Hydropyrolysis of n-Hexane and Toluene to Acetylene in Rotating-Arc Plasma
Abstract
:1. Introduction
2. Experimental and Methods
2.1. Materials
2.2. Pyrolysis Experiment
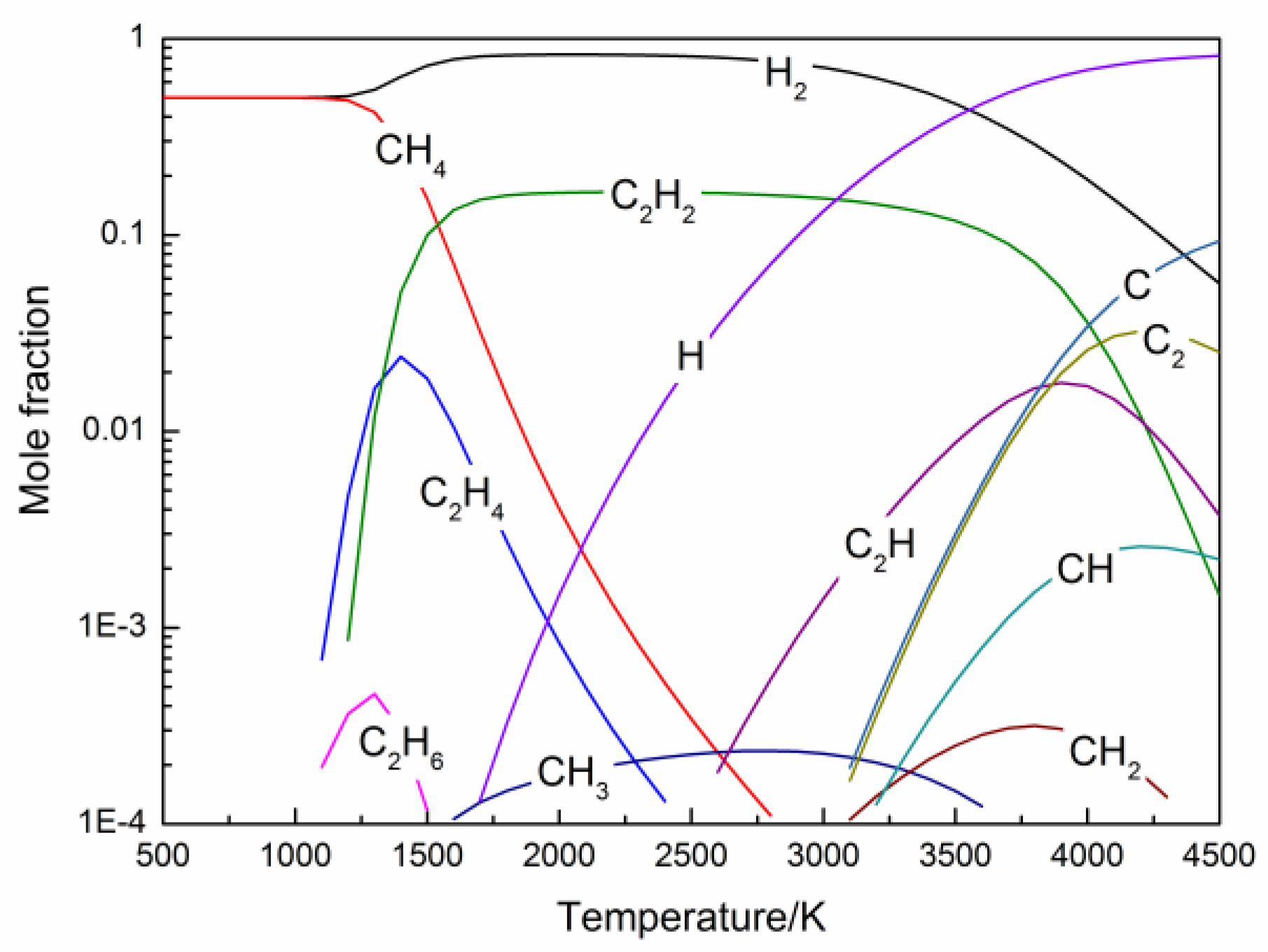
2.3. Numerical Simulation
3. Results and Discussion
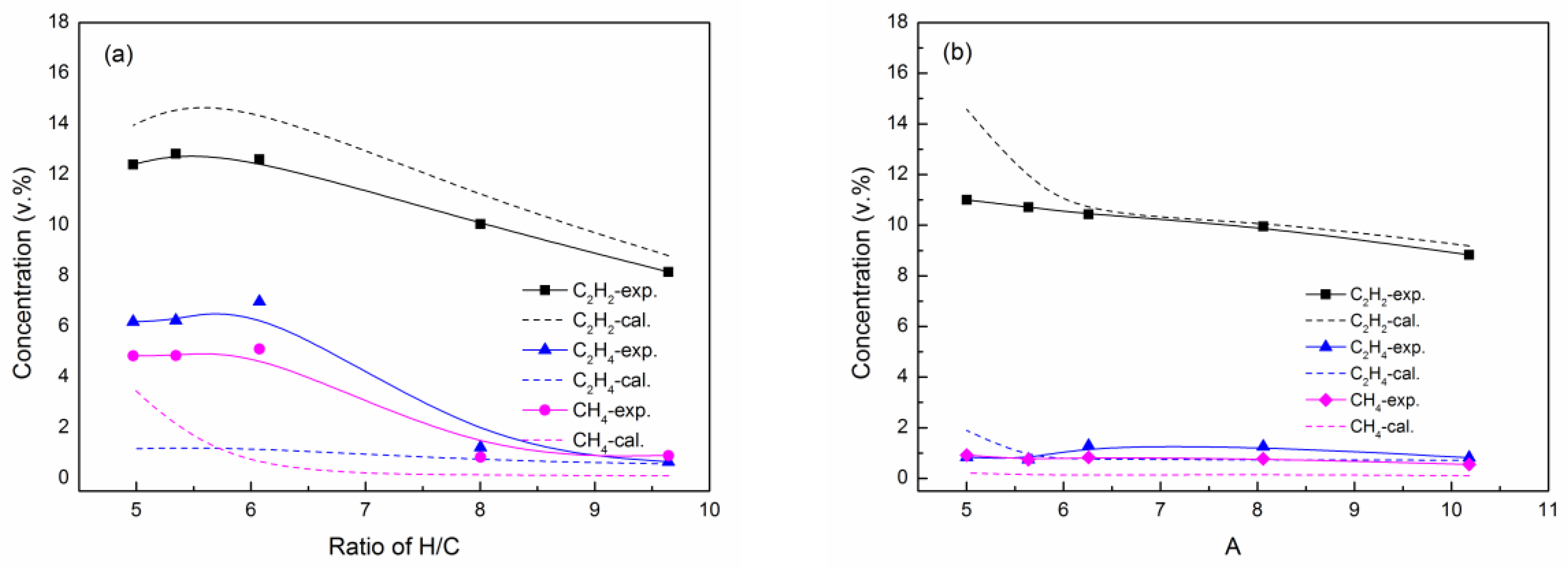
3.1. Effect of Mole Ratio of H/C
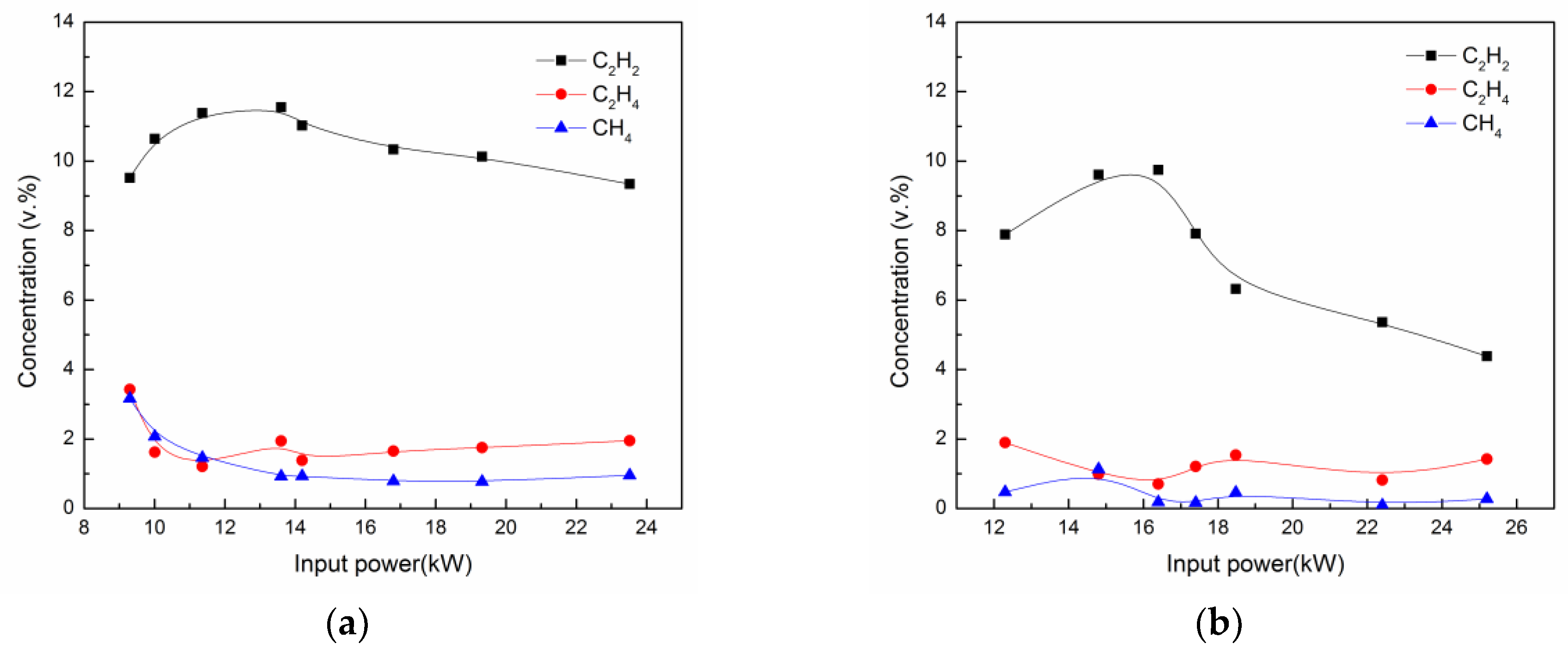
3.2. Effect of Power Input
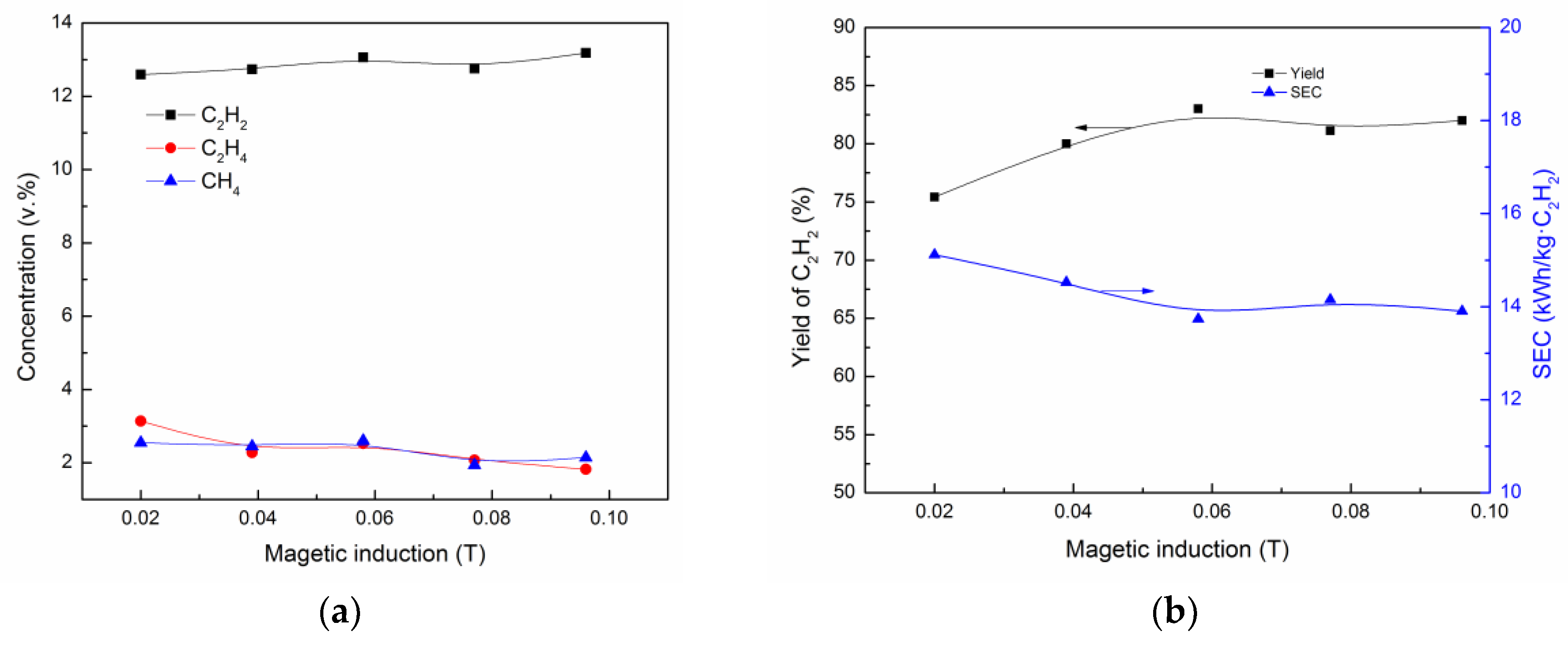
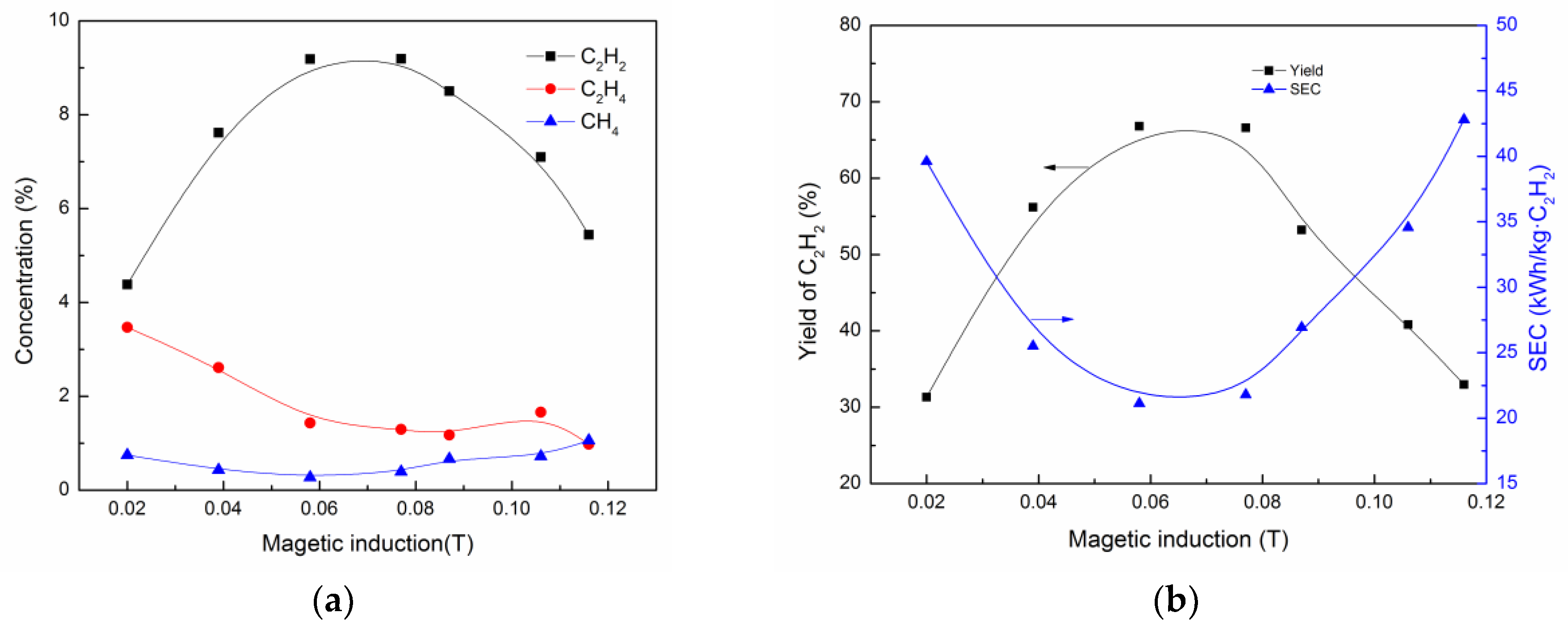
3.3. Effect of Magnetic Induction
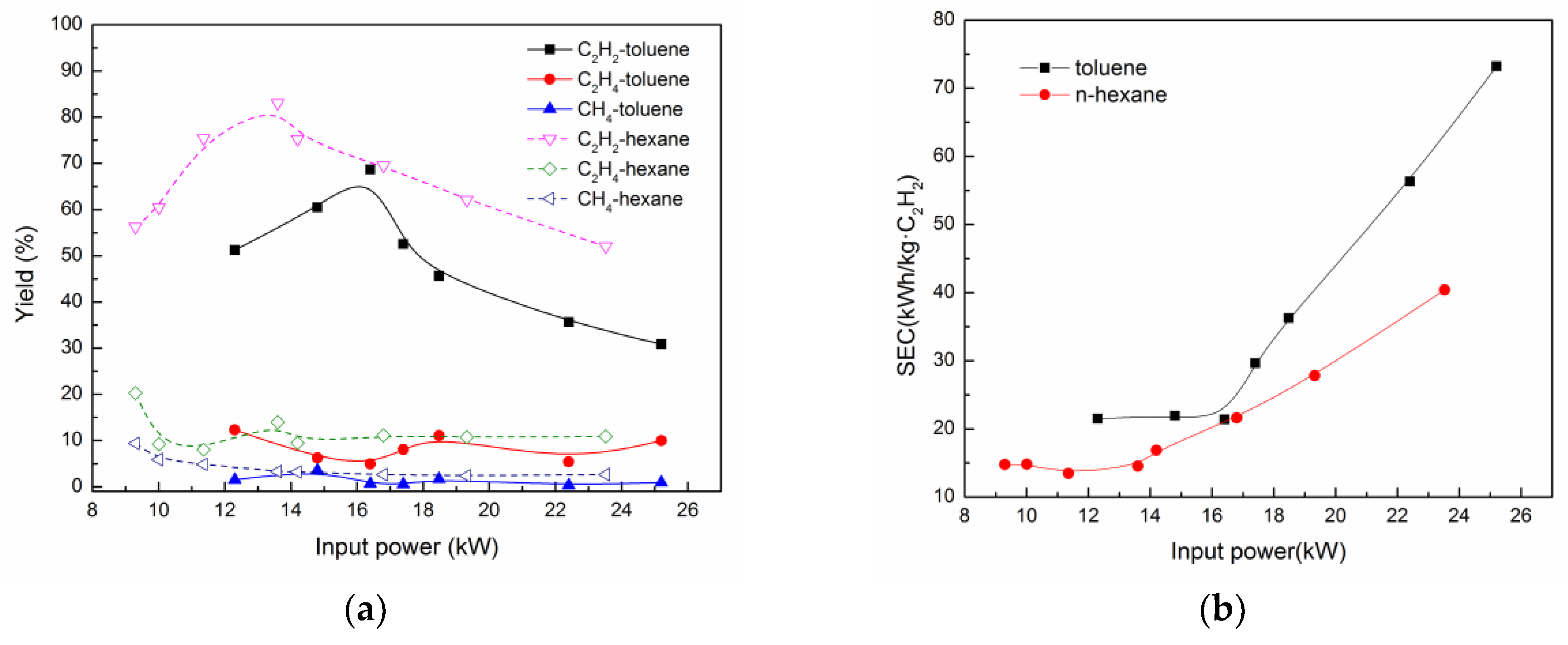
3.4. Comparison with Different Plasma Processes
4. Conclusion
Acknowledgment
Author Contributions
Conflicts of Interest
Nomenclature
| B | Magnetic induction (T) |
| I | Arc current (A) |
| m | Mass flow rate (g/min) |
| P | Power input (kW) |
| Q | Flow rate (Nm3/h) |
| BDE | Bond dissociation energy (kJ/mol) |
| PFR | Plug flow reactor |
| SEC | Specific energy consumption (kWh/kg C2H2) |
References
- Lee, D.H.; Kim, K.T.; Song, Y.H.; Kang, W.S.; Jo, S. Mapping Plasma Chemistry in Hydrocarbon Fuel Processing Processes. Plasma Chem. Plasma Process. 2013, 33, 249–269. [Google Scholar] [CrossRef]
- Fincke, J.R.; Anderson, R.P.; Hyde, T.; Detering, B.A.; Wright, R.; Bewley, R.L.; Haggard, D.C.; Swank, W.D. Plasma thermal conversion of methane to acetylene. Plasma Chem. Plasma Process. 2002, 22, 105–136. [Google Scholar] [CrossRef]
- Dors, M.; Nowakowska, H.; Jasiński, M.; Mizeraczyk, J. Chemical Kinetics of Methane Pyrolysis in Microwave Plasma at Atmospheric Pressure. Plasma Chem. Plasma Process. 2014, 34, 313–326. [Google Scholar] [CrossRef]
- Bidgoli, A.M.; Ghorbanzadeh, A.; Lotfalipour, R.; Roustaei, E.; Zakavi, M. Gliding spark plasma: Physical principles and performance in direct pyrolysis of methane. Energy 2017, 125, 705–715. [Google Scholar] [CrossRef]
- Taghvaei, H.; Jahanmiri, A.; Rahimpour, M.R.; Shirazi, M.M.; Hooshmand, N. Hydrogen production through plasma cracking of hydrocarbons: Effect of carrier gas and hydrocarbon type. Chem. Eng. J. 2013, 226, 384–392. [Google Scholar] [CrossRef]
- Yan, B.; Xu, P.; Li, X.; Guo, C.Y.; Jin, Y.; Cheng, Y. Experimental Study of Liquid Hydrocarbons Pyrolysis to Acetylene in H2/Ar Plasma. Plasma Chem. Plasma Process. 2012, 32, 1203–1214. [Google Scholar] [CrossRef]
- Yan, B.; Xu, P.; Guo, C.Y.; Jin, Y.; Cheng, Y. Experimental study on coal pyrolysis to acetylene in thermal plasma reactors. Chem. Eng. J. 2012, 207, 109–116. [Google Scholar] [CrossRef]
- Messerle, V.E.; Ustimenko, A.B.; Lavrichshev, O.A. Comparative study of coal plasma gasification: Simulation and experiment. Fuel 2016, 164, 172–179. [Google Scholar] [CrossRef]
- Cho, I.J.; Park, H.W.; Park, D.W.; Choi, S. Enhancement of synthesis gas production using gasification-plasma hybrid system. Int. J. Hydrog. Energy 2015, 40, 1709–1716. [Google Scholar] [CrossRef]
- Mohsenian, S.; Esmaili, M.S.; Fathi, J.; Shokri, B. Hydrogen and carbon black nano-spheres production via thermal plasma pyrolysis of polymers. Int. J. Hydrog. Energy 2016, 41, 16656–16663. [Google Scholar] [CrossRef]
- Marias, F.; Demarthon, R.; Bloas, A.; Robert-Arnouil, J.P.; Nebbad, F. Design of a High Temperature Reactor Fed by a Plasma Torch for Tar Conversion: Comparison between CFD Modelling and Experimental Results. Waste Biomass Valoriz. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, W.; Su, B.; Bao, Z.; Wen, G.; Xing, H.; Ren, Q. Conversion of glycerol into syngas by rotating DC arc plasma. Energy 2017, 123, 1–8. [Google Scholar] [CrossRef]
- Zhang, Q.; Dor, L.; Zhang, L.; Yang, W.; Blasiak, W. Performance analysis of municipal solid waste gasification with steam in a Plasma Gasification Melting reactor. Appl. Energy 2012, 98, 219–229. [Google Scholar] [CrossRef]
- Fabry, F.; Rehmet, C.; Rohani, V.; Fulcheri, L. Waste Gasification by Thermal Plasma: A Review. Waste Biomass Valoriz. 2013, 4, 421–439. [Google Scholar] [CrossRef]
- Petitpas, G.; Rollier, J.D.; Darmon, A.; Gonzalez-Aguilar, J.; Metkemeijer, R.; Fulcheri, L. A comparative study of non-thermal plasma assisted reforming technologies. Int. J. Hydrog. Energy 2007, 32, 2848–2867. [Google Scholar] [CrossRef]
- El-Tayeb, A.; El-Shazly, A.H.; Elkady, M.F. Investigation the Influence of Different Salts on the Degradation of Organic Dyes Using Non-Thermal Plasma. Energies 2016, 9, 874. [Google Scholar] [CrossRef]
- Samal, S. Thermal plasma technology: The prospective future in material processing. J. Clean. Prod. 2017, 142, 3131–3150. [Google Scholar] [CrossRef]
- Mostaghimi, J.; Boulos, M.I. Thermal Plasma Sources: How Well are They Adopted to Process Needs? Plasma Chem. Plasma Process. 2015, 35, 421–436. [Google Scholar] [CrossRef]
- Tamošiūnas, A.; Chouchène, A.; Valatkevičius, P.; Gimžauskaitė, D.; Aikas, M.; Uscila, R.; Ghorbel, M.; Jeguirim, M. The Potential of Thermal Plasma Gasification of Olive Pomace Charcoal. Energies 2017, 10, 710. [Google Scholar] [CrossRef]
- Gabbar, H.A.; Aboughaly, M.; Stoute, C.A. DC Thermal Plasma Design and Utilization for the Low Density Polyethylene to Diesel Oil Pyrolysis Reaction. Energies 2017, 10, 784. [Google Scholar] [CrossRef]
- Schobert, H. Production of Acetylene and Acetylene-based Chemicals from Coal. Chem. Rev. 2014, 114, 1743–1760. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, D.; Wang, T. Simulation of partial oxidation of natural gas with detailed chemistry: Influence of addition of H2, C2H6 and C3H8. Chem. Eng. Sci. 2010, 65, 2608–2618. [Google Scholar]
- Mosse, A.L.; Gorbunov, A.V.; Galinovskii, A.A.; Savchin, V.V.; Lozhechnik, A.V. Production of commercial hydrogen and acetylene from propane-butane and liquid hydrocarbons in an electric-arc plasma reactor. J. Eng. Phys. Thermophys. 2008, 81, 652–658. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, J.; Su, B.; Wen, G.; Yang, Q.; Ren, Q. Pyrolysis of Polyolefins Using Rotating Arc Plasma Technology for Production of Acetylene. Energies 2017, 10, 513. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.G.; Xie, K.C. The effect of component of coal on the yield of acetylene in arc plasma pyrolysis. Chem. Ind. Eng. 2003, 20, 195–199. (In Chinese) [Google Scholar]
- Beiers, H.G.; Baumann, H.; Bittner, D.; Klein, J.; Jüntgen, H. Pyrolysis of some gaseous and liquid hydrocarbons in hydrogen plasma. Fuel 1988, 67, 1012–1016. [Google Scholar] [CrossRef]
- Pfender, E. Thermal Plasma Technology: Where Do We Stand and Where Are We Going? Plasma Chem. Plasma Process. 1999, 19, 1–31. [Google Scholar] [CrossRef]
- Wu, A.; Li, X.; Chen, L.; Zhu, F.; Zhang, H.; Du, C.; Yan, J. Utilization of waste rapeseed oil by rotating gliding arc plasma. Int. J. Hydrog. Energy 2015, 40, 9039–9048. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, M.; Su, B.; Wen, G.; Yang, Y.; Yang, Q.; Ren, Q. Numerical simulation of the entrained flow hydropyrolysis of coal in magnetically rotating plasma reactor. Energy Convers. Manage. 2017, 148, 431–439. [Google Scholar] [CrossRef]
- Ma, J.; Wen, G.D.; Su, B.G.; Yang, Y.W.; Ren, Q.L. Current-voltage characteristics of hydrogen DC plasma torches with different sizes in an external axial magnetic field. Chinese Phys. B 2015, 24, 65205. [Google Scholar] [CrossRef]
- Ma, J.; Su, B.; Wen, G.; Ren, Q.; Yang, Y.; Yang, Q.; Xing, H. Kinetic modeling and experimental validation of the pyrolysis of propane in hydrogen plasma. Int. J. Hydrog. Energy 2016, 41, 22689–22697. [Google Scholar] [CrossRef]
- Demidov, D.V.; Mishin, I.V.; Mikhailov, M.N. Gibbs free energy minimization as a way to optimize the combined steam and carbon dioxide reforming of methane. Int. J. Hydrog. Energy 2011, 36, 5941–5950. [Google Scholar] [CrossRef]
- Wang, H.; Dames, E.; Sirjean, B.; Sheen, D.A.; Tango, R.; Violi, A.; Lai, J.Y.W.; Egolfopoulos, F.N.; Davidson, D.F.; Hanson, R.K.; et al. high-temperature chemical kinetic model of n-alkane (up to n-dodecane), cyclohexane, and methyl-, ethyl-, n-propyl and n-butyl-cyclohexane oxidation at high temperatures, JetSurF version 2.0. Available online: http://web.stanford.edu/group/haiwanglab/JetSurF/JetSurF2.0/index.html) (accessed on 19 September 2010).
- Wang, H.; You, X.; Joshi, A.V.; Davis, S.G.; Laskin, A.; Egolfopoulos, F.; Law, C.K. High-Temperature Combustion Reaction Model of H2/CO/C1–C4 Compounds, USC Mech Version II. Available online: http://ignis.usc.edu/Mechanisms/USC-Mech%20II/USC_Mech%20II.htm (accessed on 1 May 2007).
- Zhang, L.; Cai, J.; Zhang, T.; Qi, F. Kinetic modeling study of toluene pyrolysis at low pressure. Combust. Flame 2010, 157, 1686–1697. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, D.G.; Chen, F.; Zhan, X. The decomposition of aromatic hydrocarbons during coal pyrolysis in hydrogen plasma: A density functional theory study. Int. J. Hydrog. Energy 2012, 37, 18040–18049. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, D.; Chen, F.; Zhan, X. A density functional theory study on the decomposition of aliphatic hydrocarbons and cycloalkanes during coal pyrolysis in hydrogen plasma. J. Energy Chem. 2015, 24, 65–71. [Google Scholar] [CrossRef]
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energy Combust. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Agafonov, G.L.; Bilera, I.V.; Vlasov, P.A.; Zhil’tsova, I.V.; Kolbanovskii, Y.A.; Smirnov, V.N.; Tereza, A.M. Unified kinetic model of soot formation in the pyrolysis and oxidation of aliphatic and aromatic hydrocarbons in shock waves. Kinet. Catal. 2016, 57, 557–572. [Google Scholar] [CrossRef]
- Luo, Y. Handbook of Bond Dissociation Energies in Organic Compounds; CRC press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Richter, H.; Granata, S.; Green, W.H.; Howard, J.B. Detailed modeling of PAH and soot formation in a laminar premixed benzene/oxygen/argon low-pressure flame. Proc. Combust. Inst. 2005, 30, 1397–1405. [Google Scholar] [CrossRef]
- Essiptchouk, A.M.; Sharakhovsky, L.I.; Marotta, A. A new formula for the rotational velocity of magnetically driven arcs. J. Phy. D: Appl. Phy. 2000, 33, 2591. [Google Scholar] [CrossRef]


| Unit | This Work | Ref. [6] | Ref. [25] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Num. | - | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Feedstock | - | n-hexane | n-hexane | toluene | toluene | n-hexane | n-hexane | toluene | n-hexane | toluene |
| Feed rate | g/min | 27.40 | 20.54 | 18.81 | 18.81 | 3.30 | 2.55 | 2.60 | 40 | 40 |
| Input power | kW | 17.52 | 13.60 | 16.40 | 15.75 | 3.3 | 2.0 | 2.28 | 23.08 | 30.34 |
| Specific input power | kJ/kg | 3.84 × 104 | 3.97 × 104 | 5.23 × 104 | 5.03 × 104 | 6.01 × 104 | 4.70 × 104 | 5.26 × 104 | 1.04 × 105 | 7.91 × 104 |
| C2H2 | v/v % | 13.06 | 11.55 | 9.75 | 9.19 | 5.89 | 11.95 | 2.27 | 3.60 | 3.83 |
| CH4 | v/v % | 3.42 | 0.93 | 0.18 | 0.27 | 2.83 | 2.33 | 0.77 | 0.65 | 0.23 |
| C2H4 | v/v % | 2.43 | 1.95 | 0.71 | 1.43 | 4.40 | 2.85 | 0.09 | 0.55 | 0.41 |
| Yield of C2H2 | wt. % | 83.01 | 83.50 | 68.65 | 66.78 | 39.10 | 70.00 | 16.50 | 20.00 | 8.50 |
| SEC | kWh/kg C2H2 | 13.73 | 14.57 | 21.40 | 21.13 | 42.62 | 18.00 | 88.58 | 144.43 | 258.51 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhang, M.; Wu, J.; Yang, Q.; Wen, G.; Su, B.; Ren, Q. Hydropyrolysis of n-Hexane and Toluene to Acetylene in Rotating-Arc Plasma. Energies 2017, 10, 899. https://doi.org/10.3390/en10070899
Ma J, Zhang M, Wu J, Yang Q, Wen G, Su B, Ren Q. Hydropyrolysis of n-Hexane and Toluene to Acetylene in Rotating-Arc Plasma. Energies. 2017; 10(7):899. https://doi.org/10.3390/en10070899
Chicago/Turabian StyleMa, Jie, Ming Zhang, Jianhua Wu, Qiwei Yang, Guangdong Wen, Baogen Su, and Qilong Ren. 2017. "Hydropyrolysis of n-Hexane and Toluene to Acetylene in Rotating-Arc Plasma" Energies 10, no. 7: 899. https://doi.org/10.3390/en10070899
APA StyleMa, J., Zhang, M., Wu, J., Yang, Q., Wen, G., Su, B., & Ren, Q. (2017). Hydropyrolysis of n-Hexane and Toluene to Acetylene in Rotating-Arc Plasma. Energies, 10(7), 899. https://doi.org/10.3390/en10070899






