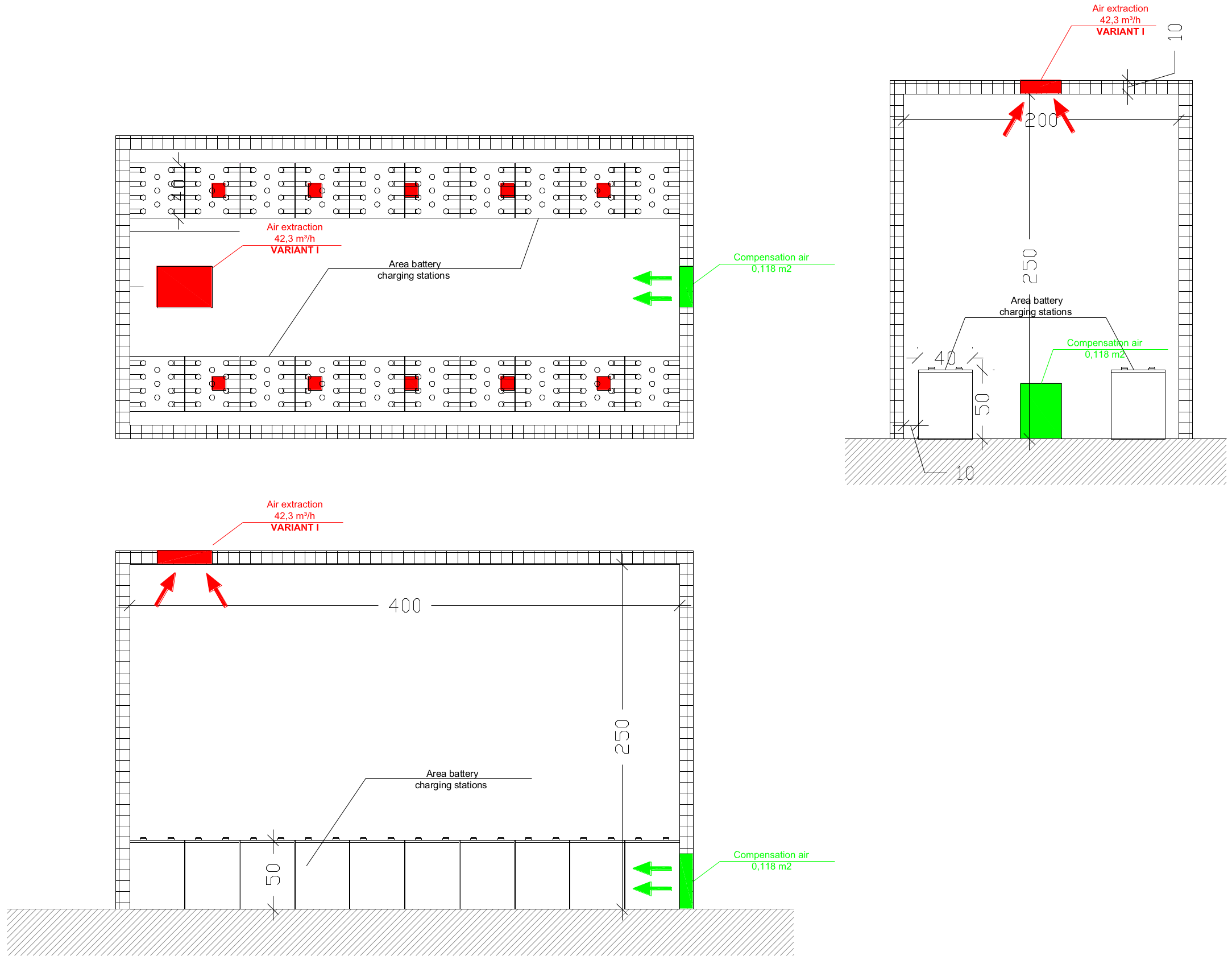

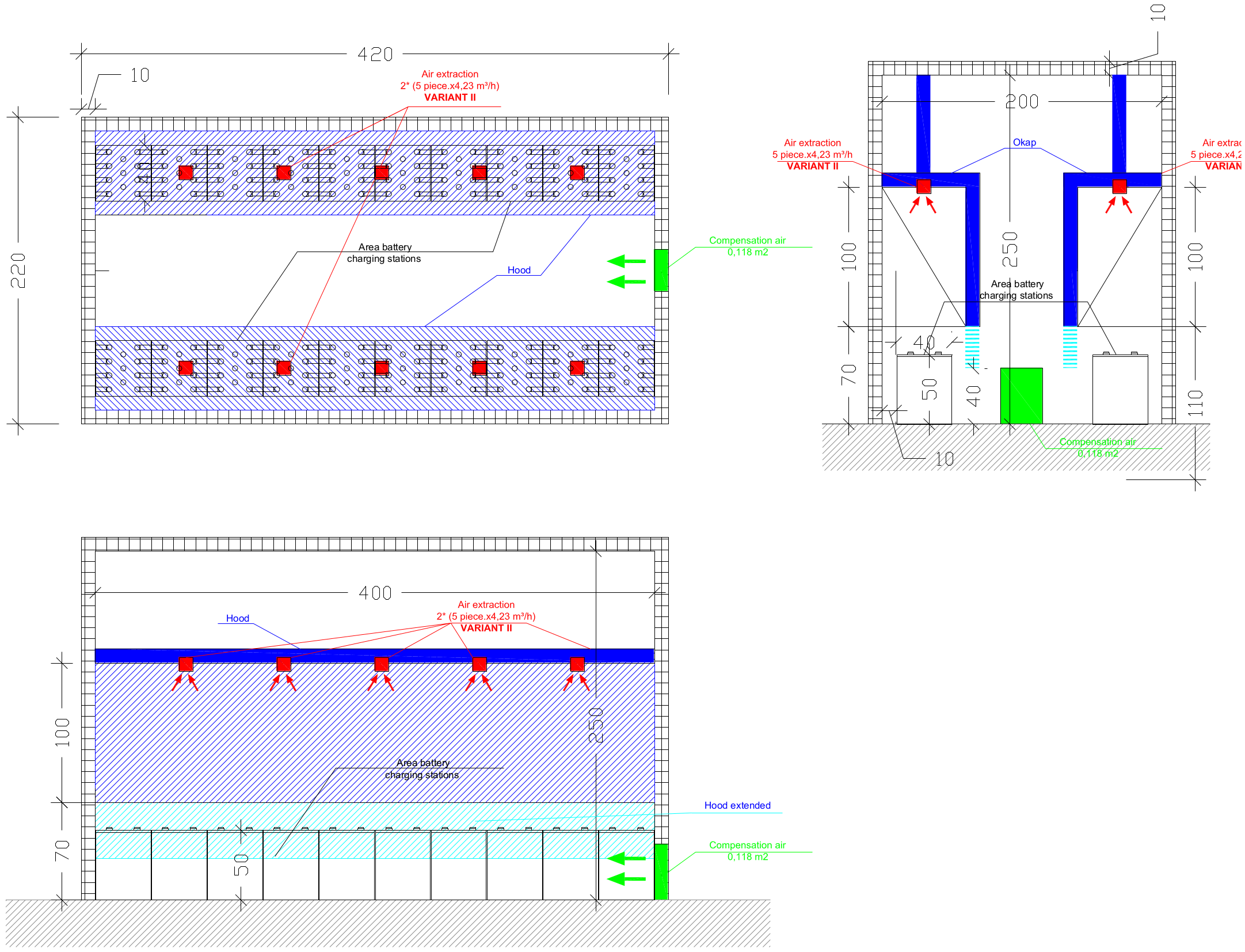
| Required System against Explosive Hazardous | Hydrogen Detection and Natural or Mechanical Ventilation System |
|---|---|
| The purpose of ventilating | To maintain the hydrogen concentration below the 4% hydrogen threshold. |
| Technical parameters of ventilation system | The volume flow of ventilation should be designed on the basis of hydrogen emission calculation (based on batteries data) and the battery room volume. The air inlet and outlet shall be located at the best possible location to create best conditions for exchange of the air. The ventilation openings shall be on opposite walls, or the minimum separation distance of 2 m shall be kept between openings on the same wall. |
| Test No. | Time Period of the Outflow [s] | Hydrogen Volume Outflow [m3/s] |
|---|---|---|
| Test 1 | 552 | 3.17 × 10−3 |
| Test 2 | 1140 | 1.63 × 10−3 |
| Test 3 | 4440 | 3.34 × 10−4 |
| The Threshold | Theoretical Time for Reaching the Threshold | Simulation Results | Scale |
|---|---|---|---|
| 10% LEL (0.4%vol) | 840 s |  |  |
| 40% LEL (1.6%vol) | 4260 s | 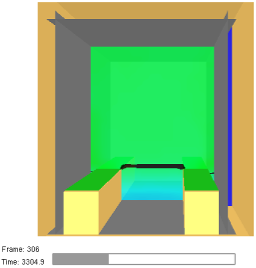 | |
| 100% LEL (4%vol) | 8520 s |  |
| Scenario Number | Description | Figure |
|---|---|---|
| Scenario 1 | Mechanical ventilation with extraction point in the ceiling of the room | 7 |
| Scenario 2 | Natural ventilation with extraction point in the ceiling of the room | 8 |
| Scenario 3 | Natural ventilation with extraction point in the wall of the room | 8 |
| Scenario 4 | Mechanical ventilation with extraction point in short hoods | 9 |
| Scenario 5 | Mechanical ventilation with extraction point in long hoods | 9 |
| Scenario Number | Simulation Results | Scale |
|---|---|---|
| Scenario 1 |  |  |
| Scenario 2 |  | |
| Scenario 3 | 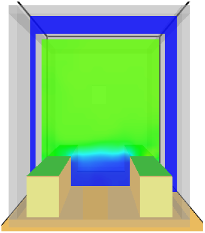 | |
| Scenario 4 | 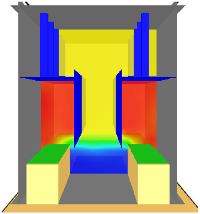 | |
| Scenario 5 |  |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzezińska, D. Ventilation System Influence on Hydrogen Explosion Hazards in Industrial Lead-Acid Battery Rooms. Energies 2018, 11, 2086. https://doi.org/10.3390/en11082086
Brzezińska D. Ventilation System Influence on Hydrogen Explosion Hazards in Industrial Lead-Acid Battery Rooms. Energies. 2018; 11(8):2086. https://doi.org/10.3390/en11082086
Chicago/Turabian StyleBrzezińska, Dorota. 2018. "Ventilation System Influence on Hydrogen Explosion Hazards in Industrial Lead-Acid Battery Rooms" Energies 11, no. 8: 2086. https://doi.org/10.3390/en11082086
APA StyleBrzezińska, D. (2018). Ventilation System Influence on Hydrogen Explosion Hazards in Industrial Lead-Acid Battery Rooms. Energies, 11(8), 2086. https://doi.org/10.3390/en11082086





