Functional Expression of the Arachis hypogaea L. Acyl-ACP Thioesterases AhFatA and AhFatB Enhances Fatty Acid Production in Synechocystis sp. PCC6803
Abstract
:1. Introduction
2. Results
2.1. Integration of AhFatA and AhFatB into Synechocystis 6803 and Their Expression Analysis
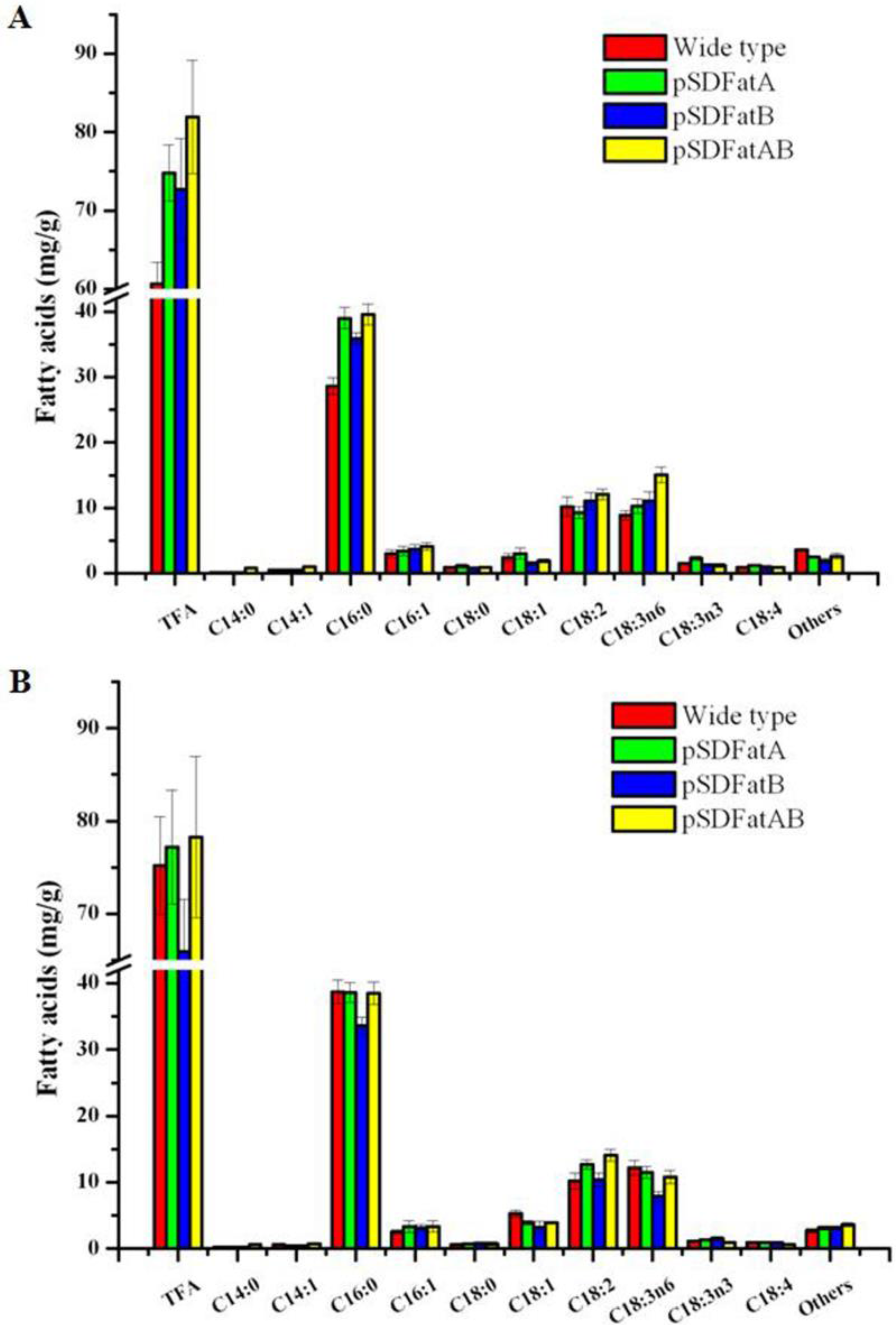
2.2. GC Analysis of Fatty Acids in the Synechocystis 6803 Strain Expressing AhFatA and AhFatB
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Salas, J.J.; Ohlrogge, J.B. Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch. Biochem. Biophys. 2002, 403, 25–34. [Google Scholar] [CrossRef]
- Wu, P.Z.; Li, J.; Wei, Q.; Zeng, L.; Chen, Y.P.; Li, M.R.; Jiang, H.W.; Wu, G.L. Cloning and functional characterization of an acyl-acyl carrier protein thioesterase (JcFATB1) from Jatropha curcas. Tree Physiol. 2009, 29, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Peng, Z.Y.; Shan, L.; Xuan, N.; Tang, G.Y.; Zhang, Y.; Li, L.; He, Q.F.; Bi, Y.P. Cloning of Acyl-ACP thioesterase FatA from Arachis hypogaea L. and its expression in Escherichia coli. J. Biomed. Biotechnol. 2012, 2012, 652579. [Google Scholar] [CrossRef] [PubMed]
- Koo, A.J.K.; Ohlrogge, J.B.; Pollard, M. On the export of fatty acids from the chloroplast. J. Biol. Chem. 2004, 279, 16101–16110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, D.; Lu, M. Cloning and expression analysis of PtFATB gene encoding the acyl-acyl carrier protein thioesterase in Populus tomentosa. Carr. J. Genet. Genom. 2007, 34, 267–274. [Google Scholar] [CrossRef]
- Chen, G.; He, Q.F.; Xuan, N.; Peng, Z.Y.; Shan, L.; Tang, G.Y.; Bi, Y.P. Functional expression analysis of an acyl-ACP thioesterase FatB1 from Arachis hypogaea L. seeds in Escherichia coli. J. Food Agric. Environ. 2012, 10, 332–336. [Google Scholar]
- Voelker, T.A.; Jones, A.; Cranmer, A.M.; Davies, H.M.; Knutzon, D.S. Broad-range and binary-range acyl-acyl-carrier protein thioesterases suggest an alternative mechanism for medium-chain production in seeds. Plant Physiol. 1997, 114, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, A.J.; Sánchez-García, A.; Salas, J.J.; Garcés, R.; Martínez-Force, E. Acyl-ACP thioesterases from macadamia (Macadamia tetraphylla) nuts: Cloning, characterization and their impact on oil composition. Plant Physiol. Biochem. 2011, 49, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, A.; Moreno-Pérez, A.J.; Muro-Pastor, A.M.; Salas, J.J.; Garcés, R.; Martínez-Force, E. Acyl-ACP thioesterases from castor (Ricinus communis L.): An enzymatic system appropriate for high rates of oil synthesis and accumulation. Phytochemistry 2010, 71, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qu, S.J.; Wang, Q.; Bian, F.; Peng, Z.Y.; Zhang, Y.; Ge, H.T.; Yu, J.H.; Xuan, N.; Bi, Y.P.; et al. Transgenic expression of delta-6 and delta-15 fatty acid desaturases enhances omega-3 polyunsaturated fatty acid accumulation in Synechocystis sp. PCC6803. Biotechnol. Biofuels 2014, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Napier, J.A.; Beaudoin, F.; Michaelson, L.V.; Sayanova, O. The production of long chain polyunsaturated fatty acids in transgenic plants by reverse engineering. Biochimie 2004, 86, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Sayanova, O.V.; Napier, J.A. Eicosapentaenoic acid: Biosynthetic routes and the potential for synthesis in transgenic plants. Phytochemistry 2004, 65, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Mutanda, T.; Ramesh, D.; Karthikeyan, S.; Kumari, S.; Anandraj, A.; Bux, F. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 2011, 102, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.; Chen, H.; Shen, G.; Hu, Z.; Chen, L.; Wang, J. Expression of fatty acid synthesis genes and fatty acid accumulation in Haematococcus pluvialis under different stressors. Biotechnol. Biofuels 2012, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.; Amaro, H.M.; Barbosa, C.R.; Pereira, R.D.; Malcata, F.X. Fatty acid composition of several wild microalgae and cyanobacteria, with a focus on eicosapentaenoic, docosahexaenoic and α-linolenic acids for eventual dietary uses. Food Res. Int. 2011, 44, 2721–2729. [Google Scholar] [CrossRef]
- Magnuson, K.; Jackowski, S.; Rock, C.O.; Cronan, J.E., Jr. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 1993, 5, 522–542. [Google Scholar]
- Liu, X.; Sheng, J.; Rd, C.R. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 6899–6904. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarzyk, D.; Fulda, M. Fatty acid activation in cyanobacteria mediated by acyl-acyl carrier protein synthetase enables fatty acid recycling. Plant Physiol. 2010, 152, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Schlich, T.; Paulsen, H.; Vermass, W. Expression of a higher plant light-harvesting chlorophyll a/b-binding protein in Synechocystis sp. PCC 6803. Eur. J. Biochem. 1999, 263, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Eriksson, J.; Osiewacz, H.D.; Jansson, C. Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. Mol. Gen. Genet. 1993, 238, 161–168. [Google Scholar] [PubMed]
- Renaud, S.M.; Thinh, L.V.; Lambrinidis, G.; Parry, D.L. Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 2002, 211, 195–214. [Google Scholar] [CrossRef]
- Mironov, K.S.; Maksimov, E.G.; Maksimov, G.V.; Los, D.A. Feedback between fluidity of membranes and transcription of the desB gene for the ω-3 desaturase in the cyanobacterium Synechocystis. Mol. Biol. 2012, 46, 134–141. [Google Scholar] [CrossRef]
- Froehlich, J.E.; Poorman, R.; Reardon, E.; Barnum, S.R.; Jaworski, J.G. Purification and characterization of acyl carrier protein from two cyanobacteria species. Eur. J. Biochem. 1990, 193, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Frentzen, M.; Heinz, E.; McKeon, T.A.; Stumpf, P.K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur. J. Biochem. 1983, 129, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae-a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Boehman, A.L. Biodiesel production and processing: Foreword. Fuel Process. Technol. 2005, 86, 1057–1058. [Google Scholar] [CrossRef]
- Dassey, A.J.; Hall, S.G.; Theegala, C.S. An analysis of energy consumption for algal biodiesel production: Comparing the literature with current estimates. Algal Res. 2014, 4, 89–95. [Google Scholar] [CrossRef]
- Pienkos, P.T.; Darzins, A. The promise and challenges of microalgal-derived biofuels. Biofuels Bioprod. Bioref. 2009, 3, 431–440. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
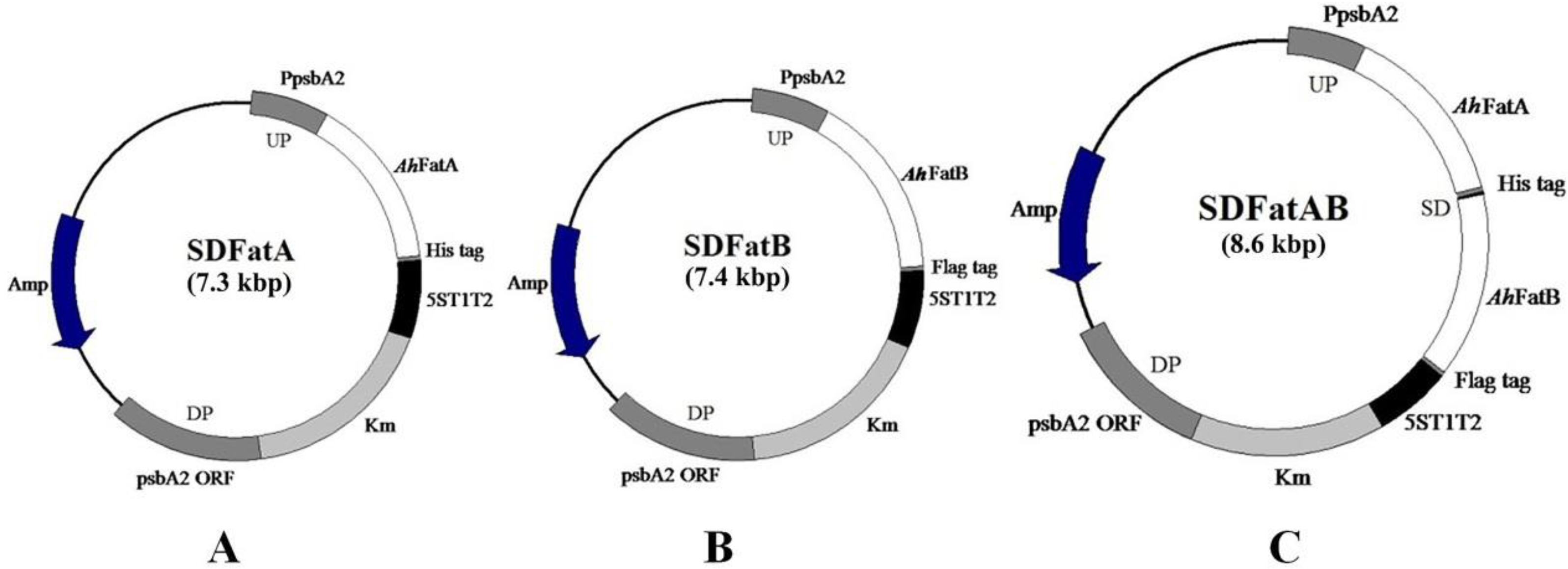
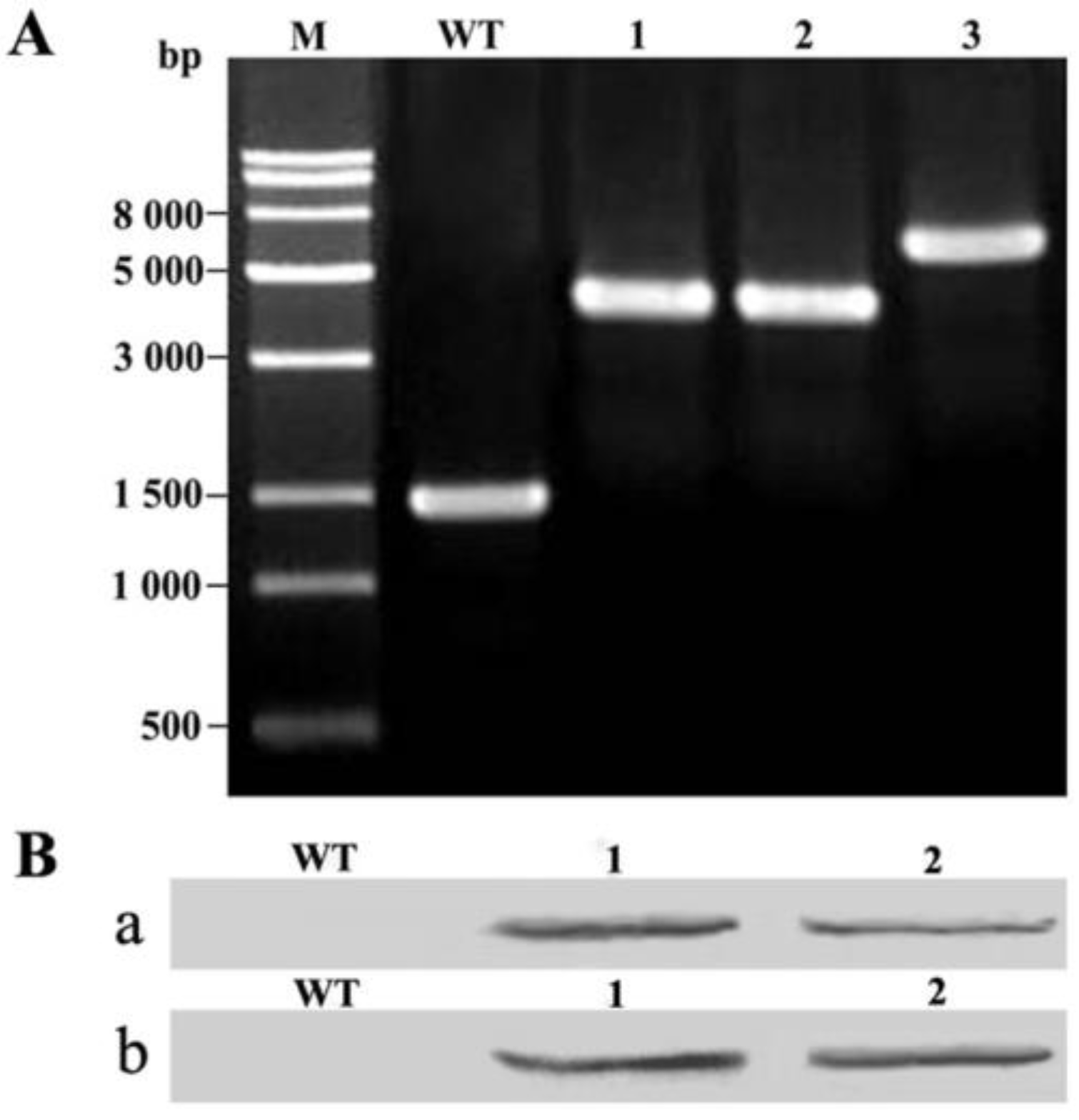
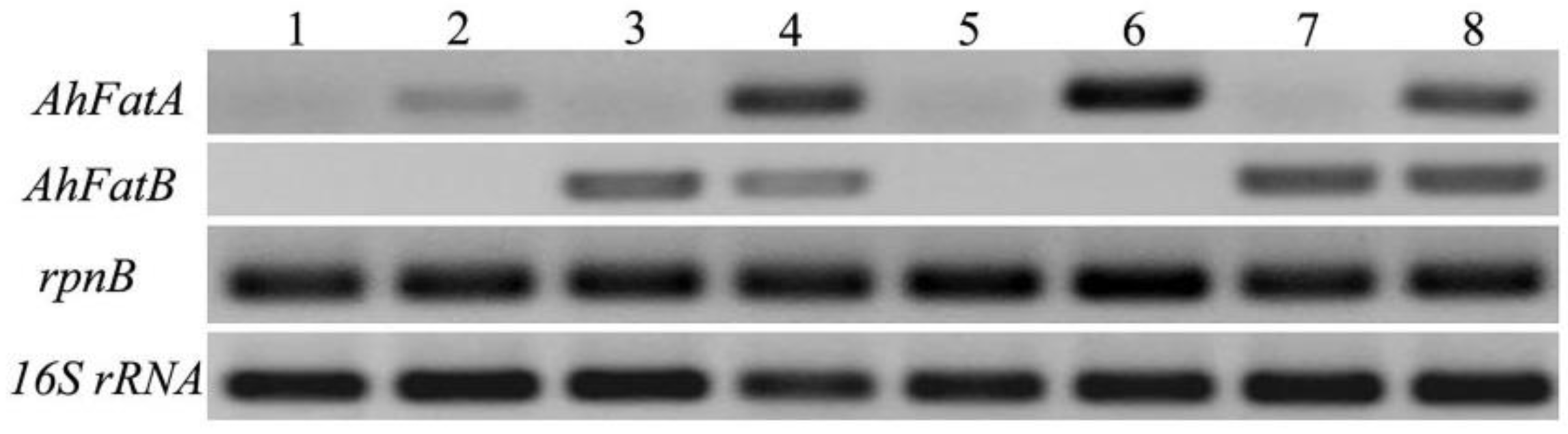
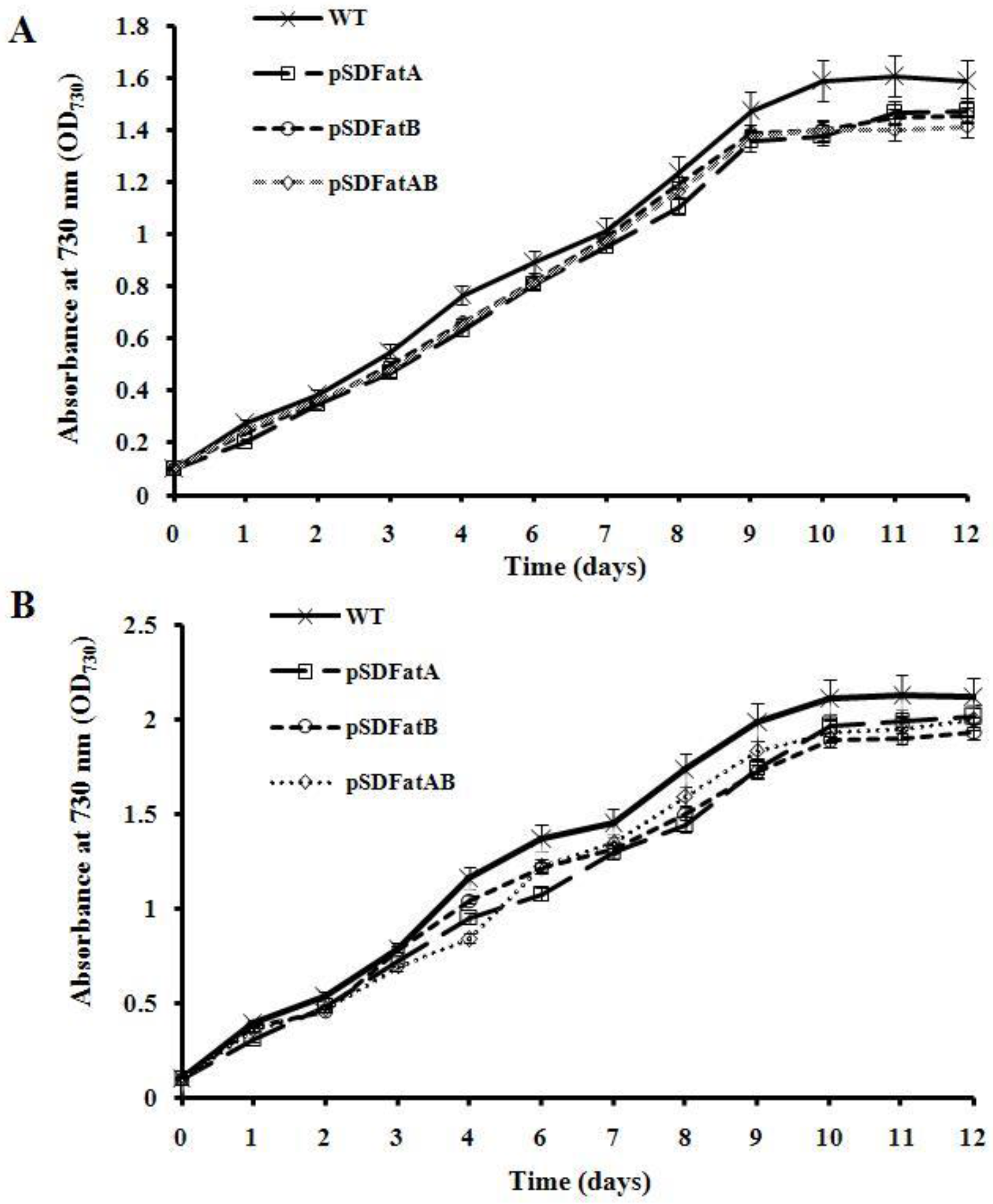
| Primer | Sequence (5′–3′) |
|---|---|
| promoter-SalI-F | GATGTCGACGCTTTAGCGTTCCAGTG |
| promoter-R | CATTTGGTTATAATTCCTTATGTAT |
| AhFatA-F | GGAATTATAACCAAATGTTGAAGGTTTCATGCAACG |
| AhFatA-SalI-His-R | CCGGTCGACTCAATGATGATGATGATGATGTAATCTTGAAGCTTTCTTTC |
| AhFatB-F | GGAATTATAACCAAATGGCAACTGCTGCTACTGCT |
| AhFatB-SalI-Flag-R | TTCGTCGACTTACTTATCGTCGTCATCCTTGTAATCGATGCTTTCGGCTGGAACC |
| AhFatA-R′ | TTGGTTATAATTCCTTATCAATGATGATGATGATGAT |
| AhFatB-F′ | TAAGGAATTATAACCAAATGGCAACTGCTGCTACTGCT |
| psbA2-SacII-F | CTTCCGCGGATGACAACGACTCTCCAAC |
| psbA2-SacI-R | AGTGAGCTCTTAACCGTTGACAGCAGG |
| rnpB-F | GTTAGGGAGGGAGTTGCGG |
| rnpB-R | AAGAGAGTTAGTCGTAAGCCG |
| 16S rRNA-F | AGCGTCCGTAGGTGGTTATG |
| 16S rRNA-R | CTACGCATTTCACCGCTACA |
| AhFatA-RT-F | CAATAAGACTGCCACCGT |
| AhFatA-RT-R | TCAAGAACCCAACCAAT |
| AhFatB-RT-F | TTCTTGGTGATGGCTTTG |
| AhFatB-RT-R | GACCCGTGCGAATGTAAT |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Chen, J.; He, Q.; Zhang, Y.; Peng, Z.; Fan, Z.; Bian, F.; Yu, J.; Qin, S. Functional Expression of the Arachis hypogaea L. Acyl-ACP Thioesterases AhFatA and AhFatB Enhances Fatty Acid Production in Synechocystis sp. PCC6803. Energies 2017, 10, 2093. https://doi.org/10.3390/en10122093
Chen G, Chen J, He Q, Zhang Y, Peng Z, Fan Z, Bian F, Yu J, Qin S. Functional Expression of the Arachis hypogaea L. Acyl-ACP Thioesterases AhFatA and AhFatB Enhances Fatty Acid Production in Synechocystis sp. PCC6803. Energies. 2017; 10(12):2093. https://doi.org/10.3390/en10122093
Chicago/Turabian StyleChen, Gao, Jun Chen, Qingfang He, Yan Zhang, Zhenying Peng, Zhongxue Fan, Fei Bian, Jinhui Yu, and Song Qin. 2017. "Functional Expression of the Arachis hypogaea L. Acyl-ACP Thioesterases AhFatA and AhFatB Enhances Fatty Acid Production in Synechocystis sp. PCC6803" Energies 10, no. 12: 2093. https://doi.org/10.3390/en10122093
APA StyleChen, G., Chen, J., He, Q., Zhang, Y., Peng, Z., Fan, Z., Bian, F., Yu, J., & Qin, S. (2017). Functional Expression of the Arachis hypogaea L. Acyl-ACP Thioesterases AhFatA and AhFatB Enhances Fatty Acid Production in Synechocystis sp. PCC6803. Energies, 10(12), 2093. https://doi.org/10.3390/en10122093





