1. Introduction
Seaweeds grow in brackish or salt water and, unlike terrestrial crops, do not require agricultural land for cultivation, thus avoiding competition for freshwater and land needed for food production [
1]. The potential biomass yield can be higher for seaweed than for terrestrial plants per unit area. Brown seaweeds which are grown “under cultured conditions” can have yields of ~13.1 kg dry weight (dw) m
−2 year
−1 compared to the yield from sugarcane of only ~10 kg dw m
−2 year
−1 [
2]. Growth systems that do not compete for land or freshwater, which are needed for crops, and high potential biomass yields have led to considerable research interest in the use of both micro- and macroalgae as sources of biofuel.
Sargassum muticum, a brown seaweed which is an invasive species to Europe, is attracting research interest as a potential feedstock for biofuels and biorefineries [
3,
4,
5,
6].
Anaerobic digestion (AD) is generally the process of choice for energy production from high water content biomass, and many groups have reported that macroalgae are a suitable feedstock for AD [
7]. Nevertheless, practical yields of biogas from the AD of seaweed are considerably below the theoretical maximum, and increasing CH
4 yield is, therefore, the most critical factor in improving process energy balance and reducing greenhouse gas emissions [
8]. A variety of biomass pretreatment methods, such as thermal, mechanical, thermochemical, and enzymatic treatments, have been shown to improve biomethane production by 19–68% [
9]. Chisti [
10], in a review of the constraints to the commercialisation of algal fuels, suggested saline algal biomass should be washed in freshwater to reduce the salt content, and washing in freshwater is a pretreatment step which is often used in a wide a variety of seaweed biofuel research studies [
6,
11,
12,
13,
14,
15,
16,
17,
18,
19]. However, the effect of washing seaweed prior to AD does not appear to have been greatly studied.
The washing of
Ulva in freshwater does not change methane yield [
20,
21], despite a 2.5% decrease in volatile solids (VS) concentration and a 23% decrease in total solids (TS) content of the green alga, due to the removal of gravel and sand [
21]. However, Adams et al. [
22] reported that washing
Laminaria digitata increased methane yield despite a reduction in soluble carbohydrate content, possibly because of the influence of the decreased salt content.
In four recent studies of ensiling seaweeds, three different washing treatments were used prior to ensiling: Redden et al. [
23] washed with seawater; Herrmann et al. [
24] and Cabrita et al. [
25] washed with cold tap water; and Milledge and Harvey [
26] did not wash the seaweed before ensiling. These differences in pretreatment could be a potential factor in the disparity in the loss of material (leaching) during ensiling between the four studies [
23]. However, the species and environmental growth conditions may also have substantial effects. In three recent studies of the AD of
S. muticum (
Sargassum muticum), two studies did not wash the seaweed prior to digestion, and one did with seawater [
5,
26,
27]. Despite these difference in washing pretreatment before ensilage and AD, there appears to have been little research on the washing pretreatment of seaweed in general and
S. muticum in particular. This research, thus, examines the effect of freshwater washing on ensilage and anaerobic digestion.
2. Methods
2.1. Sample Collection
S. muticum was collected in June 2017 from Minis Bay, Kent, England (Ordnance Survey National Grid Map reference TR287697).
2.2. Sample Preparation
Unwashed seaweed: holdfasts together with chalk and other natural contaminants, such as mud, sand, chalk, small animals, and other seaweeds attached to S. muticum, were removed by hand without washing. The samples were bagged, frozen, and stored at −20 °C.
Washed seaweed: holdfasts together with chalk and other natural contaminants, such as mud, sand, chalk, small animals, and other seaweeds attached to S. muticum, were removed by hand. The remaining S. muticum was then washed in running tap water for 30 s and allowed to drain for 5 min. The samples were bagged, frozen, and stored at −20 °C.
2.3. Dry Weight Determination
The British Standards simplified oven drying method for the determination of moisture content in solid biofuels was used to establish moisture content [
28]. All measurements were repeated in triplicate, and a mean value and standard deviation (SD) are reported. Samples after drying were stored in sealed containers at 4 °C for further experimentation.
2.4. Ash Determination
The British Standards method for determination of ash content in solid biofuels was used to establish the ash content of oven dried samples [
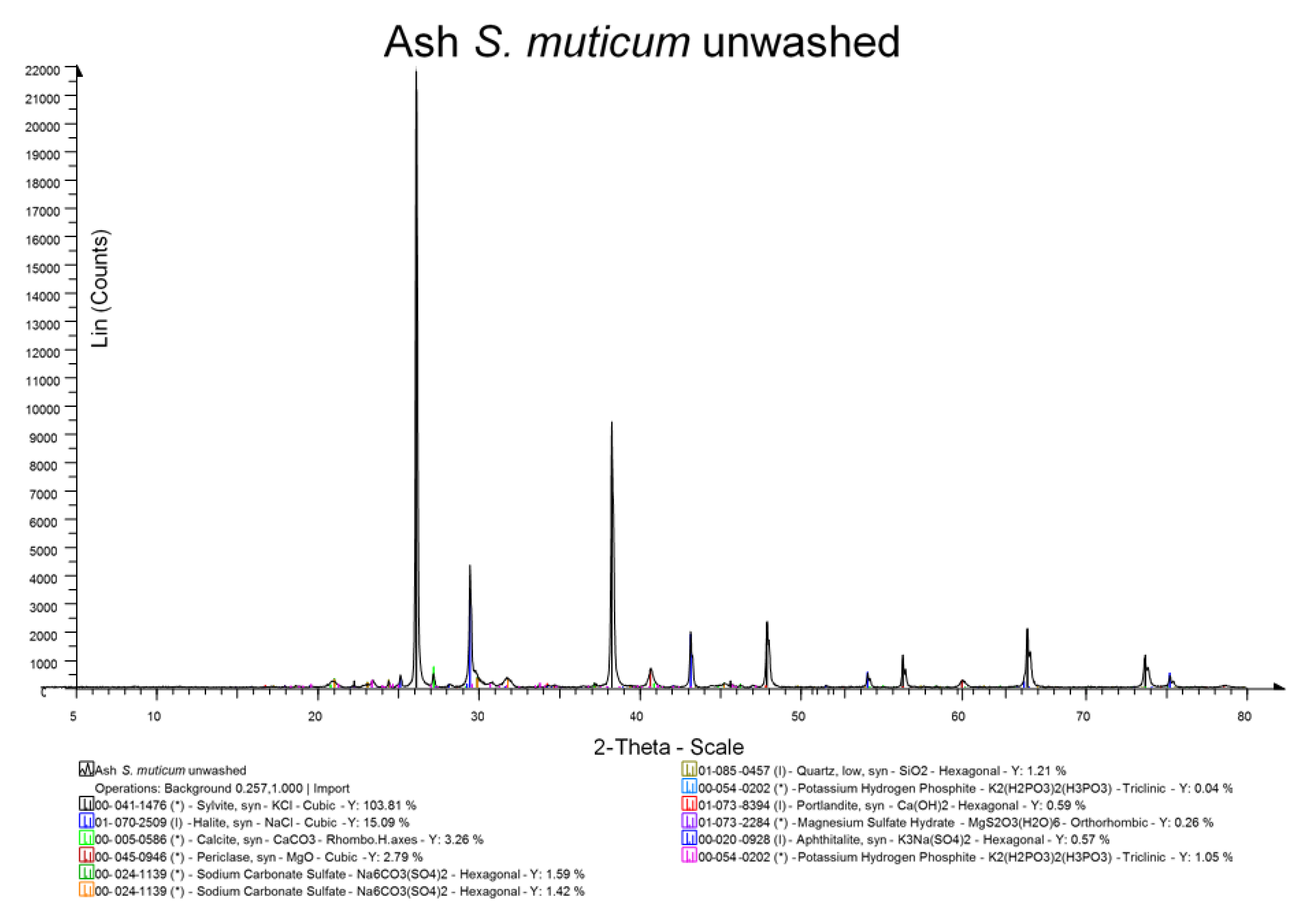
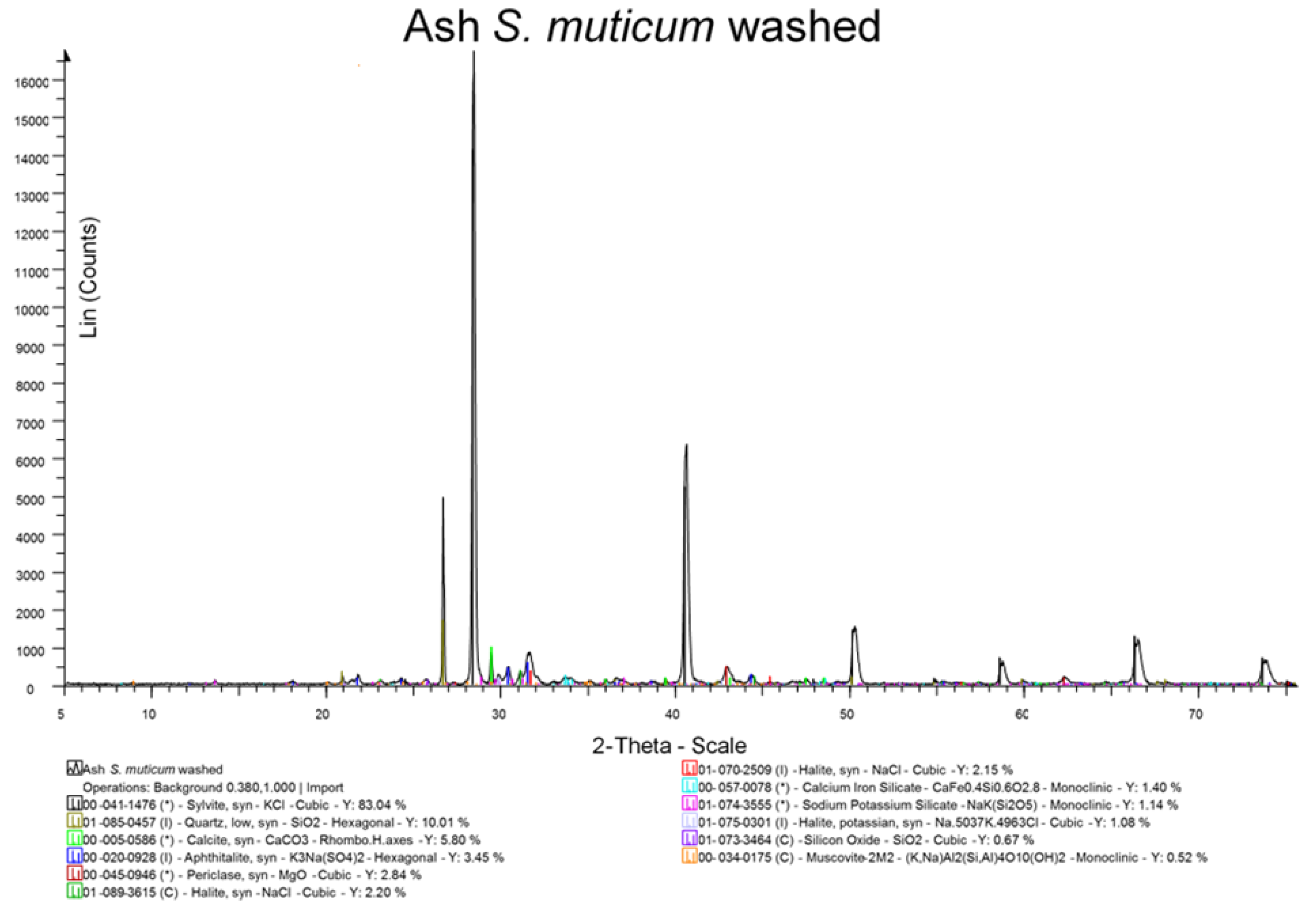
29]. All measurements were carried out in triplicate, and a mean value is reported. Ash was also examined by X-ray diffraction (XRD) analysis after grinding in a pestle and mortar to a fine powder <10 μm.
2.5. Salt Content Determination
The Mohr silver nitrate and potassium chromate titration method was used to determine the salt (sodium chloride) content of the ashed samples [
30,
31]. A mean value is reported from two determinations per sample.
2.6. Elemental Analysis
Flash dynamic combustion (Flash EA1112 CHNS Elemental Analyser, Thermo Scientific, Waltham, MA, USA) was used to determine the carbon, hydrogen, nitrogen, and sulphur content of the dried seaweed biomass. The oxygen content was calculated by difference. A mean is reported from a minimum of two determinations per sample.
2.7. Protein Content
The protein content was determined in duplicate on 0.1 g freeze-dried samples of washed and unwashed seaweeds, crushed into a fine powder with liquid nitrogen, by the Lowry method using ovalbumin as the protein standard [
32]. The blue colour of the final solution, the result of the presence of amino acids (mainly tyrosine and tryptophan), was quantified using absorbance at 750 nm with a UV-visible spectrophotometer (Jenway 6305, Bibby Scientific, Dunmow, Essex, UK).
2.8. Lipid Content
The lipid content was determined in triplicate for freeze-dried samples of washed and unwashed seaweed using a modified Bligh and Dyer method [
33].
2.9. Calorific or High Heating Value Determination
Higher heating values (HHV) or gross calorific values (CV) were measured using the UKAS (United Kingdom Accreditation Service) method for determination of calorific value with a Parr Model 1341 Bomb Calorimeter [
34]. Combustion in an adiabatic bomb containing oxygen under pressure oxidised the samples. The HHV was determined by measuring the temperature change in a known mass of water. The dissolved sulphate and nitrate were calculated from titration to adjust for their contribution. A mean is reported from a minimum of two determinations per sample.
2.10. Ensiling
Approximately 200 g of either washed or unwashed seaweed were placed in preweighed 300 mm × 200 mm food-grade composite (polyamide and polyethylene) bags (Andrew James Worldwide, Ferryhill, Co Durham. DL17 8JH), vacuum sealed (Andrew James Worldwide VS517), and weighed. The bags were then stored in the dark for 60 days in a temperature controlled cabinet at 20 °C ± 1 °C. At the end of the 60-day storage period, the bag was suspended by one corner with a 5-mm diameter hole in the upper corner of the bag releasing the vacuum. The lowest corner of the bag was cut to create a 30-mm opening. Leachate was allowed to drain from the bag for 10 min into a preweighed measuring cylinder via a funnel and 1-mm sieve. Then, the bag and its contents were weighed to calculate the mass of ensiled material. The funnel, sieve, and measuring cylinder were also weighed to calculate the mass of the leachate. The pH of the leachate was also measured (Hauna Instruments HI2210, Woonsocket, RI, USA).
2.11. Methane Potential Determination
The biomethane potential (BMP) of the washed and unwashed seaweed (excluding leachate) was analysed using a biomethane potential test system (CJC Labs Ltd., Nether Wasdale, Seascale, UK), shown in
Figure 1. The equipment consists of a controlled temperature water bath with 8 × 1 L plastic digestion containers, with each digester connected via a CO
2 fixing bottle to a tipping cup volumetric gas measuring device.
The 1 L digestion vessels were filled with inoculum and substrate, and made-up to a volume of 400 mL with deionised water. The inoculum was collected from an internal recirculation granular sludge anaerobic digester treating papermaking liquid waste at Smurfit Kappa Townsend Hook Paper Makers (Mill Street, Snodland, Kent, UK).
Table 1 gives the analysis of the inoculum solids. Three experimental replicates using 10 g wet weight of each variant, at an inoculum-to-substrate ratio of 9:1 on a volatile solids basis, were carried out, together with a control containing no substrate but containing inoculum.
After filling the digesters, the headspace was flushed with nitrogen, and the digestion bottles were sealed. The digesters were incubated for 28 days at a mesophilic temperature of 37 °C in a water bath. The contents of each digester were continuously mixed throughout the test by a slowly rotating paddle (~40 rpm). Biogas from each digester was passed through a fixing bottle containing 80 mL of 3 M NaOH solution (containing thymolphthalein indicator) for fixation of carbon dioxide. The resultant methane produced was subsequently measured in a tipping cup volumetric gas measuring device submerged in deionised water. Methane volume, pressure, and temperature data were recorded continuously, and gas volumes were normalised (100 kPa, 0 °C, dry gas).
The pH was measured (Hauna Instruments HI221) for each sample at the beginning and end of the BMP test.
2.12. Statistical Analysis
IBM SPSS Statistics (version 23) was used for two-way analysis of variance (ANOVA) with data tests for skewness (0.5 to −0.5), kurtosis (1 to −1), normality (Kolmogorov–Smirnov (>0.05), and Shapiro–Wilks (>0.05). A two-way ANOVA was performed to examine the effect washing and time and their interaction on daily cumulative methane production from the BMP test.
Microsoft Excel 2013was used for one-way ANOVA and all other statistical analyses. One-way ANOVAs were conducted to compare the effect of washing on water, ash, salt, lipid content, protein content, and calorific value.
5. Conclusions
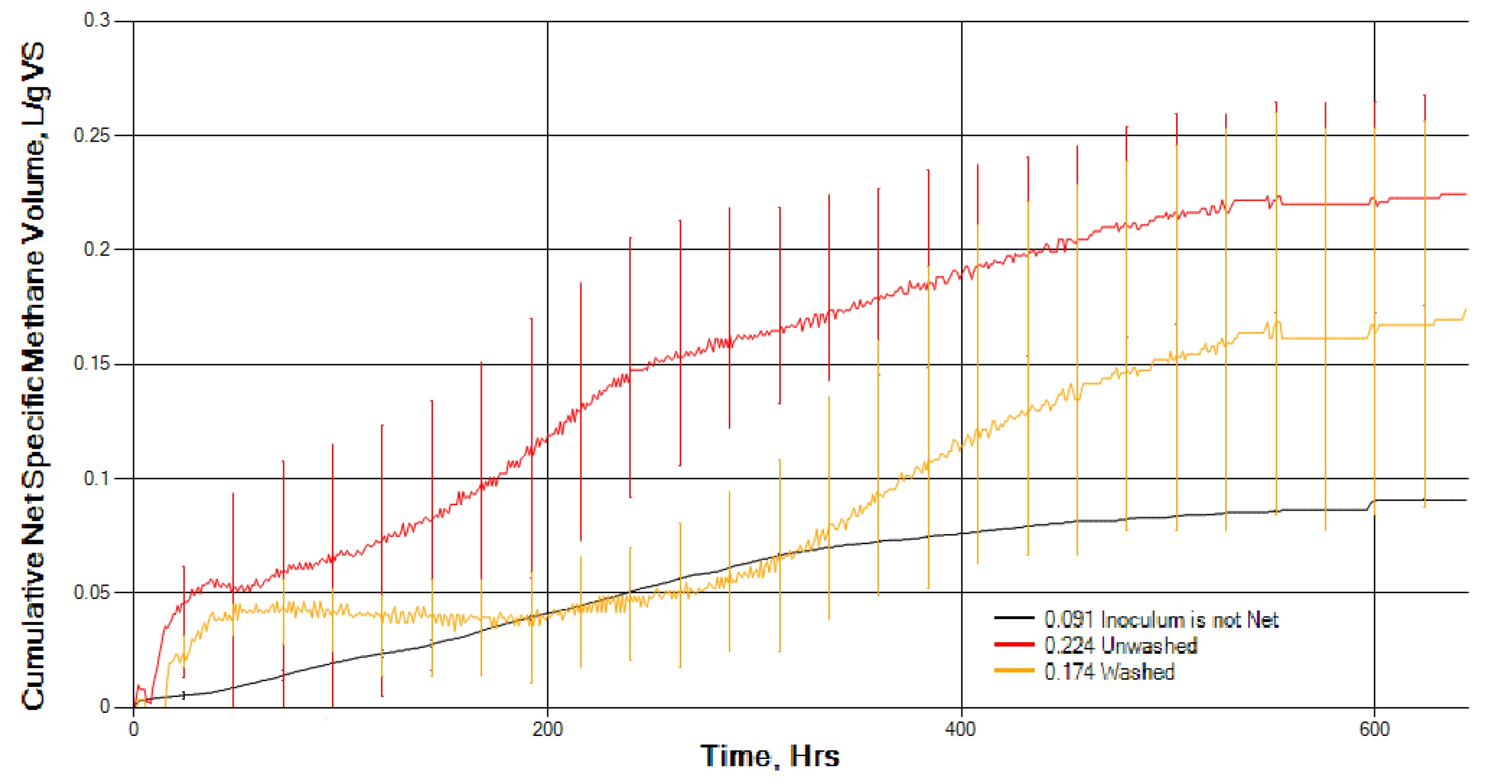
Washing in freshwater seems to have an effect on the composition of seaweed and must be taken into account when comparing research studies. Although washing seaweeds in freshwater prior to anaerobic digestion can reduce salt, an inhibitor of AD, it will add to the overall processing costs. Washing S. muticum did not affect overall BMP gas production during AD, but it did significantly slow down the initial rate of biomethane production. This could be due to the removal of readily digested soluble molecules in washing and/or the removal of naturally present hydrolytic bacteria from the surface of the seaweed. However, further research work appears to be warranted on the effect of washing on seaweed composition, particularly amino acids and carbohydrates, and natural bacterial biota and its effect on ensilage and anaerobic digestion.