The Effect of Fuel Injection Equipment of Water-In-Diesel Emulsions on Micro-Explosion Behaviour
Abstract
:1. Introduction
2. Experimental Method
2.1. Stability and Physical Properties of Emulsified Blends
2.2. Fuel Injection System
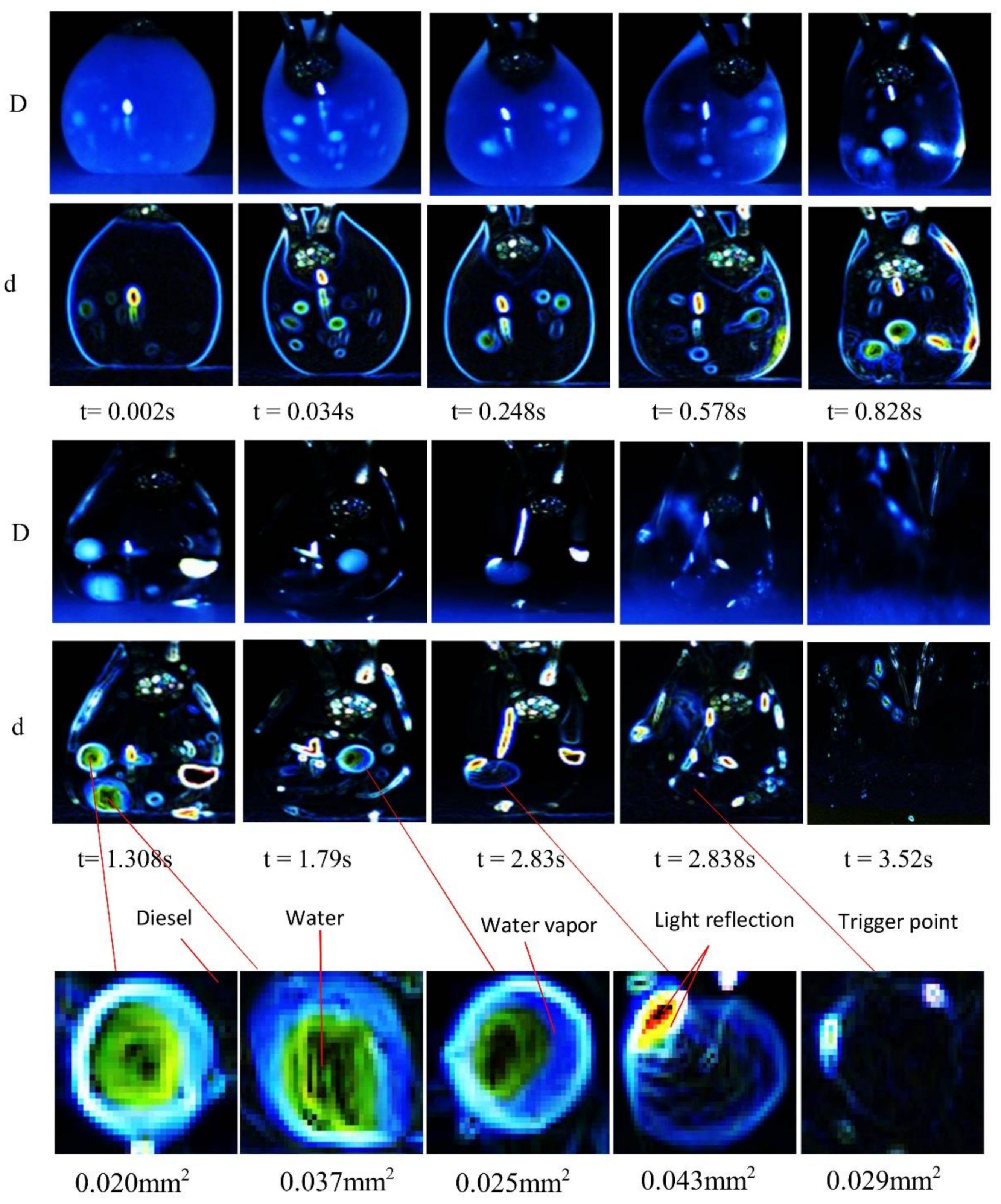
2.3. Image acquisition, Processing and Analysis
2.4. Hot Plate Technique
3. Results and Discussion
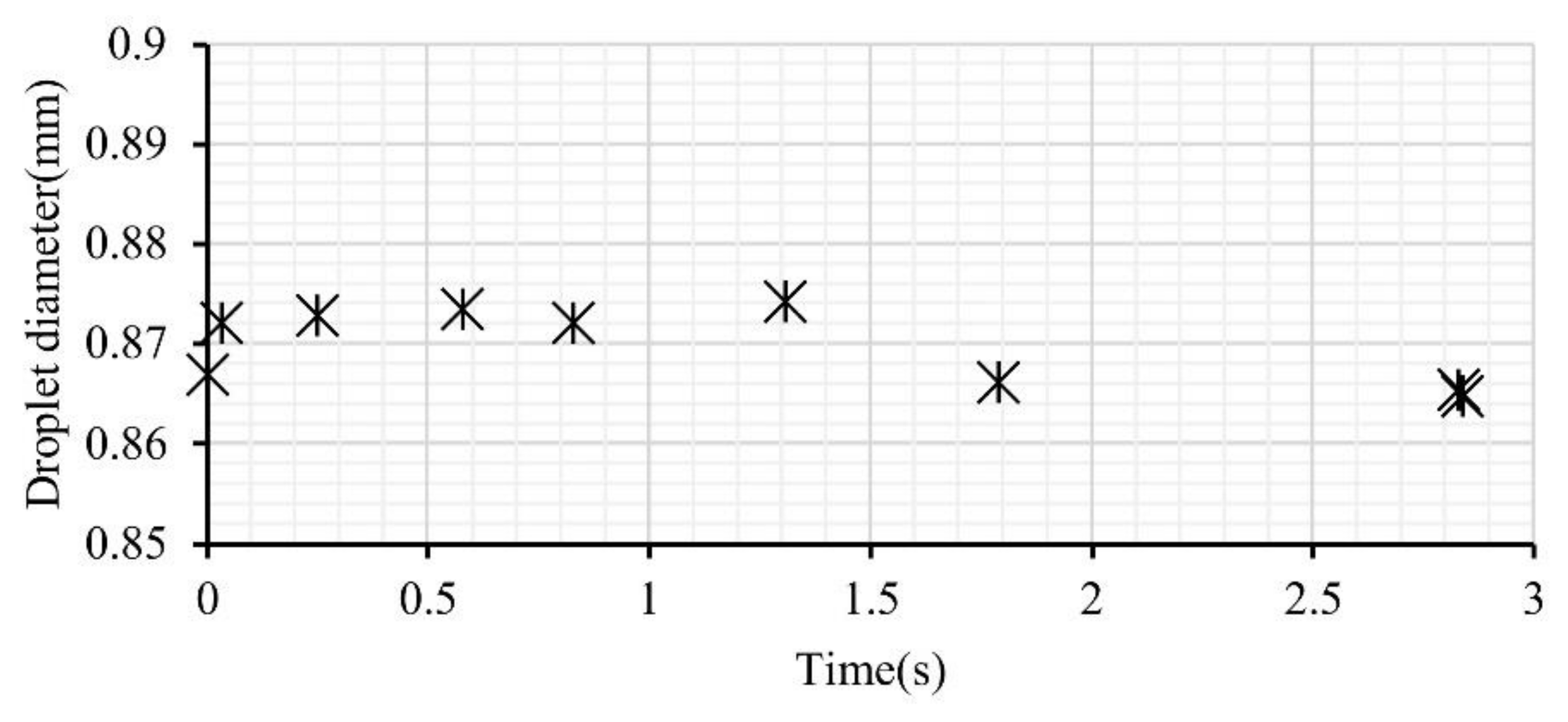
3.1. The Effect of Injector Shearing on Droplet Size Distribution
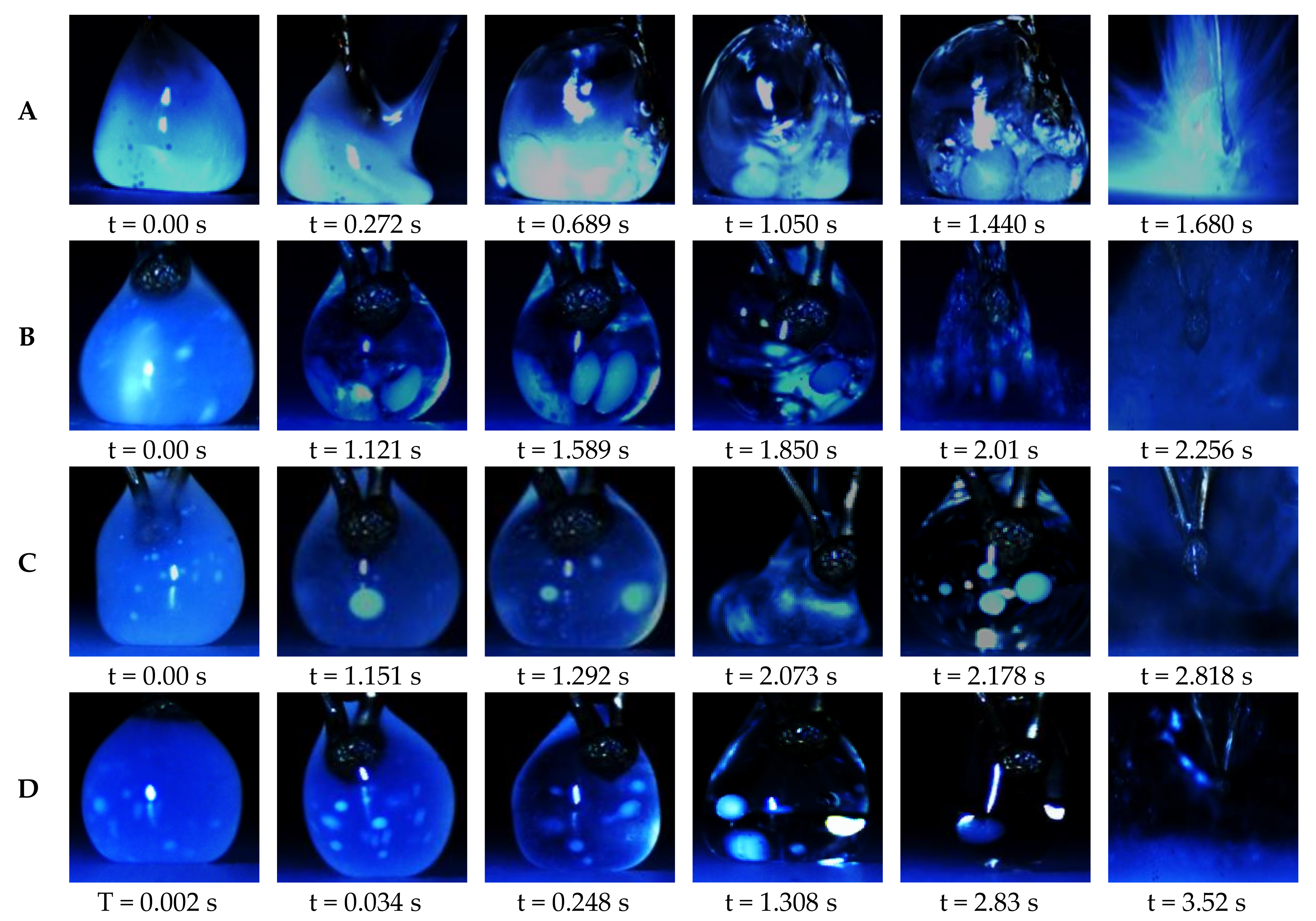
3.2. Micro-Explosion Phenomenon
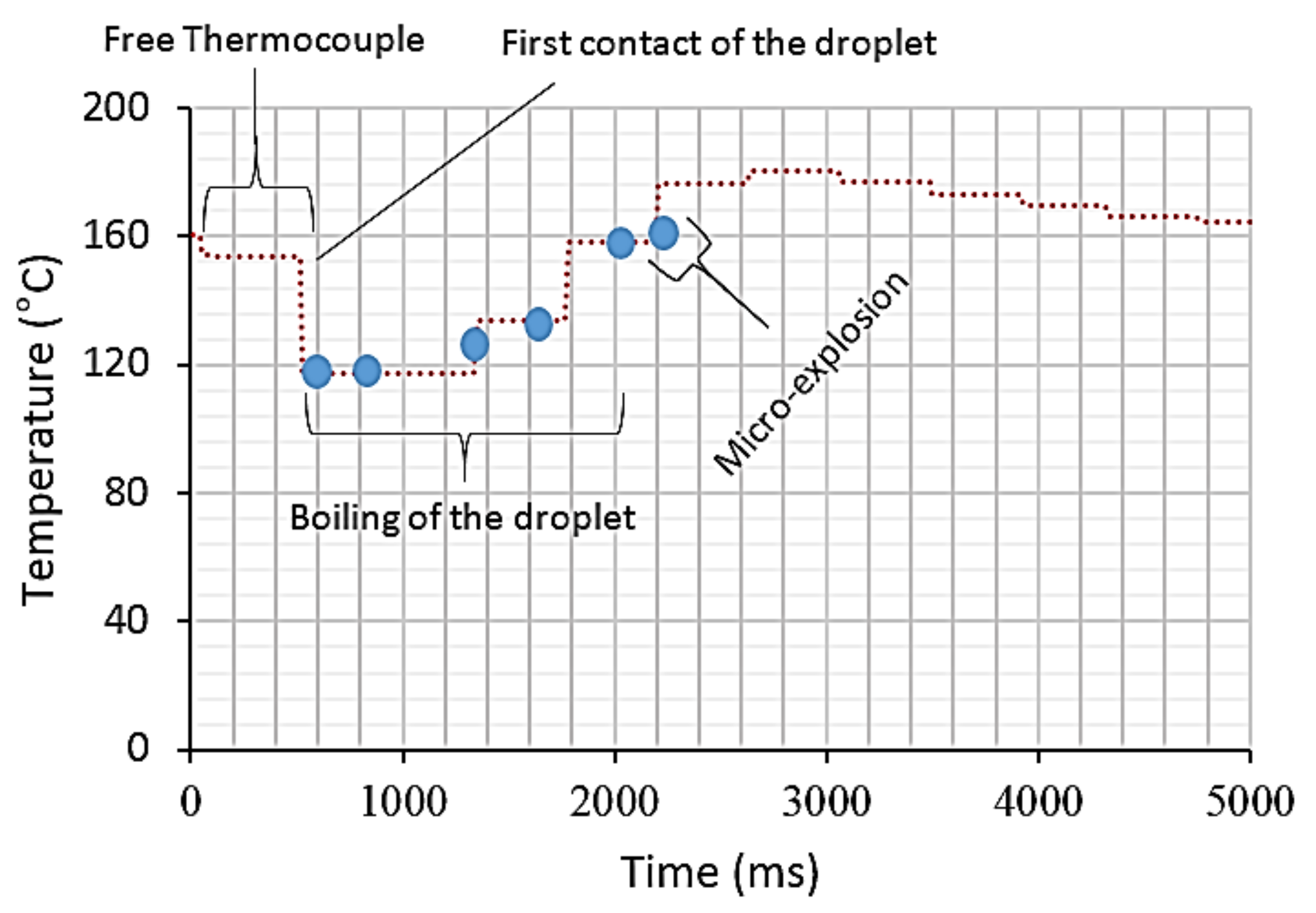
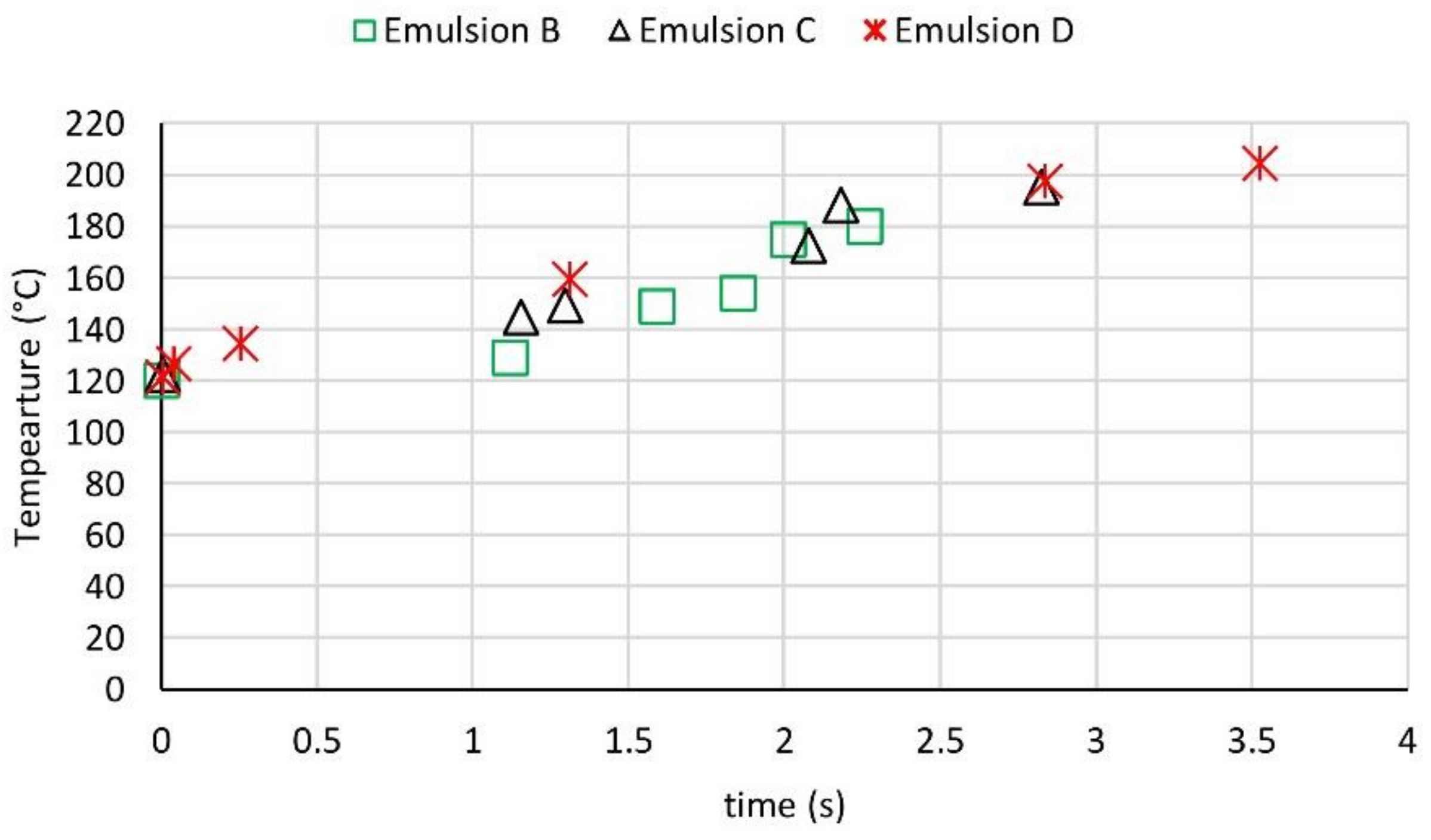
3.3. Temporal Temperature Profiles
3.4. The Effect of Dispersed Water Droplet Coalescence on Micro-Explosion Behavior
4. Conclusions
- (1)
- The dispersed droplet sizes reduce significantly after the emulsion is injected through the nozzle’s orifices, and some of the water is lost through evaporation during the injection.
- (2)
- Micro-explosions were delayed as a result of the shear in the injector nozzle. This was found to be the result of a shift of the emulsion towards a nano-emulsion, leading to the deceleration of the coalescence rate which, in turn, increased the stability of the emulsion.
- (3)
- For fine dispersed water droplets, the micro-explosion time depends on the explosive boiling of multiple water sub-droplet coalescence into larger droplet.
- (4)
- The temperature of micro-explosion increases with injection pressure.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sazhin, S.; Crua, C.; Kennaird, D.; Heikal, M. The initial stage of fuel spray penetration. Fuel 2003, 82, 875–885. [Google Scholar] [CrossRef]
- Raju, V.R.K.; Rao, S.S. Effect of Fuel Injection Pressure and Spray Cone Angle in DI Diesel Engine Using CONVERGETM CFD Code. Procedia Eng. 2015, 127, 295–300. [Google Scholar]
- Yu, X.; Zuo, X.; Wu, H.; Du, Y.; Sun, Y.; Wang, Y. Study on Combustion and Emission Characteristics of a Combined Injection Engine with Hydrogen Direct Injection. Energy Fuels 2017, 31, 5554–5560. [Google Scholar] [CrossRef]
- Yang, S.; Moon, H.; Lee, C. A Study of Spill Control Characteristics of JP-8 and Conventional Diesel Fuel with a Common Rail Direct Injection System. Energies 2017, 10, 2104. [Google Scholar] [CrossRef]
- Kadota, T.; Yamasaki, H. Recent advances in the combustion of water fuel emulsion. Prog. Energy Combust. Sci. 2002, 28, 385–404. [Google Scholar] [CrossRef]
- Ithnin, A.M.; Noge, H.; Kadir, H.A.; Jazair, W. An overview of utilizing water-in-diesel emulsion fuel in diesel engine and its potential research study. J. Energy Inst. 2014, 87, 273–288. [Google Scholar] [CrossRef]
- Feng, L.; Du, B.; Tian, J.; Long, W.; Tang, B. Combustion performance and emission characteristics of a diesel engine using a water-emulsified heavy fuel oil and light diesel blend. Energies 2015, 8, 13628–13640. [Google Scholar] [CrossRef]
- Lif, A.; Holmberg, K. Water-in-diesel emulsions and related systems. Adv. Colloid Interface Sci. 2006, 123–126, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, T.; Ma, P.; Wang, B. Spray Combustion Characteristics and Soot Emission Reduction of Hydrous Ethanol Diesel Emulsion Fuel Using Color-Ratio Pyrometry. Energies 2017, 10, 2062. [Google Scholar] [CrossRef]
- Saad, M.A. Evaporation and Combustion of Single Fuel Deoplet in a Hot Atmosphere; University of Michigan: Ann Arbor, MI, USA, 1956. [Google Scholar]
- Law, C.K.; Lee, C.H.; Srinivasan, N. Combustion characteristics of water-in-oil emulsion droplets. Combust. Flame 1980, 37, 125–143. [Google Scholar] [CrossRef]
- Kadota, T.; Tanaka, H.; Segawa, D.; Nakaya, S.; Yamasaki, H. Microexplosion of an emulsion droplet during Leidenfrost burning. Proc. Combust. Inst. 2007, 31, 2125–2131. [Google Scholar] [CrossRef]
- Ivanove, V.M.; Nefedov, P.I. Experimental investigation of ths combustion process of natural and emulsified liquid fuels. Natl. Aeronaut. Space Adm. Natl. Sci. Found. 1965, 19, 35–45. [Google Scholar]
- Avulapati, M.M.; Ganippa, L.C.; Xia, J.; Megaritis, A. Puffing and micro-explosion of diesel-biodiesel-ethanol blends. Fuel 2016, 166, 59–66. [Google Scholar] [CrossRef]
- Califano, V.; Calabria, R.; Massoli, P. Experimental evaluation of the effect of emulsion stability on micro-explosion phenomena for water-in-oil emulsions. Fuel 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Fu, W.B.; Hou, L.Y.; Wang, L.; Ma, F.H. A unified model for the micro-explosion of emulsified droplets of oil and water. Fuel Process. Technol. 2002, 79, 107–119. [Google Scholar] [CrossRef]
- Girin, O.G. Dynamics of Secondary Breakup of Emulsified Fuel Drop. In Proceedings of the 25th ICDERS, Leeds, UK, 2–7 August 2015; pp. 1–6. [Google Scholar]
- Jeong, I.; Lee, K.-H.; Kim, J. Characteristics of auto-ignition and micro-explosion behavior of a single droplet of water-in-fuel. J. Mech. Sci. Technol. 2008, 22, 148–156. [Google Scholar] [CrossRef]
- Park, S.; Woo, S.; Kim, H.; Lee, K. The characteristic of spray using diesel water emulsified fuel in a diesel engine. Appl. Energy 2016, 176, 209–220. [Google Scholar] [CrossRef]
- Arabaci, E.; Içingür, Y.; Solmaz, H.; Uyumaz, A.; Yilmaz, E. Experimental investigation of the effects of direct water injection parameters on engine performance in a six-stroke engine. Energy Convers. Manag. 2015, 98, 89–97. [Google Scholar] [CrossRef]
- Yang, W.M.; An, H.; Chou, S.K.; Chua, K.J.; Mohan, B.; Sivasankaralingam, V.; Raman, V.; Maghbouli, A.; Li, J. Impact of emulsion fuel with nano-organic additives on the performance of diesel engine. Appl. Energy 2013, 112, 1206–1212. [Google Scholar] [CrossRef]
- Bedford, F.; Rutland, C.; Dittrich, P.; Wirbeleit, F. Effects of Direct Water Injection on di Diesel Engine Combustion; SAE Paper 01-2938; SAE: Warrendale, PA, USA, 2000. [Google Scholar]
- Lin, C.Y.; Chen, L.W. Comparison of fuel properties and emission characteristics of two- and three-phase emulsions prepared by ultrasonically vibrating and mechanically homogenizing emulsification methods. Fuel 2008, 87, 2154–2161. [Google Scholar] [CrossRef]
- Attia, A.M.A.; Kulchitskiy, A.R. Influence of the structure of water-in-fuel emulsion on diesel engine performance. Fuel 2014, 116, 703–708. [Google Scholar] [CrossRef]
- Khan, M.Y.; Karim, Z.A.A.; Aziz, A.R.A.; Morgan, R.; Crua, C. Puffing and Microexplosion Behavior of Water in Pure Diesel Emulsion Droplets During Leidenfrost Effect. Combust. Sci. Technol. 2017, 189, 1186–1197. [Google Scholar] [CrossRef]
- Volkov, R.S.; Kuznetsov, G.V.; Strizhak, P.A. Influence of droplet concentration on evaporation in a high-temperature gas. Int. J. Heat Mass Transf. 2016, 96, 20–28. [Google Scholar] [CrossRef]
- Morozumi, Y.; Saito, Y. Effect of physical properties on microexplosion occurrence in water-in-oil emulsion droplets. Energy Fuels 2010, 24, 1854–1859. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, X.; He, Z.; Wu, J. Experimental study on the combustion and microexplosion of freely falling gelled unsymmetrical dimethylhydrazine (UDMH) fuel droplets. Energies 2012, 5, 3126–3136. [Google Scholar] [CrossRef]
- Khan, M.Y.; Karim, Z.A.A.; Aziz, A.R.A.; Tan, I.M. Experimental Investigation of Microexplosion Occurrence in Water in Diesel Emulsion Droplets during the Leidenfrost Effec. Energy Fuels 2014, 28, 7079–7084. [Google Scholar] [CrossRef]
- Kimoto, Y.; Owashi, K.; Omae, Y. The vaporizing behavior of the fuel droplet of water-in-oil emulsion on the hot surface. Bull. JSME 1986, 258, 4247–4255. [Google Scholar] [CrossRef]
- Mura, E.; Massoli, P.; Josset, C.; Loubar, K.; Bellettre, J. Study of the micro-explosion temperature of water in oil emulsion droplets during the Leidenfrost effect. Exp. Therm. Fluid Sci. 2012, 43, 63–70. [Google Scholar] [CrossRef]
- Mura, E.; Josset, C.; Loubar, K.; Huchet, G.; Bellettre, J. Effect of dispersed water droplet size in microexplosion phenomenon for water in oil emulsion. At. Sprays 2010, 20, 791–799. [Google Scholar] [CrossRef]
- Ismael, M.A.; Heikal, M.R.; Baharom, M.B. Spray characteristics of a diesel-cng dual fuel jet using the schlieren imaging technique. Int. J. Mater. Mech. Manuf. 2015, 3, 145–151. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Mura, E.; Calabria, R.; Califano, V.; Massoli, P.; Bellettre, J. Emulsion droplet micro-explosion: Analysis of two experimental approaches. Exp. Therm. Fluid Sci. 2014, 56, 69–74. [Google Scholar] [CrossRef]
- Shinjo, J.; Xia, J.; Ganippa, L.C.; Megaritis, A. Physics of puffing and microexplosion of emulsion fuel droplets. Phys. Fluids 2014, 26, 103302. [Google Scholar] [CrossRef] [Green Version]
- Dittmann, P.; Dörksen, H.; Steiding, D.; Kremer, F. Influence of Micro Emulsions on Diesel Engine Combustion. MTZ Worldw. 2015, 76, 38–44. [Google Scholar] [CrossRef]
- Tarlet, D.; Bellettre, J.; Tazerout, M.; Rahmouni, C. Prediction of micro-explosion delay of emulsified fuel droplets. Int. J. Therm. Sci. 2009, 48, 449–460. [Google Scholar] [CrossRef]
- Abu-Zaid, M. An experimental study of the evaporation characteristics of emulsified liquid droplets. Heat Mass Transf. 2004, 40, 737–741. [Google Scholar] [CrossRef]
- Mohan, S.; Narsimhan, G. Coalescence of protein-stabilized emulsions in a high-pressure homogenizer. J. Colloid Interface Sci. 1997, 192, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Harada, T.; Watanabe, H.; Shoji, M.; Matsushita, Y.; Aoki, H.; Miura, T. Visualization of aggregation process of dispersed water droplets and the effect of aggregation on secondary atomization of emulsified fuel droplets. Proc. Combust. Inst. 2011, 33, 2063–2070. [Google Scholar] [CrossRef]
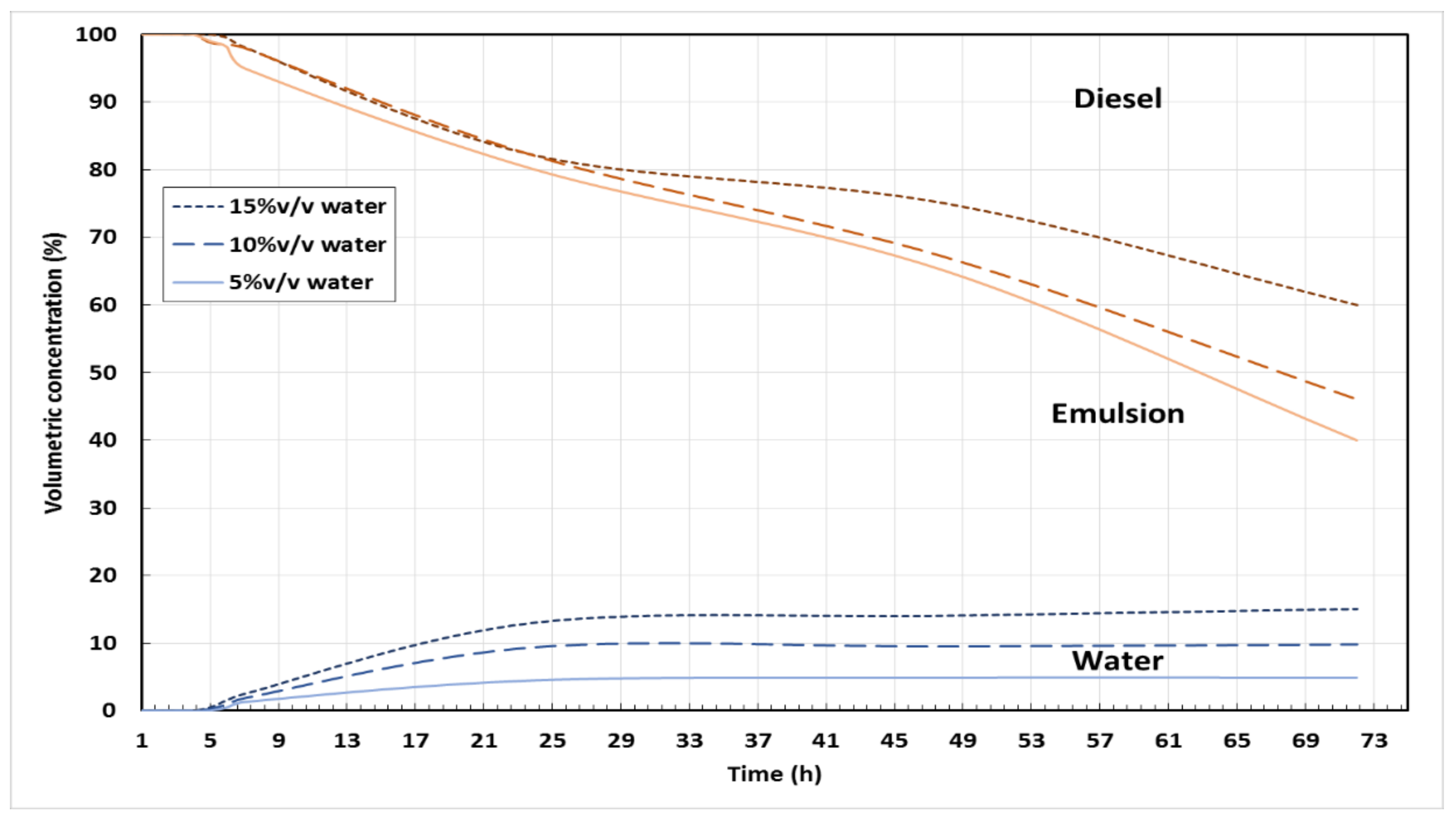
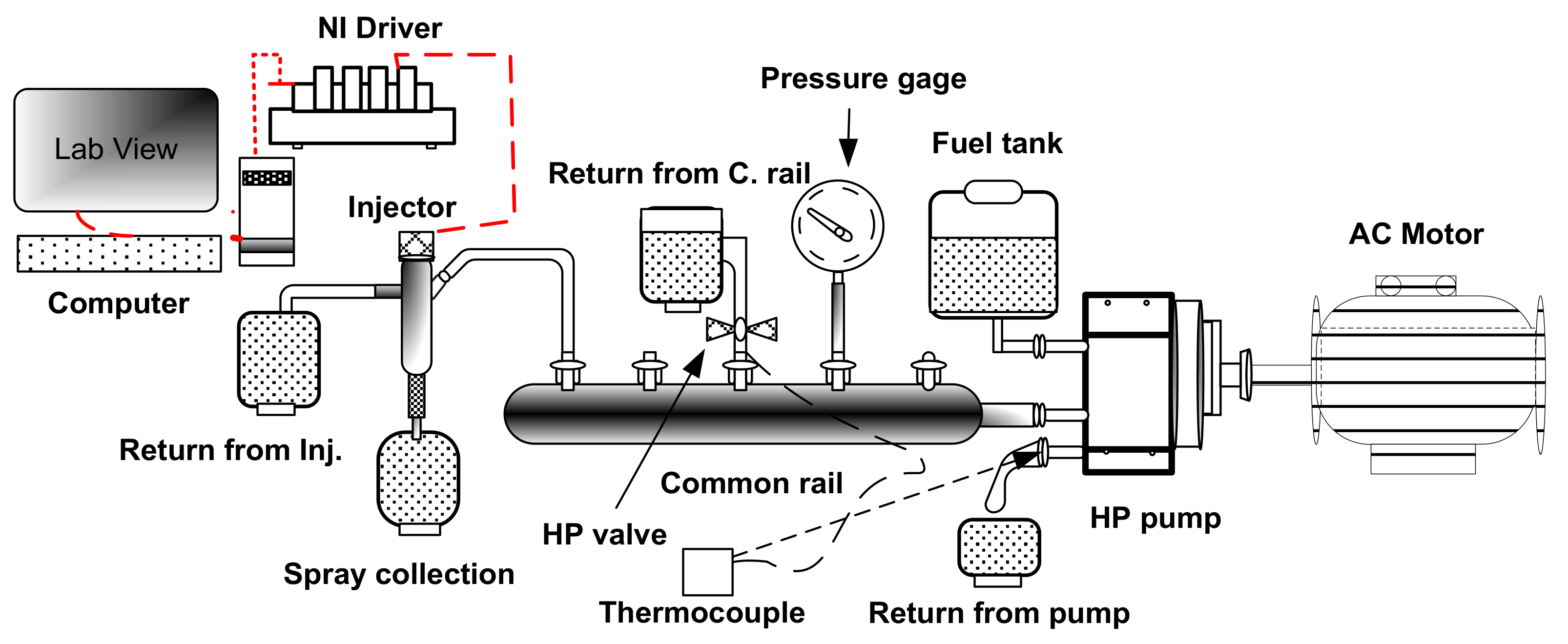
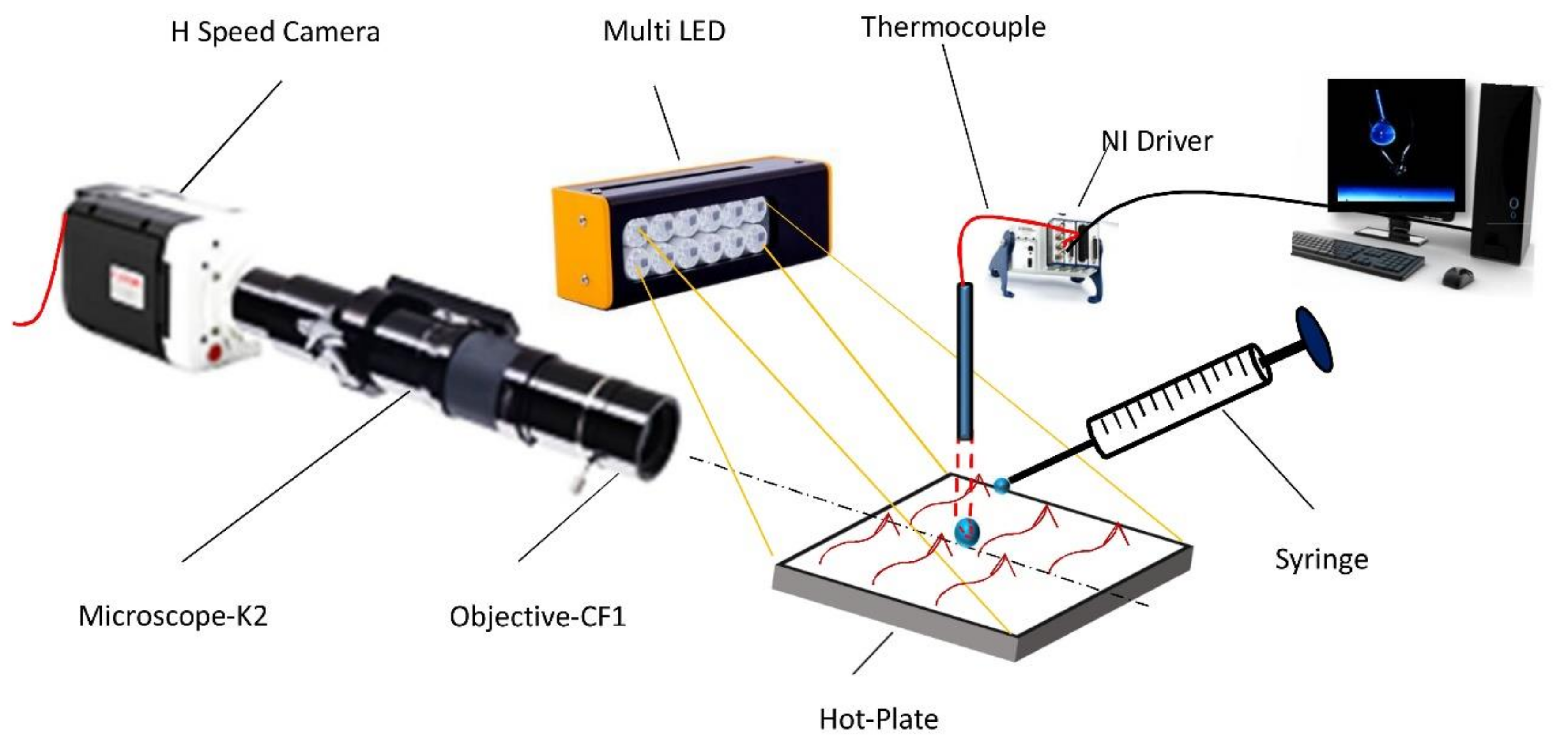
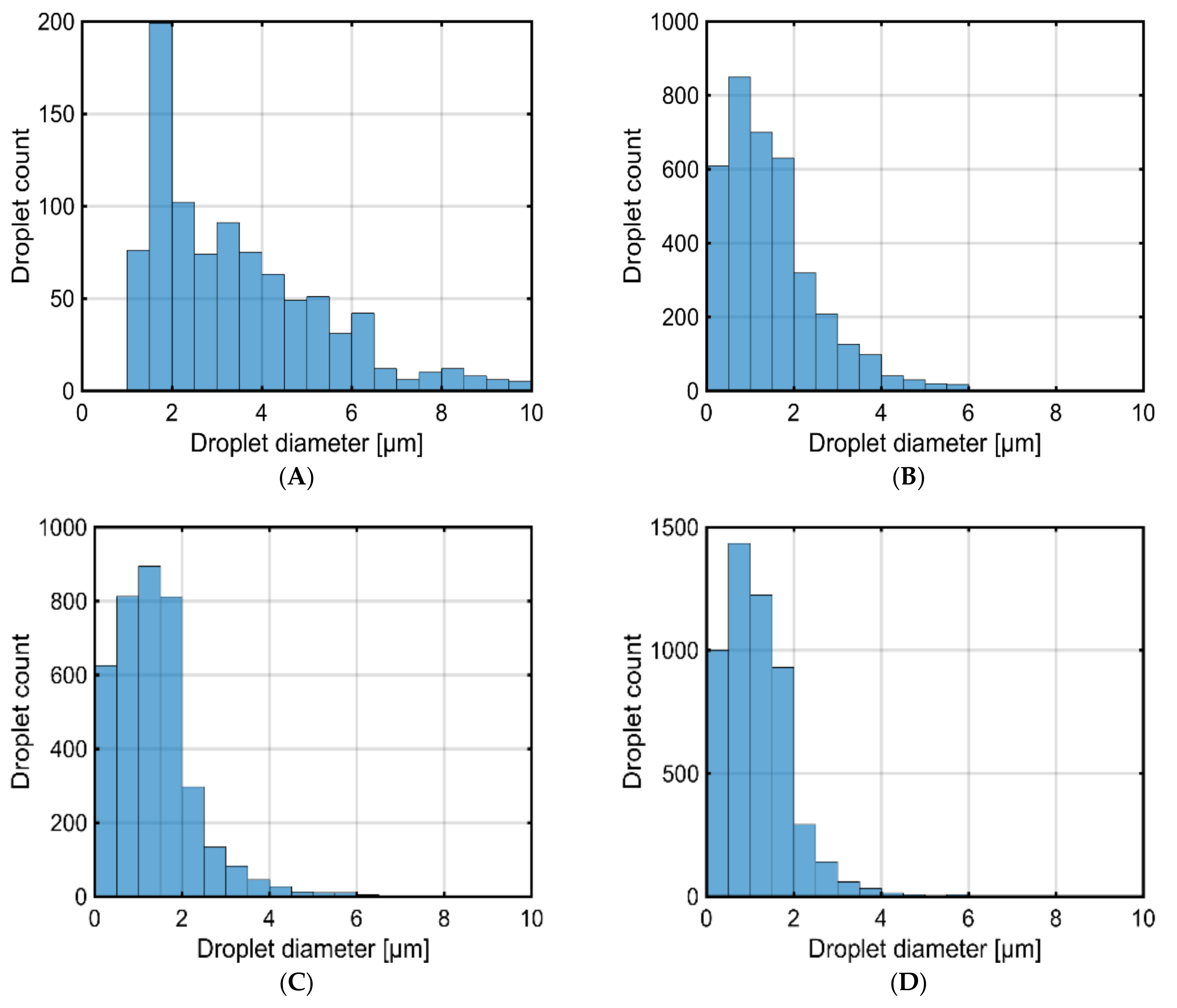
| Water Content (% v/v) | Density at 25 °C (kg/m3) | Viscosity at 40 °C (mm2/s) | Calorific Value (MJ/kg) | Surface Tension @ 25 °C (N.m) |
|---|---|---|---|---|
| 0 (neat diesel) | 825 | 3.21 | 43.20 | 27.1 |
| 5 | 848 | 6.12 | 41.89 | 26.1 |
| 10 | 855 | 9.53 | 39.15 | 23.9 |
| 15 | 863 | 11.22 | 36.33 | 21.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Ismael, M.; R. Heikal, M.; A. Aziz, A.R.; Crua, C. The Effect of Fuel Injection Equipment of Water-In-Diesel Emulsions on Micro-Explosion Behaviour. Energies 2018, 11, 1650. https://doi.org/10.3390/en11071650
A. Ismael M, R. Heikal M, A. Aziz AR, Crua C. The Effect of Fuel Injection Equipment of Water-In-Diesel Emulsions on Micro-Explosion Behaviour. Energies. 2018; 11(7):1650. https://doi.org/10.3390/en11071650
Chicago/Turabian StyleA. Ismael, Mhadi, Morgan R. Heikal, A. Rashid A. Aziz, and Cyril Crua. 2018. "The Effect of Fuel Injection Equipment of Water-In-Diesel Emulsions on Micro-Explosion Behaviour" Energies 11, no. 7: 1650. https://doi.org/10.3390/en11071650
APA StyleA. Ismael, M., R. Heikal, M., A. Aziz, A. R., & Crua, C. (2018). The Effect of Fuel Injection Equipment of Water-In-Diesel Emulsions on Micro-Explosion Behaviour. Energies, 11(7), 1650. https://doi.org/10.3390/en11071650







