Drivers of Wettability Alteration for Oil/Brine/Kaolinite System: Implications for Hydraulic Fracturing Fluids Uptake in Shale Rocks
Abstract
1. Introduction
2. Fluids and Procedures
2.1. Oil
2.2. Brines
2.3. Minerals
2.4. Experimental Procedure
3. Theoretical Background
3.1. Van der Waals Forces
3.2. Electrostatic Forces
3.3. Structural Forces
4. Results and Discussion
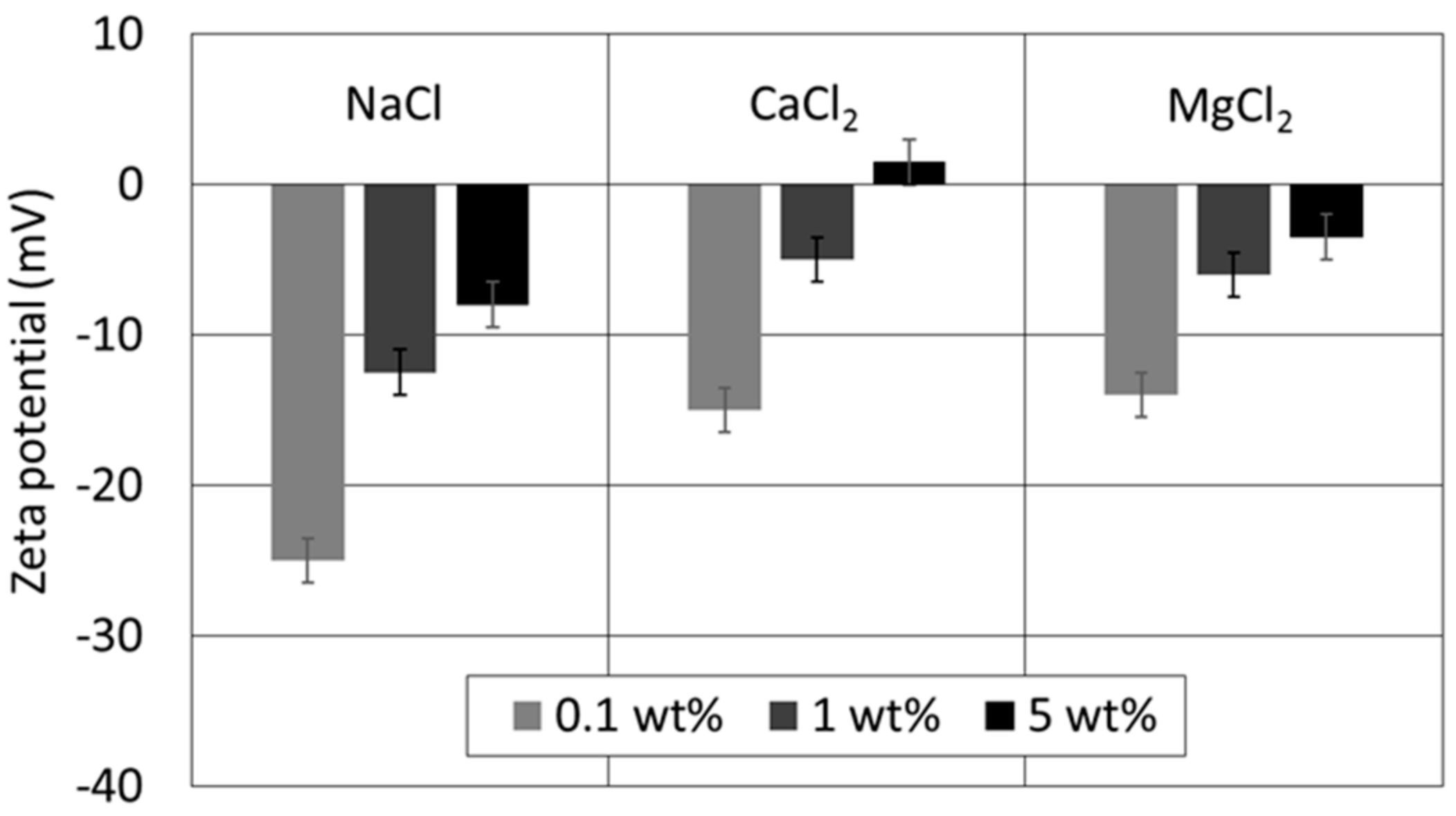
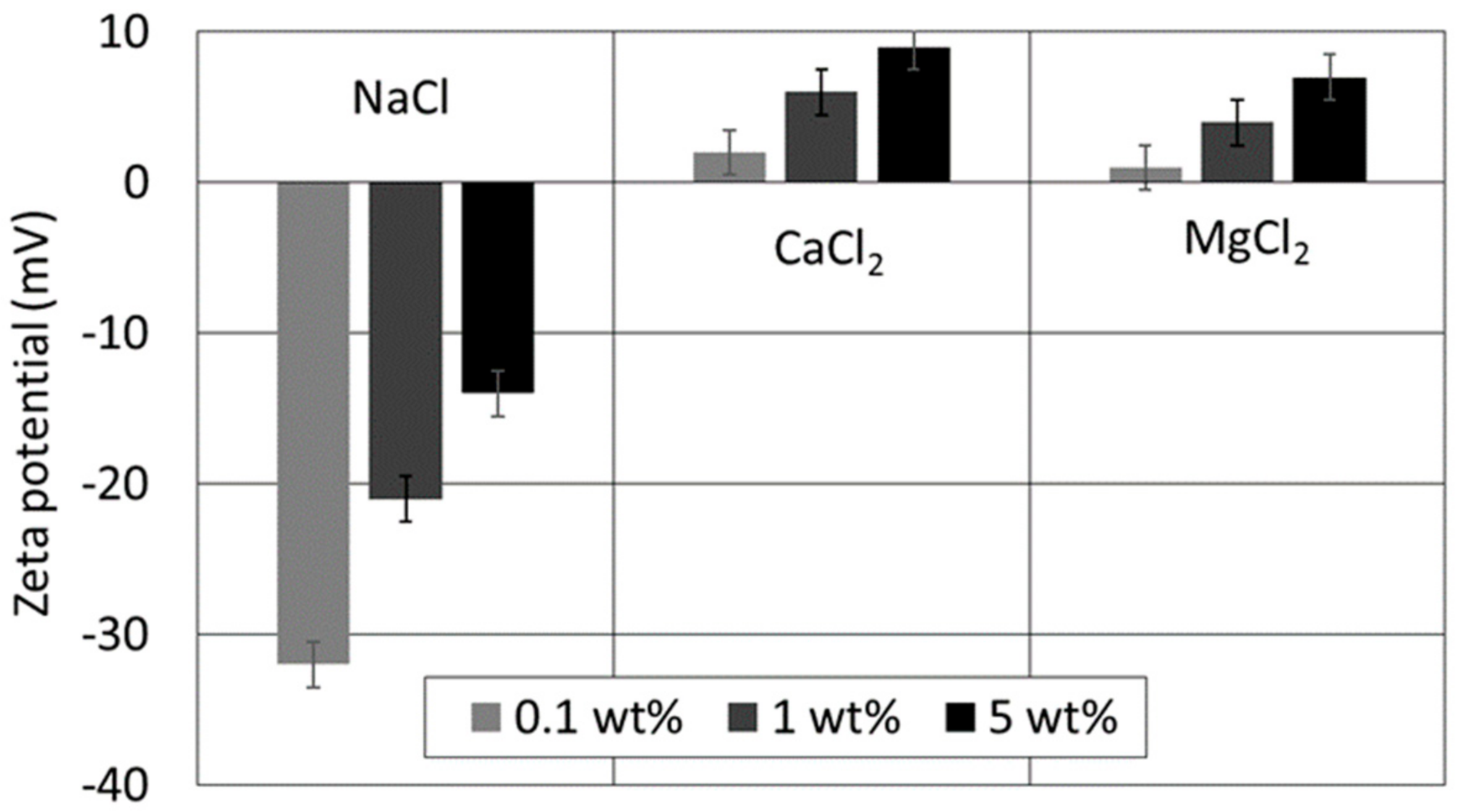
4.1. Effect of Ion Type and Concentration on Zeta Potential of Oil/Brines
4.2. Effect of Ion Type and Concentration on Zeta Potential of Brine/Kaolinite
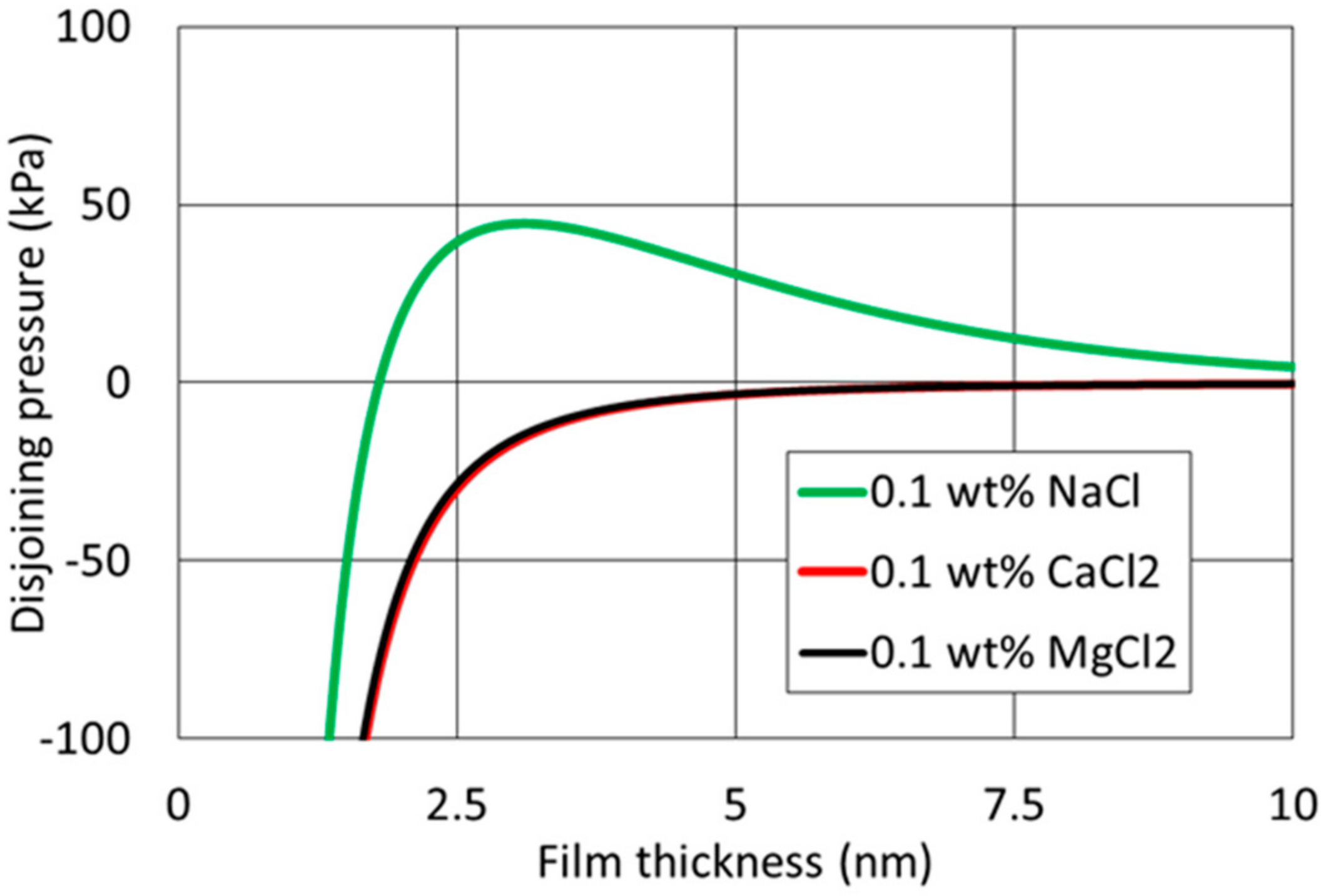
4.3. Effect of Cation Type and Concentration on Disjoining Pressure
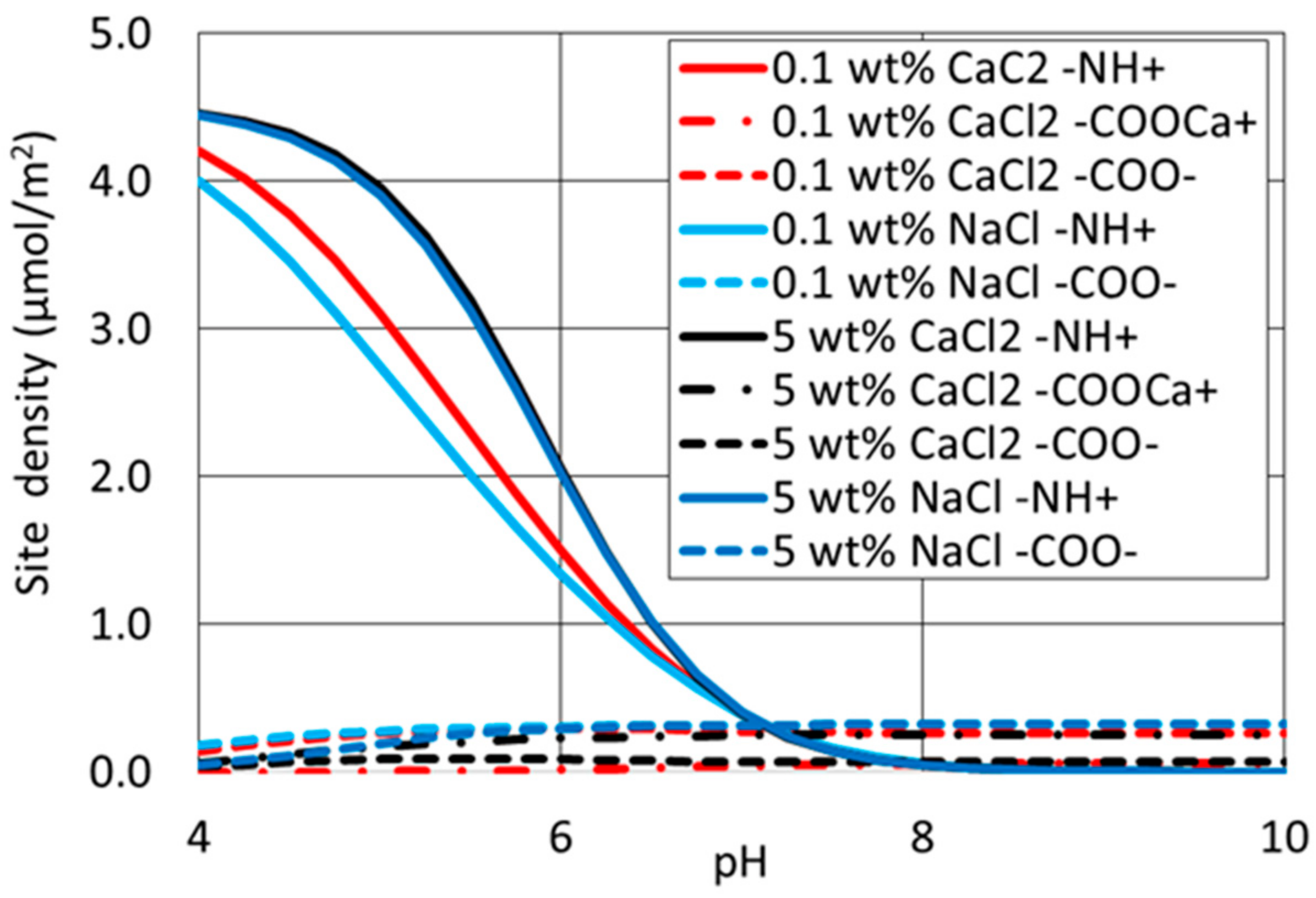
4.4. Surface Complexation Modelling
5. Implications and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pierre Gadonneix, A.S.; Liu, T. 2013 World Energy Issues Monitor; Pierre Gadonneix, World Energy Council: London, UK, 2013. [Google Scholar]
- Roshan, H.; Al-Yaseri, A.Z.; Sarmadivaleh, M.; Iglauer, S. On wettability of shale rocks. J. Colloid Interface Sci. 2016, 475, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.J. Critical review of field EOR projects in shale and tight reservoirs. J. Pet. Sci. Eng. 2017, 159, 654–665. [Google Scholar] [CrossRef]
- Engelder, T.; Cathles, L.M.; Bryndzia, L.T. The fate of residual treatment water in gas shale. J. Unconv. Oil Gas Resour. 2014, 7, 33–48. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Zubair, H.A.; Chhabra, A.; Ullah, A. Liquid Intake of Organic Shales. Energy Fuels 2012, 26, 5750–5758. [Google Scholar] [CrossRef]
- Ghanbari, E.; Dehghanpour, H. Impact of rock fabric on water imbibition and salt diffusion in gas shales. Int. J. Coal Geol. 2015, 138, 55–67. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Lan, Q.; Saeed, Y.; Fei, H.; Qi, Z. Spontaneous Imbibition of Brine and Oil in Gas Shales: Effect of Water Adsorption and Resulting Microfractures. Energy Fuels 2013, 27, 3039–3049. [Google Scholar] [CrossRef]
- Makhanov, K.; Habibi, A.; Dehghanpour, H.; Kuru, E. Liquid uptake of gas shales: A workflow to estimate water loss during shut-in periods after fracturing operations. J. Unconv. Oil Gas Resour. 2014, 7, 22–32. [Google Scholar] [CrossRef]
- Binazadeh, M.; Xu, M.; Zolfaghari, A.; Dehghanpour, H. Effect of Electrostatic Interactions on Water Uptake of Gas Shales: The Interplay of Solution Ionic Strength and Electrostatic Double Layer. Energy Fuels 2016, 30, 992–1001. [Google Scholar] [CrossRef]
- Roshan, H.; Andersen, M.S.; Rutlidge, H.; Marjo, C.E.; Acworth, R.I. Investigation of the kinetics of water uptake into partially saturated shales. Water Resour. Res. 2016, 52, 2420–2438. [Google Scholar] [CrossRef]
- Roshan, H.; Ehsani, S.; Marjo, C.E.; Andersen, M.S.; Acworth, R.I. Mechanisms of water adsorption into partially saturated fractured shales: An experimental study. Fuel 2015, 159, 628–637. [Google Scholar] [CrossRef]
- Fakcharoenphol, P.; Kurtoglu, B.; Kazemi, H.; Charoenwongsa, S.; Wu, Y.-S. The Effect of Osmotic Pressure on Improve Oil Recovery from Fractured Shale Formations; Society of Petroleum Engineers: Woodlands, TX, USA, 2014. [Google Scholar]
- Xu, M.; Dehghanpour, H. Advances in Understanding Wettability of Gas Shales. Energy Fuels 2014, 28, 4362–4375. [Google Scholar] [CrossRef]
- Buckley, J.S.; Takamura, K.; Morrow, R.N. Influence of electrical surface charges on the wetting properties of crude oils. SPE Reserv. Eng. 1989, 4, 332–342. [Google Scholar] [CrossRef]
- Hirasaki, G.J. Wettability: Fundamentals and surface forces. SPE Form. Eval. 1991, 6, 217–226. [Google Scholar] [CrossRef]
- Matthiesen, J.; Hassenkam, T.; Bovet, N.; Dalby, K.N.; Stipp, S.L.S. Adsorbed Organic Material and Its Control on Wettability. Energy Fuels 2017, 31, 55–64. [Google Scholar] [CrossRef]
- Wu, J.; Liu, F.; Yang, H.; Xu, S.; Xie, Q.; Zhang, M.; Chen, T.; Hu, G.; Wang, J. Effect of specific functional groups on oil adhesion from mica substrate: Implications for low salinity effect. J. Ind. Eng. Chem. 2017, 56, 342–349. [Google Scholar] [CrossRef]
- Myint, P.C.; Firoozabadi, A. Thin liquid films in improved oil recovery from low-salinity brine. Curr. Opin. Colloid Interface Sci. 2015, 20, 105–114. [Google Scholar] [CrossRef]
- Brady, P.V.; Bryan, C.R.; Thyne, G.; Li, H. Altering wettability to recover more oil from tight formations. J. Unconv. Oil Gas Resour. 2016, 15, 79–83. [Google Scholar] [CrossRef]
- Brady, P.V.; Morrow, N.R.; Fogden, A.; Deniz, V.; Loahardjo, N. Electrostatics and the Low Salinity Effect in Sandstone Reservoirs. Energy Fuels 2015, 29, 666–677. [Google Scholar] [CrossRef]
- Austad, T.; Rezaeidoust, A.; Puntervold, T. Chemical Mechanism of Low Salinity Water Flooding in Sandstone Reservoirs; Society of Petroleum Engineers: Tulsa, Oklahoma, USA, 2010. [Google Scholar]
- Zhang, D. Surfactant-Enhanced Oil Recovery Process for a Fractured, Oil-Wet Carbonate Reservoir; RICE University: Houston, TX, USA, 2006. [Google Scholar]
- Busireddy, C.; Rao, D.N. Application of DLVO Theory to Characterize Spreading in Crude Oil-Brine-Rock Systems. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2004; Society of Petroleum Engineers: Tulsa, Oklahoma, USA, 2004. [Google Scholar]
- Melrose, J. Interpretation of mixed wettability states in reservoir rocks. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 26–29 September 1982. [Google Scholar]
- Gregory, J. Interaction of unequal double layers at constant charge. J. Colloid Interface Sci. 1975, 51, 44–51. [Google Scholar] [CrossRef]
- Hirasaki, G. Interfacial Phenomina in Petroleum Recovery; Marcel Dekker: New York, NY, USA, 1991; Chapter 3. [Google Scholar]
- Nasralla, R.A.; Nasr-El-Din, H.A. Impact of cation type and concentration in injected brine on oil recovery in sandstone reservoirs. J. Pet. Sci. Eng. 2014, 122, 384–395. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, G.; Wang, L.; Wu, W.; Ge, J. Zeta potential of limestone in a large range of salinity. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 1–8. [Google Scholar] [CrossRef]
- Tokunaga, T.K. DLVO-based estimates of adsorbed water film thicknesses in geologic CO2 reservoirs. Langmuir 2012, 28, 8001–8009. [Google Scholar] [CrossRef] [PubMed]
- Nyström, R.; Lindén, M.; Rosenholm, J.B. The Influence of Na+, Ca2+, Ba2+, and La3+ on the ζ Potential and the Yield Stress of Calcite Dispersions. J. Colloid Interface Sci. 2001, 242, 259–263. [Google Scholar] [CrossRef]
- Takahashi, S.; Kovscek, A.R. Wettability estimation of low-permeability, siliceous shale using surface forces. J. Pet. Sci. Eng. 2010, 75, 33–43. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Chapter 14—Electrostatic Forces between Surfaces in Liquids. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 291–340. [Google Scholar]
- Israelachvili, J.N. Chapter 13—Van der Waals Forces between Particles and Surfaces. In Intermolecular and Surface Forces, 3rd ed.; Israelachvili, J.N., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 253–289. [Google Scholar]
- Xie, Q.; Saeedi, A.; Pooryousefy, E.; Liu, Y. Extended DLVO-based estimates of surface force in low salinity water flooding. J. Mol. Liq. 2016, 221, 658–665. [Google Scholar] [CrossRef]
- Xie, Q.; Brady, P.V.; Pooryousefy, E.; Zhou, D.; Liu, Y.; Saeedi, A. The low salinity effect at high temperatures. Fuel 2017, 200, 419–426. [Google Scholar] [CrossRef]
- Brady, P.V.; Thyne, G. Functional Wettability in Carbonate Reservoirs. Energy Fuels 2016, 30, 9217–9225. [Google Scholar] [CrossRef]
- RezaeiDoust, A.; Puntervold, T.; Austad, T. Chemical Verification of the EOR Mechanism by Using Low Saline/Smart Water in Sandstone. Energy Fuels 2011, 25, 2151–2162. [Google Scholar] [CrossRef]

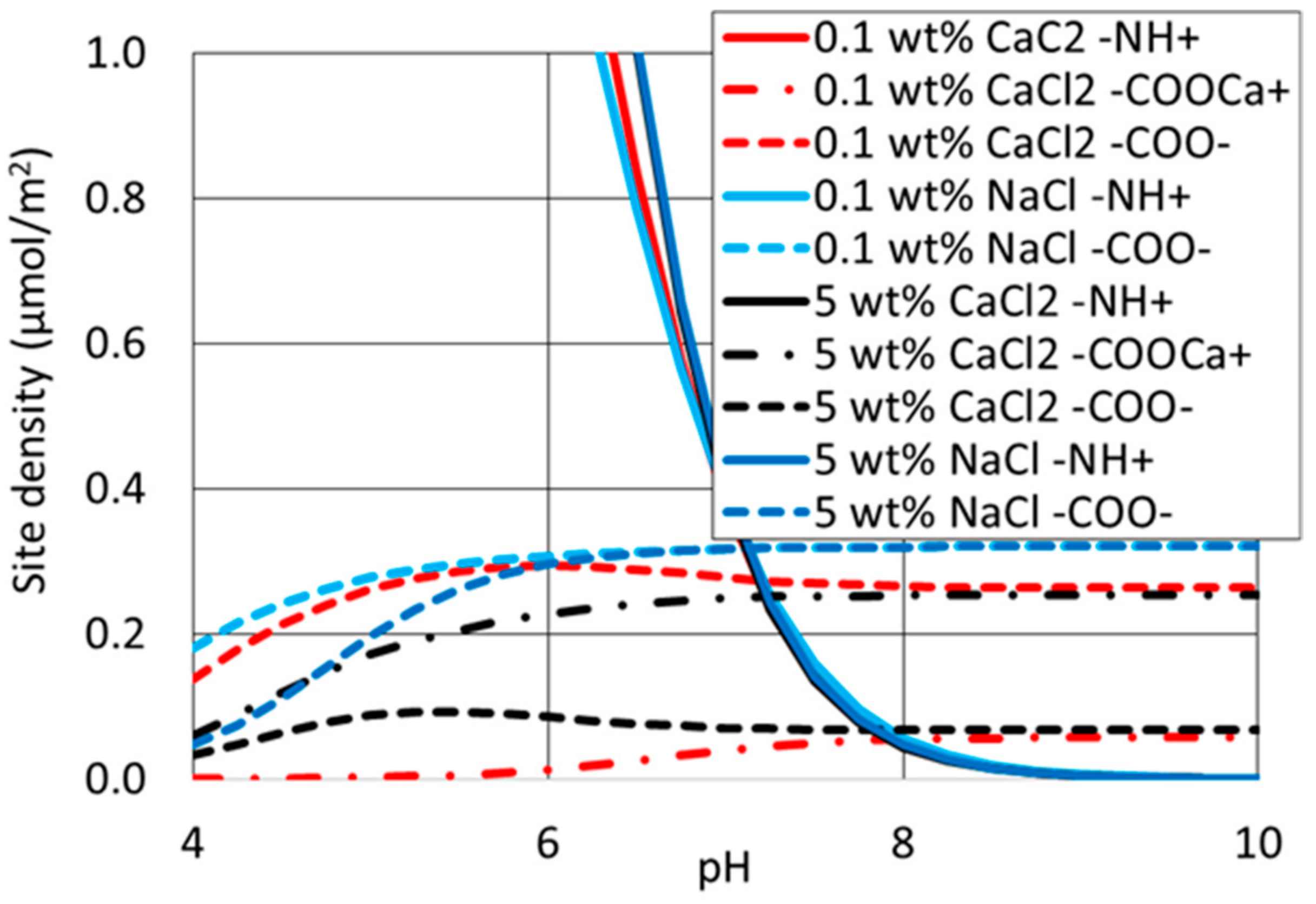
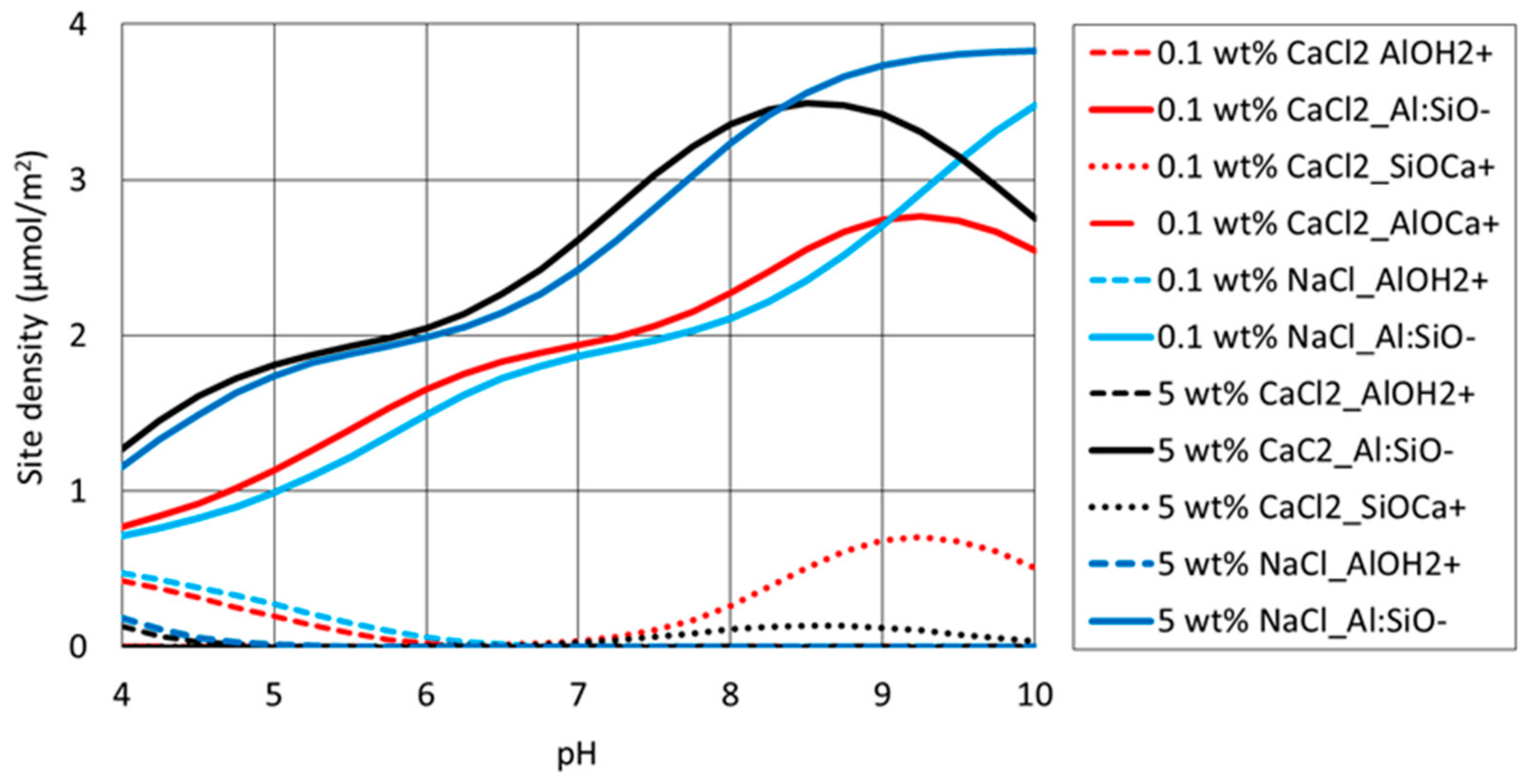
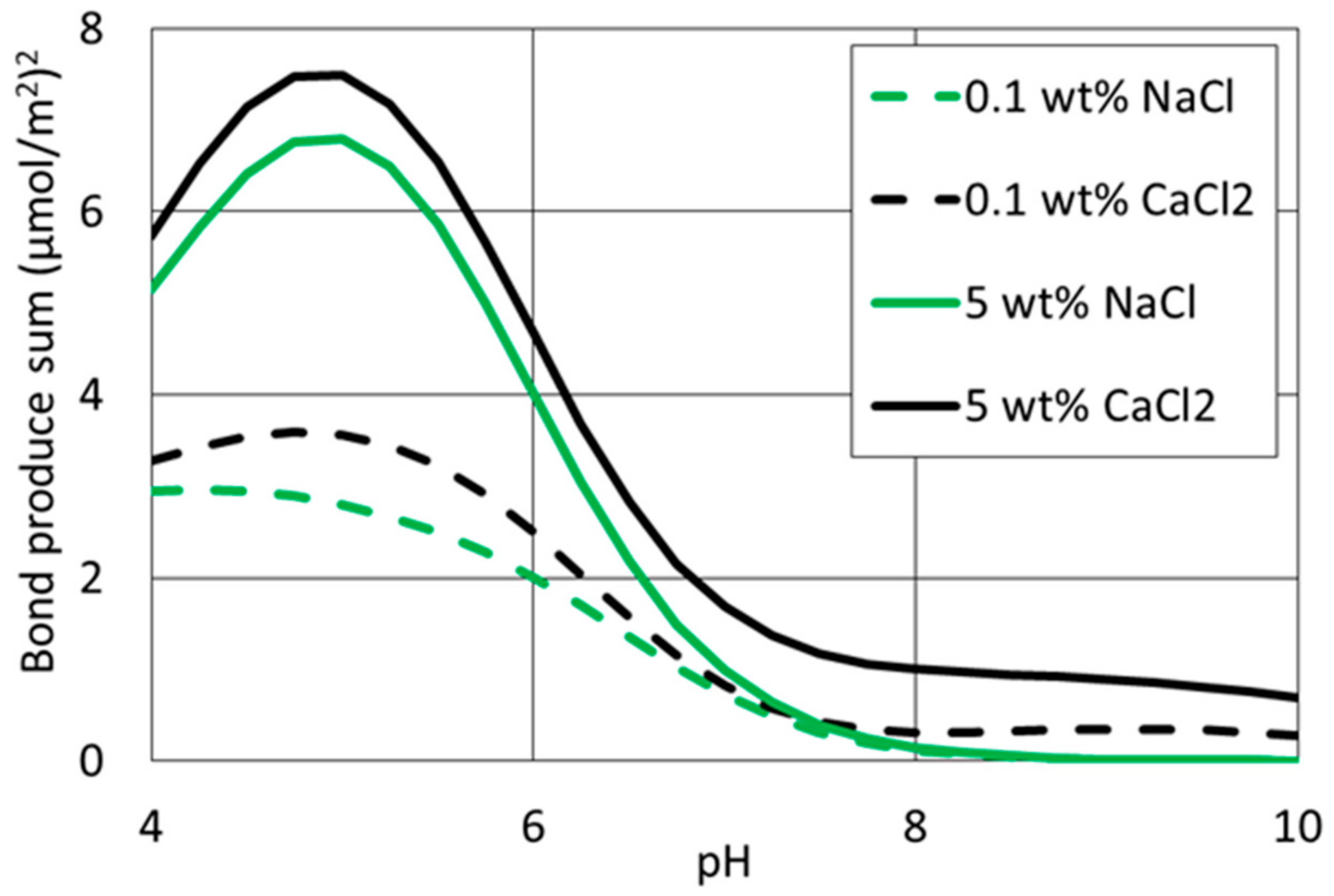
| Interface | Reaction | Log K (25 °C) | Reaction |
|---|---|---|---|
| oil/brine | -NH+ ↔ -N + H+ | −6.0 | 1 |
| -COOH ↔ -COO- + H+ | −5.0 | 2 | |
| -COOH + Ca2+ ↔ -COOCa+ + H+ | −3.8 | 3 | |
| kaolinite/brine | >AlOH2+ ↔ >AlOH + H+ | −3.0 | 4 |
| >AlOH ↔ >AlO− + H+ | −3.8 | 5 | |
| >SiOH ↔ >SiO− + H+ | −7.0 | 6 | |
| >AlOH + Ca2+ ↔ >AlOCa+ + H+ | −9.7 | 7 | |
| >SiOH + Ca2+ ↔ >SiOCa+ + H+ | −9.7 | 8 | |
| >AlOH + CaOH+ ↔ >AlOCaOH + H+ | −4.5 | 9 | |
| >SiOH + CaOH+ ↔ >SiOCaOH + H+ | −4.5 | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Chen, Y.; You, L.; Hossain, M.M.; Saeedi, A. Drivers of Wettability Alteration for Oil/Brine/Kaolinite System: Implications for Hydraulic Fracturing Fluids Uptake in Shale Rocks. Energies 2018, 11, 1666. https://doi.org/10.3390/en11071666
Xie Q, Chen Y, You L, Hossain MM, Saeedi A. Drivers of Wettability Alteration for Oil/Brine/Kaolinite System: Implications for Hydraulic Fracturing Fluids Uptake in Shale Rocks. Energies. 2018; 11(7):1666. https://doi.org/10.3390/en11071666
Chicago/Turabian StyleXie, Quan, Yongqiang Chen, Lijun You, Md Mofazzal Hossain, and Ali Saeedi. 2018. "Drivers of Wettability Alteration for Oil/Brine/Kaolinite System: Implications for Hydraulic Fracturing Fluids Uptake in Shale Rocks" Energies 11, no. 7: 1666. https://doi.org/10.3390/en11071666
APA StyleXie, Q., Chen, Y., You, L., Hossain, M. M., & Saeedi, A. (2018). Drivers of Wettability Alteration for Oil/Brine/Kaolinite System: Implications for Hydraulic Fracturing Fluids Uptake in Shale Rocks. Energies, 11(7), 1666. https://doi.org/10.3390/en11071666







