Abstract
Palmitoleic acid (C16:1) and stearic acid (C18:0) are precursors of polyunsaturated fatty acids, which are the focus of intensive global research due to their nutritional value, medicinal applications, and potential use as biofuel. Acyl-acyl carrier protein (ACP) thioesterases are intraplastidial enzymes that determine the types and amounts of fatty acids produced in plants and release fatty acids into the cytosol to be incorporated into glycerolipids. Based on amino acid sequence identity and substrate specificity, these enzymes are classified into two families, FatA and FatB. In this study, we cloned FatA and FatB thioesterases from Arachis hypogaea L. seeds and functionally expressed these genes, both individually and in tandem, in a blue-green alga Synechocystis sp. PCC6803. The heterologous expression of these genes in Synechocystis altered the fatty acid composition of lipids, resulting in a 29.5–31.6% increase in palmitoleic acid production and a 22.5–35.5% increase in stearic acid production. Moreover, the transgenic Synechocystis cells also showed significant increases in levels of oleic acid (C18:1, OA), linoleic acid (C18:2, LA), and α-linolenic acid (C18:3n3, ALA). These results suggest that the fatty acid profile of algae can be significantly improved by the heterologous expression of exogenous genes. This study not only provides insight into fatty acid biosynthesis, but also lays the foundation for manipulating the fatty acid content of cyanobacteria.
1. Introduction
In higher plants, de novo fatty acid (FA) biosynthesis mainly takes place in plastids. In this pathway, malonylacyl carrier protein (ACP) is condensed with acyl-ACP derivatives to form two-carbon units, which are successively added to the acyl-ACP chain [1,2,3]. Acyl chain elongation is terminated by an acyl-ACP thioesterase (FAT), which hydrolyzes the thioester bond of acyl-ACP and releases free fatty acids, which are quickly transported to the cytosol via acyl-CoA synthetase in prokaryotic and eukaryotic cells [4,5,6]. Therefore, acyl-ACP FAT proteins are important regulators of lipid storage. Based on their amino acid sequence identity and substrate specificity, acyl-ACP FAT proteins are classified into two families: FatA and FatB [2,7]. Whereas FatA has a higher specificity for 18:1-ACP substrates and a lower specificity for 18:0-ACP and 16:0-ACP [1,8], FatB has a preference for saturated FAs containing 8–18 carbons [1,7,9].
FAs are carboxylic acids with hydrocarbon chains of 4 to 36 carbon atoms. Unsaturated fatty acids (UFAs) are hydrocarbon chains that have at least one double bond in their backbone structure, and are 18 to 22 carbons in length. Polyunsaturated fatty acids (PUFAs) are UFAs that possess two or more double bonds. Based on the position of the first double bond from the methyl end of the molecule, PUFAs are classified as omega-3 (ω-3) or omega-6 (ω-6) FAs. ω-3 PUFAs are essential dietary molecules with potential medicinal and nutritional applications, and have recently become the focus of intensive research due to their perceived benefits to human health [10]. Furthermore, PUFAs were found to have potential applications in biofuel production, and may thus represent an alternative energy source to meet the world’s increasing energy demands.
Genetic engineering provides an effective approach for enhancing the production of FAs, and the FA biosynthetic pathways in higher plants and microbes have been well studied. Much research has focused on developing metabolic engineering methods to increase the FA content in oilseed crops [11,12] and microalgae [10,13,14,15]. Cyanobacteria are photosynthetic prokaryotes that produce valuable metabolites using solar energy and carbon. These microorganisms grow readily in a broad range of environments, exhibit rapid growth rates, and produce high yields of metabolites. The unicellular cyanobacterium Synechocystis sp. PCC6803 is a facultative phototroph that is considered a model organism for studying FA biosynthesis [10,16].
Recently, several Fat genes have been cloned and characterized [1,3,8,9]. It has been reported that overproduction of thioesterase stimulates fatty acid synthesis by decreasing feedback inhibition and reducing the cellular acyl-ACP concentrations [17,18]. In cyanobacteria, acyl-CoA synthetases are essential for the incorporation of exogenously supplied free fatty acids into cellular lipid metabolism [19]. However, the study of peanut Fat genes in microalgae has not previously been reported. The primary objective of this work was to create a strain of Synechocystis sp. PCC6803 that effectively produces useful FAs. Here, we cloned the genes encoding FatA and FatB thioesterases (AhFatAand AhFatB) from Arachis hypogaeaL. seeds and functionally expressed these genes in the blue-green alga, Synechocystis sp. PCC6803. By heterologously expressing exogenous thioesterases in Synechocystis sp. PCC6803, the accumulation of the PUFAs palmitoleic acid and stearic acid in this cyanobacterium were markedly increased.
2. Results
2.1. Integration of AhFatA and AhFatB into Synechocystis 6803 and Their Expression Analysis
We constructed three homologous recombination plasmids, harboring AhFatA (SDFatA), AhFatB (SDFatB), and both AhFatA and AhFatB (SDFatAB; Figure 1). We selected psbA2 as the integration site, because this gene belongs to the psbA multi-gene family, and inactivation of psbA2 has no effect on Syenchocystis [20,21]. Therefore, to construct the homologous recombination vectors, we used the 500-bp fragment upstream of the psbA2 ORF and the psbA2 ORF as the upstream and downstream arms, respectively, and cloned AhFatA, AhFatB, or both AhFatA and AhFatB between these arms. The fragment upstream of the psbA2 ORF was used as the promoter to drive the expression of AhFatA, AhFatB, or both AhFatA and AhFatB in Synechocystis sp. PCC6803. psbA2 was replaced with the target gene(s) (i.e., AhFatA, AhFatB, or both AhFatA and AhFatB) and an antibiotics selection gene by double homologous recombination. To isolate transgenic Synechocystis cells, the transformants were subcultured in BG-11 solid medium in the presence of kanamycin (50 μg/mL). To verify that AhFatA, AhFatB, or both AhFatA and AhFatB were stably integrated into the genome, the complete segregation of the transformants was confirmed by PCR. Genomic DNA from the wild-type strain was used as a control. In the transgenic Synechocystis cells, the DNA fragment containing AhFatA, AhFatB, or AhFatAB with the psbA2promoter were amplified using the promoter-SalI-F and psbA2-SacI-R primers (Figure 2A). The PCR products were recycled and sequenced. Sequence analysis showed the amplified fragments were the same as the recombinant fragments. No DNA fragment corresponding to the wild-type psbA2 gene and its promoter (1.5 kb) was detected, confirming that we had indeed isolated Synechocystis transformants.
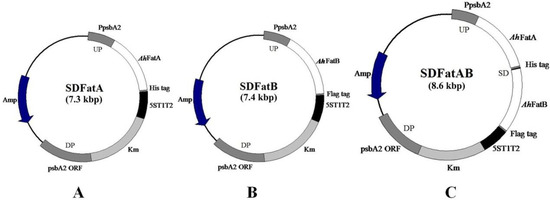
Figure 1.
Structure of homologous recombination vectors harboring thioesterase genes from Arachis hypogaea L. Fat genes (AhFatA, AhFatB, or both AhFatA and AhFatB) were cloned into the pBluescript II SK (+) plasmid. The psbA2 promoter (PpsbA2), consisting of the 0.5-kb fragment upstream (UP) of psbA2, the kanamycin resistance cassette (Km), the 1.0-kb fragment downstream of psbA2 (psbA2 ORF, DP), and the E. coli 5ST1T2 terminator were also cloned into the plasmids. Amp: ampicillin resistance cassette. (A) SDFatA, for the heterologous expression of AhFatA tailed with a His tag; (B) SDFatB, for the heterologous expression of AhFatB tailed with a Flag-tag; (C) SDFatAB, tandem expression of AhFatA tailed with a His tag and AhFatB tailed with a Flag-tag.
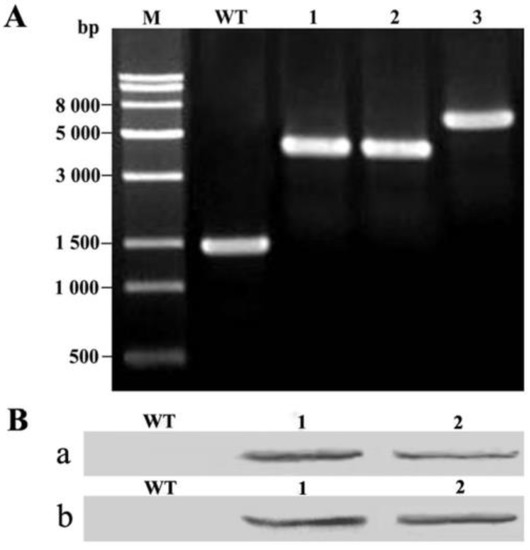
Figure 2.
PCR and immunoblot analysis of wild-type Synechocystis and thioesterase transformants. (A): PCR analysis of transgenic Synechocystis in which a fragment of psbA2 was deleted and replaced with various exogenous genes. M: Trans15k DNA Marker; WT: wild type Syenchocystis sp. PCC6803; Lane 1: pSDFatA; Lane 2: pSDFatB; Lane 3: pSDFatAB; (B): Immunoblot analysis of Synechocystis wild type and thioesterase transformants grown at 30 °C under mixotrophic conditions. a: Immunoblot analysis using His-tag antibodies. WT: wild type Synechocystis sp. PCC6803; Lane 1: pSDFatA Synechocystis transformant; Lane2: pSDFatAB Synechocystis transformant; b: Immunoblot analysis using Flag-tag antibodies. WT: wild type Synechocystis sp. PCC6803; Lane 1: pSDFatB Synechocystis transformant; Lane 2: pSDFatAB Synechocystis transformant.
To detect the expression of AhFatA or AhFatB in the transformants, we incubated the wild-type and SDFatA/SDFatB/SDFatAB transformant cells at 30 °C. Immunoblot analysis confirmed the expression of AhFatA and AhFatB, cloned in-frame with the psbA2 promoter and the T1T2 terminator, in the Synechocystis transformants. Furthermore, immunoblot analysis using antibodies to the His tag and Flag-tag confirmed that AhFatA and AhFatB, respectively, were localized to the cell extracts of transgenic pSDFatA, pSDFatB, and pSDFatAB Synechocystis cell extracts (Figure 2B). Reverse transcription PCR (RT-PCR) analysis showed that both AhFatA and AhFatB were indeed expressed at 20 °C and 30 °C in these transformants (Figure 3). Furthermore, we found that AhFatA expression was greater at 20 °C than at 30 °C in transgenic Synechocystis cells expressing AhFatA. However, there was no significant difference in AhFatB expression when cells were cultured under 20 °C or 30 °C.
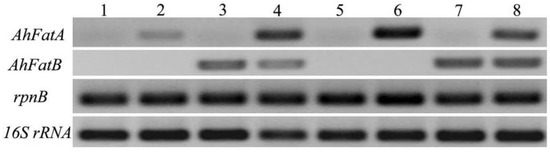
Figure 3.
Semi-quantitative RT-PCR analysis of Acyl-ACP thioesterases from Arachis hypogaea L. in Synechocystis transformants. Lane 1: wild type Syenchocystis sp. PCC6803; Lane 2: pSDFatA Synechocystis transformant; Lane 3: pSDFatB Synechocystis transformant; Lane 4: pSDFatAB Synechocystis transformant; Lane 5: WT Syenchocystis sp. PCC6803; Lane 6: pSDFatA Synechocystis transformant; Lane 7: pSDFatB Synechocystis transformant; Lane 8: pSDFatAB Synechocystis transformant. All samples were cultured under mixotrophic culture conditions. Those in Lanes 1 to 4 were cultured at 20 °C, whereas those in Lanes 5 to 8 were cultured at 30 °C.
Growth rates of the AhFatA/AhFatB transformants and wild-type strains were measured in BG-11 medium at 20 °C and 30 °C. The growth rates of the transformant strains were similar to those of wild-type cells (Figure 4). These results indicate that the exogenous expression of AhFatA and/or AhFatB and the interruption of the neutral site (psbA2 promoter and psbA2 gene) have no negative effect on the cell physiology and growth of Synechocystis.
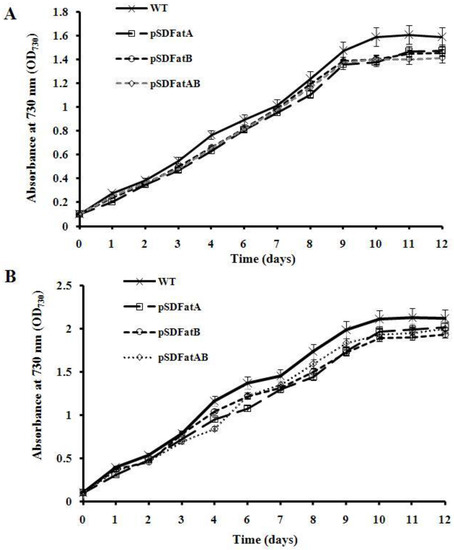
Figure 4.
Growth curves of wild-type and transgenic Synechocystis. Cells were grown under mixotrophic conditions at (A) 20 °C or (B) 30 °C. Cultures were grown in BG-11 medium and bubbled with air under an illumination of 40 μmol photon m−2 s−1. The optical density of cells at 730 nm was measured at the indicated time points. Values are means ± SD (bars) of three independent experiments conducted on different days. Absence of a bar indicates that the SD falls within the symbol.
2.2. GC Analysis of Fatty Acids in the Synechocystis 6803 Strain Expressing AhFatA and AhFatB
Next, we cultivated wild-type and transgenic Synechocystis sp. PCC6803 cells under mixotrophic conditions at 20 °C and 30 °C and then analyzed the FA content in membranes by GC (Figure 5, see Supplementary Materials, Table S1). The FA profiles of the Synechocystis 6803 strains expressing AhFatA, AhFatB, or AhFatAB exhibited striking changes.
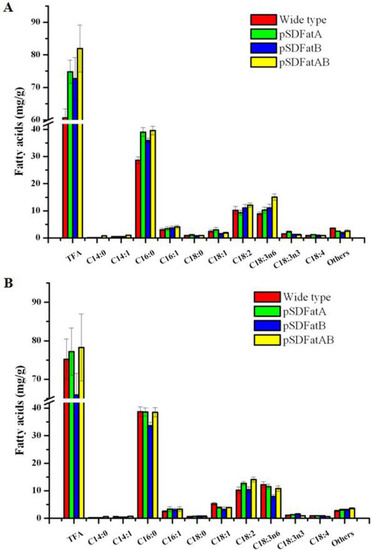
Figure 5.
Fatty acid content of wild-type and transgenic Synechocystis sp. PCC6803 a cultured under mixotrophic growth conditions at (A) 20 °C and (B) 30 °C. TFA: Total fatty acids; FA: fatty acids. a Values are means and S.D. of triplicate experiments. Cells were grown under a light intensity of 40 μmol photon m−2 s−1 for 10 d in BG-11 medium. The membrane lipids were extracted from the wild-type and genetically engineered Synechocystis sp. PCC6803. The enzymes overexpressed are indicated in parentheses (FatA: Acyl-ACP Thioesterase A from ‘Luhua 14’ cultivar of Arachis hypogaea L.; FatB: Acyl-ACP Thioesterase B from ‘Luhua 14’ cultivar of Arachis hypogaea L.).
In wild-type cells grown under mixotrophic conditions at 20 °C, the total FA content was about 60.66 mg/g, but the heterologous expression of AhFatA or AhFatB increased the total FA content to 74.79 mg/g and 72.73 mg/g, respectively (i.e., resulted in increases of 23.3% and 19.9%). The FA content of cells heterologously expressing both AhFatA and AhFatB was 81.94 mg/g, which was 35.1% more than that of the wild type. These results suggest that the heterologous expression of AhFatA has a greater effect than that of AhFatB or both AhFatA and AhFatB on the FA content under mixotrophic conditions at 20 °C.
Gas chromatography (GC) analysis revealed that the cultivated temperature affected the FA content. Temperature has a large effect on the composition of FAs produced by microalgae which, inturn, affects the physiology of the organism. Several studies indicate that the exponential growth rates of many microalgal species increase in response to elevated growth temperatures, up to an optimal temperature. In response to cold stress, several microalgal species enhance the biosynthesis and accumulation of total FAs [10,22,23]. The higher temperature (30 °C) enhanced the expression of AhFatA, and increased the FA content of transgenic Synechocystis expressing AhFatA or AhFatAB, which is in accordance with the results of reverse transcription PCR (Figure. 3). The FA content of transgenic Synechocystis expressing AhFatA was 3.1% greater under mixotrophic conditions at 30 °C than at 20 °C, whereas the FA content of transgenic Synechocystis expressing AhFatB and AhFatAB were decreased 10.3% and 4.7% under mixotrophic conditions at 30 °C than at 20 °C.These results suggest that transgenic Synechocystis cells expressing AhFatA should be cultured under mixotrophic conditions at 30 °C to increase the yield of FAs. While for transgenic Synechocystis cells expressing AhFatB and AhFatAB should be cultured under mixotrophic conditions at 20 °C to increase the yield of FAs.
In wild-type cells grown under mixotrophic conditions at 30 °C, the most abundant FAs are C14:1 (0.8%), C16:0 (51.5%), C16:1 (3.4%), C18:0 (0.8%), and C18:1 (7.1%). By contrast, at 20 °C, C14:1 (0.8%), C16:0 (47.1%), C16:1 (5.0%), C18:0 (1.4), and C18:1 (3.9) were most abundant.
The heterologous expression of AhFatA in Synechocystis 6803 increased the content of palmitoleic acid (C16:1) and stearic acid (C18:0) significantly. Under mixotrophic cultivation, the concentration of C16:1 and C18:0 in cells heterologously expressing AhFatA was about 3.36 mg/g and 0.76 mg/g at 30 °C, respectively, which was 31.2% and 22.5% greater than the corresponding concentrations in wild-type cells, and 3.39 mg/g and 1.08 mg/g at 20 °C, which was 12.7% and 22.7% greater than the corresponding concentrations in wild-type cells. The content of C14:0 and C18:1 in cells heterologously expressing AhFatA was about 0.14mg/g and 3.05 mg/g at 20 °C under mixotrophic conditions, which was 27.2% and 27.6% greater than those of wild-type cells grown under the same conditions.
The heterologous expression of AhFatB in Synechocystis 6803 markedly increased the content of C16:1, C18:0, and C18:3n3 at 30 °C under mixotrophic condition, resulting in concentrations of about 3.13 mg/g, 0.84 mg/g, and 1.51 mg/g, respectively, which were 29.5%, 35.5%, and 38.5% greater than those of the wild type. Similarly, the heterologous expression of AhFatB in Synechocystis 6803 increased the content of C14:0, C16:1, and C18:3n6, at 20 °C under mixotrophic cultivation, by 31.4, 24.0, and 23.6%, respectively.
Tandemly expressed AhFatA and AhFatB increased the content of C16:1, C18:0, and C18:2, to about 3.37 mg/g, 0.78 mg/g, and 13.12 mg/g, respectively, at 30 °C under mixotrophic conditions, which was 31.6%, 25.8%, and 28.4% greater than the content in wild-type cells, while the content of C16:1, C18:0, and C18:2 was about 4.07 mg/g, 0.92 mg/g, and 12.08 mg/g, at 20 °C under mixotrophic cultivation, which was 35.7, 4.5, and 18.9% greater than levels in wild-type cells grown under the same conditions, respectively.
The heterologous expression of AhFatA in Synechocystis 6803 decreased the content of C14:1 and C18:1. At 30 °C under mixotrophic cultivation, the concentration of C14:1 and C18:1 in cells heterologously expressing AhFatA was about 0.44 mg/g and 3.94 mg/g, respectively, which was 27.9% and 25.8% lower than that in the wild type. Furthermore, the content of C14:1 and C18:2 was about 0.48 mg/g and 9.24 mg/g, respectively, at 20 °C under mixotrophic conditions, which was 4.3% and 9.1% lower than that of wild-type cells grown under the same conditions. While heterologously expressing of AhFatB in Synechocystis 6803 decreased the content of C14:1, C18:1, and C18:3n6, by 29.5%, 38.6%, and 35.5% at 30 °C under mixotrophic cultivation, and decreased the content of C18:0, C18:1, and C18:3n3, by 1.1%, 37.7%, and 12.4% at 20 °C under mixotrophic cultivation. Tandem expression of AhFatA and AhFatB decreased the content of C18:1 and C18:3n6 to 3.96 mg/g and 10.82 mg/g, which was 25.4% and 11.5% lower than the corresponding levels in wild-type cells grown at 30 °C under mixotrophic cultivation, while the content of C18:1 and C18:3n3 decreased by 20.9% and 19.0%, respectively, at 20 °C under mixotrophic cultivation, respectively.
Therefore, Synechocystis lines heterologously expressing AhFatA and AhFatB exhibit similar changes in FA profiles. For example, they both had increased levels of C14:0, C16:1, C18:0, and C18:2, and decreased levels of C14:1, C16:0, C18:1, and C18:3n6 at 30 °C under mixotrophic cultivation, and increased levels of C14:0, C16:0, C16:1, and C18:3n6 at 20 °C under mixotrophic cultivation. However, there were also some differences in FA content between cells expressing AhFatA and those expressing AhFatB. For example, AhFatA expression increased the content of C18:3n3 and C18:4, whereas AhFatB expression increased the content of C18:3n3, but tandem expression of AhFatA and AhFatB decreased the content of C18:3n3 and C18:4 at 30 °C under mixotrophic cultivation. While the expression of AhFatA increased the content of C18:3n3 and C18:4, that of AhFatB decreased the content of C18:4 at 20 °C under mixotrophic conditions.
3. Discussion
Thioesterase play an important role intransferring acyl chains to the extraplastidial glycerolipid and determine the metabolic flux into triacylglycerols [3]. Over the past few decades, several thioesterases have been cloned and functionally characterized, including representatives from Jatropha curcas, Arachis hypogaea L., Synechocystis, Populus tomentosa, Ricinus communis L., and so on [2,3,5,21]. However, more thioesterases need to be characterized to exploit their potential uses, especially to adjust the fatty acid composition from microorganisms and various plants.
In cyanobacteria, fatty acids are synthesized by the type II fatty acid synthase complex, which interacts with acyl carrier protein [24]. The products of fatty acid synthase are released as acyl-ACPs and incorporated into membrane lipids [25]. Acyl-ACP thioesterase hydrolyzes the thioester bond of acyl-ACP, releaseing free fatty acids, which are quickly transported into the cytosol via acyl-CoA synthetase in prokaryotic and eukaryotic cells [4,5,6]. However, such a mechanism would be detrimental to cyanobacteria, causing a loss of fatty acids; as expected, no homologs of acyl-ACP thioesterases have been identified in cyanobacteria [19].
In this study, we sought to develop a method to increase the FA content in Synechocystis sp. PCC6803. We cloned the genes encoding FatA and FatB thioesterases from Arachis hypogaea L. seeds and then functionally expressed these genes in a blue-green alga Synechocystis sp. PCC6803, both individually and in tandem. We then analyzed the expression of exogenous genes (AhFatA and AhFatB thioesterases) in transgenic Syenchocystis under mixotrophic conditions at 30 °C and 20 °C Immunoblot analysis confirmed the presence of AhFatA and AhFatB proteins in the soluble fraction of Synechocystis transformant cell extracts. We also used RT-PCR analysis to detect the expression of AhFatA and AhFatB. Both AhFatA and AhFatB were indeed expressed at 20 °C and 30 °C, demonstrating that the presence of AhFatA and AhFatB mRNA in the transformants.
The heterologous expression of these genes altered the FA profiles of the transgenic Synechcystis cells, increasing the content of palmitoleic acid (C16:1, PA) and stearic acid (C18:0, SA). Moreover, the altered FA profiles in transgenic Syenchocystis also showed striking increases in the levels of oleic acid (C18:1, OA) and linoleic acid (C18:2, LA), along with increases in the content of α-linolenic acid (C18:3n3, ALA). These results suggest that the heterologous expression of Fat genes can alter FA production. Our finding that transgenic Syenchocystis cells heterologously expressing AhFatA had significantly higher levels of C16:0, C18:0, and C18:1 at 20 °C under mixotrophic cultivation conditions is in accordance with the finding that FatA encodes a thioesterase with a higher specificity for 18:1-ACP substrates and lower specificities for 18:0-ACP and 16:0-ACP [1,8]. Furthermore, under mixotrophic cultivation, transgenic Syenchocystis cells heterologously expressing AhFatB had significantly higher levels of C16:0 at 20 °C and of C18:0 at 30 °C, which is in accordance with the conclusion that FatB encodes a thioesterase with a preference for saturated FAs containing 8-18 carbons [1,7,9]. It is interesting that the tandem expression of AhFatA and AhFatB in Syenchocystis decreased the content of C18:1 and increased the content of C18:2 at 30 °C and increased the content of C18:2 and C18:3n6 at 20 °C under mixotrophic cultivation. It is possible that the tandem expression of AhFatA and AhFatB increased the activity of delta-9 and delta-6 fatty acid desaturases during FA biosynthesis.
The increase in the world’s population coupled with dwindling supplies of fossil fuel and environmental degradation have prompted intensive research aimed at developing renewable and potentially carbon neutral liquid, solid, and gaseous biofuels as alternative energy resources [26]. Among many potential sources of biodiesel fuel, the FA methyl esters derived from triglycerides have emerged as a promising alternative to diesel fuels. Although current biodiesels, generally produced by transesterification of vegetable oils and animal fats, are considered to be a renewable transportation fuel, they have the disadvantage of having low volatilities, high viscosities, and poor cold flow properties [27]. Microalgae, which have simple growth requirements and grow rapidly, are photosynthetic microorganisms that are able to produce lipids, proteins, and carbohydrates in large amounts. These products can be processed into both biofuels and other valuable co-products [27,28,29]. As the third generation of biofuel feedstocks, microalgae are considered to be a technically viable alternative energy resource.
4. Materials and Methods
The cyanobacterium Synechocystis sp. PCC6803 was cultivated in BG-11 medium (5 mM glucose) at 30 °C [30]. The culture was bubbled with air under a light intensity of 40 μmol photon m−2 s−1. For growth on solid medium, cells were cultured on plates containing 1.5% (w/v) DifcoBacto Agar (Becton Dickinson, Sparks, MD, USA), 0.3% (w/v) sodium thiosulfate, and 10 mM TES (pH8.2). For resistance selection of the transformed strains, 50μg/mL kanamycin (Dingguo Company, Beijing, China) was added to the liquid and solid BG-11 medium. Cell density was determined by measuring the optical density (OD) of the suspension at 730 nm (OD730) with a spectrophotometer (DU-70, Beckman Coulter, Brea, CA, USA).
For heterologous expression of AhFatA and AhFatB (individually or in tandem) in Synechocystis sp. PCC6803, the 1.1-kb AhFatA gene (GenBank: GU324446) and 1.2-kb AhFatB gene (GenBank: GU324447) were amplified from Arachis hypogaea L. cultivar ‘Luhua 14’ using primers AhFatA-F andAhFatA-SalI-His-R and AhFatB-F and AhFatB-SalI-Flag-R primers, respectively (Table 1). For fusion PCR of AhFatA and AhFatB, the AhFatA-F and AhFatA-R′ promoters and AhFatB-F and AhFatB-SalI-Flag-R primers were used, and then the AhFatA and AhFatB fragments (AhFatAB) were combined by fusion PCR. The termination codon of AhFatA was deleted and a 17-bp Shine-Dalgarno (SD) sequence, 5′-TTGGTTATAATTCCTTA-3′, was added to the 3′ end of AhFatA. For heterologous expression of AhFatA and AhFatB (individually or in tandem) in Synechocystis sp. PCC6803, plasmid constructs were generated in which a His-tag was added to the 3′ end of AhFatA and a Flag-tag to the 3′ end of AhFatB. The resulting plasmid constructs (i.e., SDFatA, SDFatB, and SDFatAB) were used to replace the psbA2 gene of Synechocystis with AhFatA, AhFatB, or both of these genes via double homologous recombination. The psbA2 promoter (0.5-kb fragment) in Synechocystis genomic DNA upstream of the psbA2, ORF was amplified by PCR as the upstream region and the promoter. To fuse the psbA2 promoter to AhFatA (AhFatB/AhFatAB), the psbA2 promoter was amplified using promoter-SalI-F and promoter-R, and the 1.0-kb fragment of Synechocystis genomic DNA that encodes the psbA2 ORF was amplified by PCR as the downstream region of the homologous recombination vector, using the primers psbA2-SacII-F and psbA2-SacI-R. The downstream fragment was cloned into the SacII and SacI sites of pBluescript SK+T1T2, to form plasmid p5ST1T2psbA2. Then the kanamycin resistance cassette carrying npt was cloned into the single BamHI site of p5ST1T2psbA2, to form p5ST1T2psbA2npt. Then the fragments of AhFatA, AhFatB, and AhFatAB that fused with promoter were cloned into the SalI site of p5ST1T2psbA2npt, to form the pSDFatA, pSDFatB, and pSDFatAB plasmids.

Table 1.
Primer sequences used in this study a.
Synechocystis sp. PCC 6803 was grown in liquid BG-11 medium at 30 °C under a light intensity of 40 μmol of photon m−2 s−1 until the OD730 reached 0.6, and the cells were harvested by centrifugation and resuspended in fresh BG-11 medium to an OD730 value of 4.8. Then, 2 mg plasmid DNA was added to 500 μL of cell suspension and gently mixed, and the mixture was incubated at 30 °C under low light for 6 h and then spread on BG-11 agar plates containing kanamycin (20μg/mL). Transformants were isolated after about 10 days of incubation, and subcultured on BG-11 agar plates containing 50μg/mL kanamycin. The transformants were then grown in liquid culture for analysis.
Wild-type and transformant cell lines were cultured and harvested in the exponential growth phase, and total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA ), following the manufacturer’s instructions. First strand cDNA was synthesized using M-MLV reverse transcriptase and modified oligo (dT) following the manufacturer’s instructions (TaKaRa, Dalian, China). The resulting cDNA molecules were amplified by PCR using the following gene-specific primers: rnpB-F and rnpB-R for amplification of the rnpB gene and 16S rRNA-F and 16S rRNA-R for amplification of the 16S rRNA gene, which were used as loading controls; AhFatA-RT-F and AhFatA-RT-R for amplification of AhFatA; AhFatB-RT-F and AhFatB-RT-R for amplification of AhFatB. The PCR products were analyzed by 1.0% agarose gel electrophoresis. The PCR products were then cloned in to pGEX-T Easy Cloning Vector (Promega, Madison, WI, USA) and sequenced by the Biotechnology Research Center, Shandong Academy of Agricultural Science (Ji’nan, China).
The suspension cultures grown at 30 °C were diluted to an OD730 nm of 0.1, and were further incubated at 20 °C and 30 °C. The growth of wild-type and transformant were measured by monitoring absorbance of the culture medium at 730 nm after a regular interval of 1 day using SP-723 UV–VIS Spectrophotometer (Shanghai Spectrum Instruments Co., Ltd., Shanghai, China).
Crude extracts of wild-type and transformant cells were collected and dissolved in lysis buffer (1 mL of 40 mM Tris–HCl pH 8.0). After sonication, incubation, and centrifugation, the insoluble material was removed and the supernatants were subjected to immunoblot analysis. The soluble proteins were separated on 12% SDS-PAGE gels and then blotted onto 0.45 μm PVDF membranes (Beijing CoWin Biotech Co., Ltd., Beijing, China), stained with antibody to His tag or FLAG tag (1: 5000, Beijing CoWin Biotech Co., Ltd., Beijing, China) for 1 h, and then incubated with goat anti-rabbit IgG HRP at 1: 5000 for 1 h. The cross-reactions between protein bands and antibodies were detected using an HRP-DAB Color Development Kit (Tiangen Biotech, Beijing, China), according to the manufacturer’s instructions.
Membrane lipid extractions of wild-type cyanobacteria Synechocystis sp. PCC6803 and genetically engineered cyanobacteria Synechocystis sp. PCC6803 were carried out as described by Bligh and Dyer [31]. The colonies were collected and transferred to flasks containing 400 mL sterile BG-11 medium, respectively, and grown for 10 days at a light intensity of 40 μmol photons m−2 s−1 and a constant temperature of 30 °C. Cultures were harvested when OD730 reached 3.0, and were then washed with distilled water by centrifugation (6000× g for 10 min at room temperature). The wet cell pellets were heated at 40 °C to obtain 600 mg dry cell paste. The dry cell paste was diluted with 4 mL chloroform/methanol (1:10, v/v), and a suspension of 1 mL hexane containing C19:0 internal standard (1 mg/mL) was added. The mixture was heated at 80 °C for 2 h in a water bath, and then after cooling, 5 mL of 7% potash was added and mixed. After 10 min, the mixture was centrifuged at 10,000 × g for 10 min. The supernatants (bacterial sample FAME eluate) were subjected to gas chromatography (GC) using the Elite-WAX column in an ASXL instrument (Perkin-Elmer, Waltham, MA, USA). The flame-ionization detection (FID) temperature was 250 °C, and the operating temperature was maintained at 220 °C. The data presented in this paper are the average of three experiments for each sample.
5. Conclusions
In this study, we cloned two thioesterase genes from Arachis hypogaea L. seeds and then functionally expressed these genes in a blue-green alga Synechocystis sp. PCC6803 individually and in tandem. The heterologous expression of AhFatA and AhFatB altered the FA profiles of the transgenic cells. This study lays the foundation for increasing the content of desirable FAs.
Supplementary Materials
The following are available online at www.mdpi.com/1996-1073/10/12/2093/s1, Table S1: Fatty acid content of wild-type and transgenic Synechocystis sp. PCC6803 a cultured under mixotrophic growth conditions at 30 °C and 20 °C.
Acknowledgments
This work was financially supported by Natural Science Foundation of Shandong Province, China (ZR2016CM48), Young Talents Training Program of Shandong Academy of Agricultural Sciences (2016), China Postdoctoral Science Foundation (2014M551942), and National Key Research and Development Program—China (2016YFF0202304).
Author Contributions
Gao Chen conceived and designed the experiments; Gao Chen, Jun Chen, Yan Zhang, FeiBian, and Jinhui Yu performed the experiments; Gao Chen, Qingfang He, Zhenying Peng, Zhongxue Fan, and Songqin analyzed the data; Gao Chen contributed reagents/materials/analysis tools; Gao Chen wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salas, J.J.; Ohlrogge, J.B. Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch. Biochem. Biophys. 2002, 403, 25–34. [Google Scholar] [CrossRef]
- Wu, P.Z.; Li, J.; Wei, Q.; Zeng, L.; Chen, Y.P.; Li, M.R.; Jiang, H.W.; Wu, G.L. Cloning and functional characterization of an acyl-acyl carrier protein thioesterase (JcFATB1) from Jatropha curcas. Tree Physiol. 2009, 29, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Peng, Z.Y.; Shan, L.; Xuan, N.; Tang, G.Y.; Zhang, Y.; Li, L.; He, Q.F.; Bi, Y.P. Cloning of Acyl-ACP thioesterase FatA from Arachis hypogaea L. and its expression in Escherichia coli. J. Biomed. Biotechnol. 2012, 2012, 652579. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.K.; Ohlrogge, J.B.; Pollard, M. On the export of fatty acids from the chloroplast. J. Biol. Chem. 2004, 279, 16101–16110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, D.; Lu, M. Cloning and expression analysis of PtFATB gene encoding the acyl-acyl carrier protein thioesterase in Populus tomentosa. Carr. J. Genet. Genom. 2007, 34, 267–274. [Google Scholar] [CrossRef]
- Chen, G.; He, Q.F.; Xuan, N.; Peng, Z.Y.; Shan, L.; Tang, G.Y.; Bi, Y.P. Functional expression analysis of an acyl-ACP thioesterase FatB1 from Arachis hypogaea L. seeds in Escherichia coli. J. Food Agric. Environ. 2012, 10, 332–336. [Google Scholar]
- Voelker, T.A.; Jones, A.; Cranmer, A.M.; Davies, H.M.; Knutzon, D.S. Broad-range and binary-range acyl-acyl-carrier protein thioesterases suggest an alternative mechanism for medium-chain production in seeds. Plant Physiol. 1997, 114, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, A.J.; Sánchez-García, A.; Salas, J.J.; Garcés, R.; Martínez-Force, E. Acyl-ACP thioesterases from macadamia (Macadamia tetraphylla) nuts: Cloning, characterization and their impact on oil composition. Plant Physiol. Biochem. 2011, 49, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, A.; Moreno-Pérez, A.J.; Muro-Pastor, A.M.; Salas, J.J.; Garcés, R.; Martínez-Force, E. Acyl-ACP thioesterases from castor (Ricinus communis L.): An enzymatic system appropriate for high rates of oil synthesis and accumulation. Phytochemistry 2010, 71, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qu, S.J.; Wang, Q.; Bian, F.; Peng, Z.Y.; Zhang, Y.; Ge, H.T.; Yu, J.H.; Xuan, N.; Bi, Y.P.; et al. Transgenic expression of delta-6 and delta-15 fatty acid desaturases enhances omega-3 polyunsaturated fatty acid accumulation in Synechocystis sp. PCC6803. Biotechnol. Biofuels 2014, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Napier, J.A.; Beaudoin, F.; Michaelson, L.V.; Sayanova, O. The production of long chain polyunsaturated fatty acids in transgenic plants by reverse engineering. Biochimie 2004, 86, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Sayanova, O.V.; Napier, J.A. Eicosapentaenoic acid: Biosynthetic routes and the potential for synthesis in transgenic plants. Phytochemistry 2004, 65, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, T.; Ramesh, D.; Karthikeyan, S.; Kumari, S.; Anandraj, A.; Bux, F. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 2011, 102, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.; Chen, H.; Shen, G.; Hu, Z.; Chen, L.; Wang, J. Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnol. Biofuels 2012, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.; Amaro, H.M.; Barbosa, C.R.; Pereira, R.D.; Malcata, F.X. Fatty acid composition of several wild microalgae and cyanobacteria, with a focus on eicosapentaenoic, docosahexaenoic and α-linolenic acids for eventual dietary uses. Food Res. Int. 2011, 44, 2721–2729. [Google Scholar] [CrossRef]
- Magnuson, K.; Jackowski, S.; Rock, C.O.; Cronan, J.E., Jr. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 1993, 5, 522–542. [Google Scholar]
- Liu, X.; Sheng, J.; Rd, C.R. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 6899–6904. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarzyk, D.; Fulda, M. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol. 2010, 152, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Schlich, T.; Paulsen, H.; Vermass, W. Expression of a higher plant light-harvesting chlorophyll a/b-binding protein in Synechocystis sp. PCC 6803. Eur. J. Biochem. 1999, 263, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Eriksson, J.; Osiewacz, H.D.; Jansson, C. Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. Mol. Gen. Genet. 1993, 238, 161–168. [Google Scholar] [PubMed]
- Renaud, S.M.; Thinh, L.V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Mironov, K.S.; Maksimov, E.G.; Maksimov, G.V.; Los, D.A. Feedback between fluidity of membranes and transcription of the desB gene for the ω-3 desaturase in the cyanobacterium Synechocystis. Mol. Biol. 2012, 46, 134–141. [Google Scholar] [CrossRef]
- Froehlich, J.E.; Poorman, R.; Reardon, E.; Barnum, S.R.; Jaworski, J.G. Purification and characterization of acyl carrier protein from two cyanobacteria species. Eur. J. Biochem. 1990, 193, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Frentzen, M.; Heinz, E.; McKeon, T.A.; Stumpf, P.K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur. J. Biochem. 1983, 129, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Boehman, A.L. Biodiesel production and processing: Foreword. Fuel Process. Technol. 2005, 86, 1057–1058. [Google Scholar] [CrossRef]
- Dassey, A.J.; Hall, S.G.; Theegala, C.S. An analysis of energy consumption for algal biodiesel production: Comparing the literature with current estimates. Algal Res. 2014, 4, 89–95. [Google Scholar] [CrossRef]
- Pienkos, P.T.; Darzins, A. The promise and challenges of microalgal-derived biofuels. Biofuels Bioprod. Bioref. 2009, 3, 431–440. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).