Novel Carbon Materials in the Cathode Formulation for High Rate Rechargeable Hybrid Aqueous Batteries
Abstract
1. General Introduction of Aqueous Rechargeable Battery
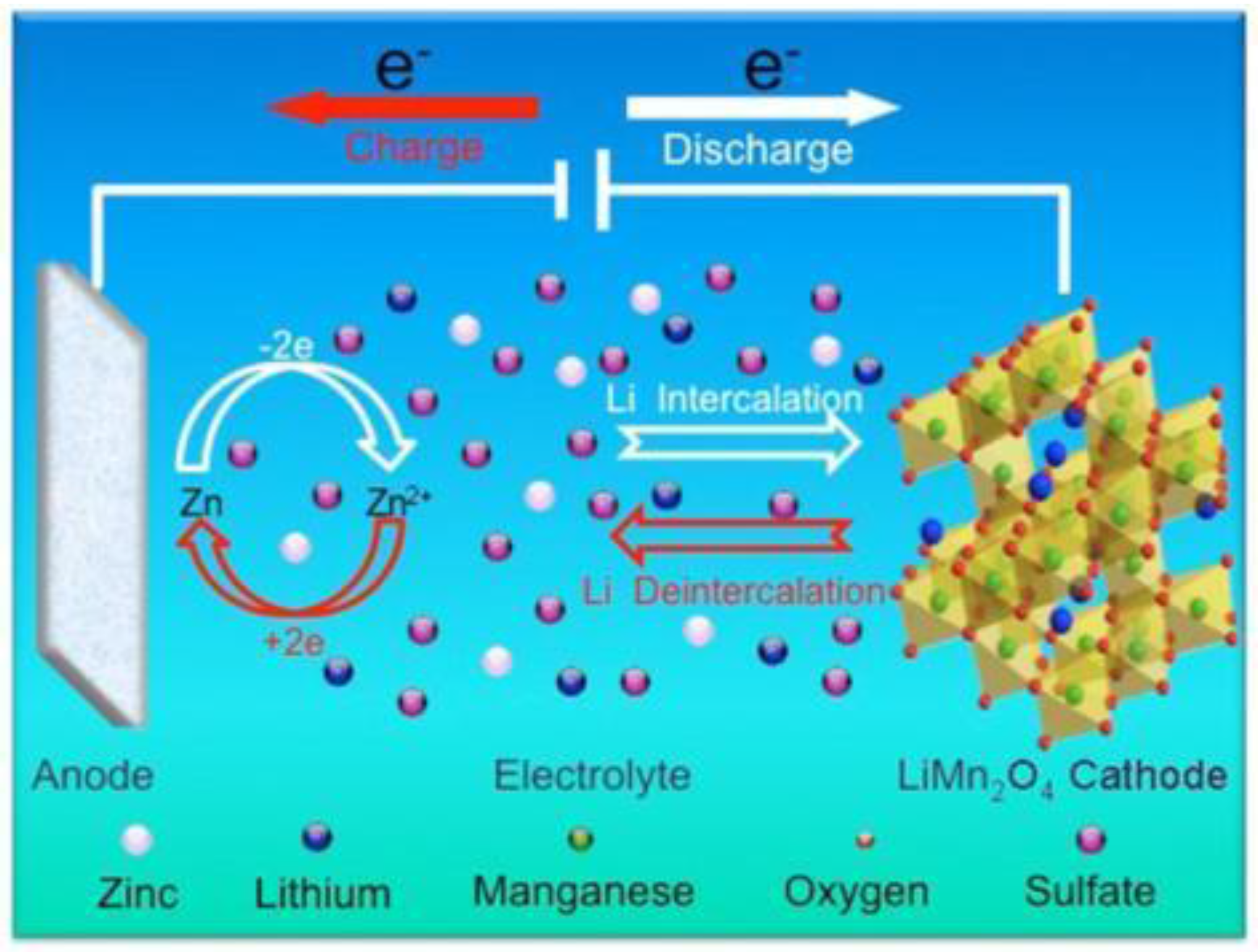
2. Introduction of Rechargeable Hybrid Aqueous Battery
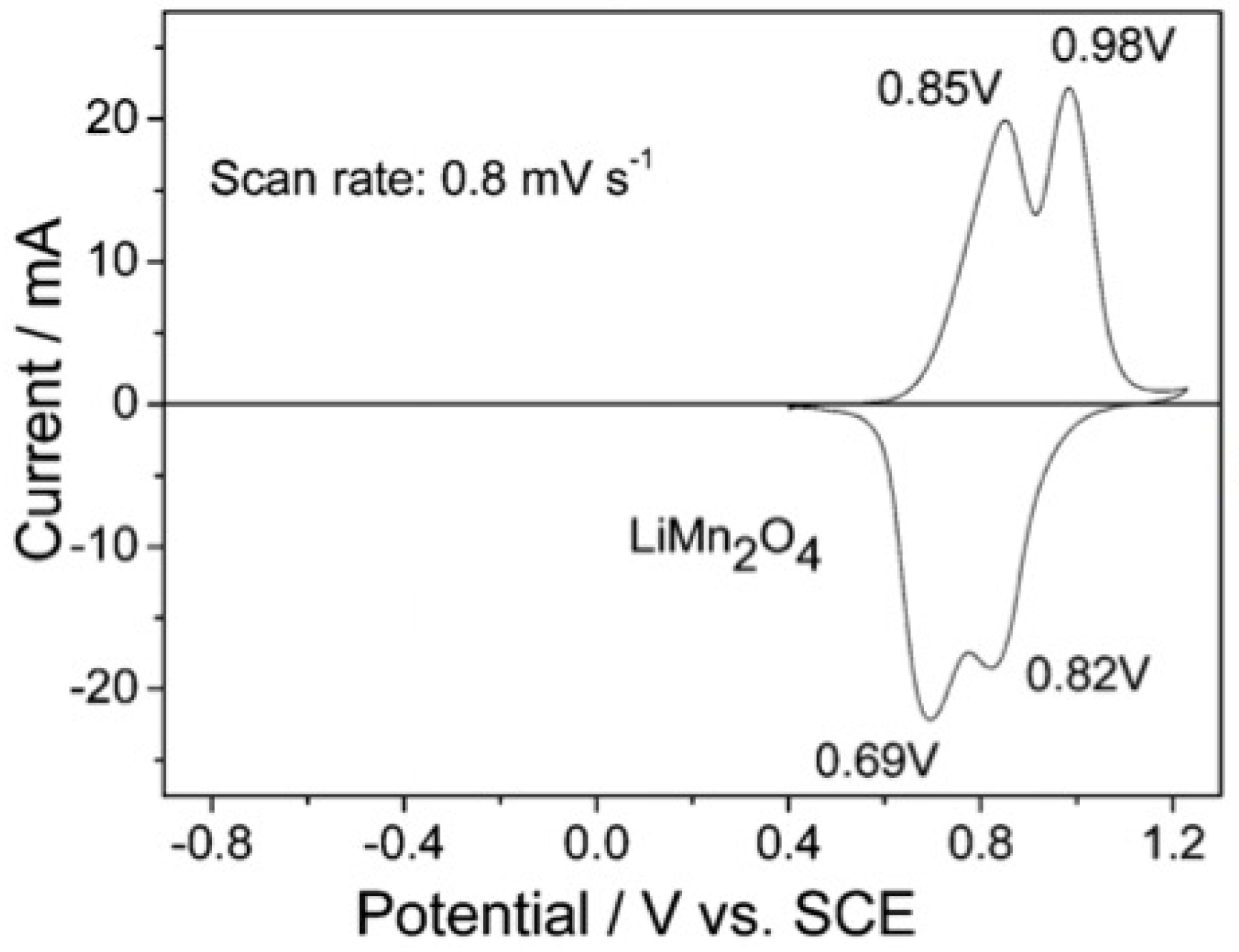
3. Introduction of LiMn2O4
4. Increase the Electrical Conductivity of LiMn2O4 Cathode by Adding CNTs or Graphene as Conductive Additive
4.1. Increase the Electrical Conductivity of LiMn2O4 Cathode by Adding CNTs as Conductive Additive
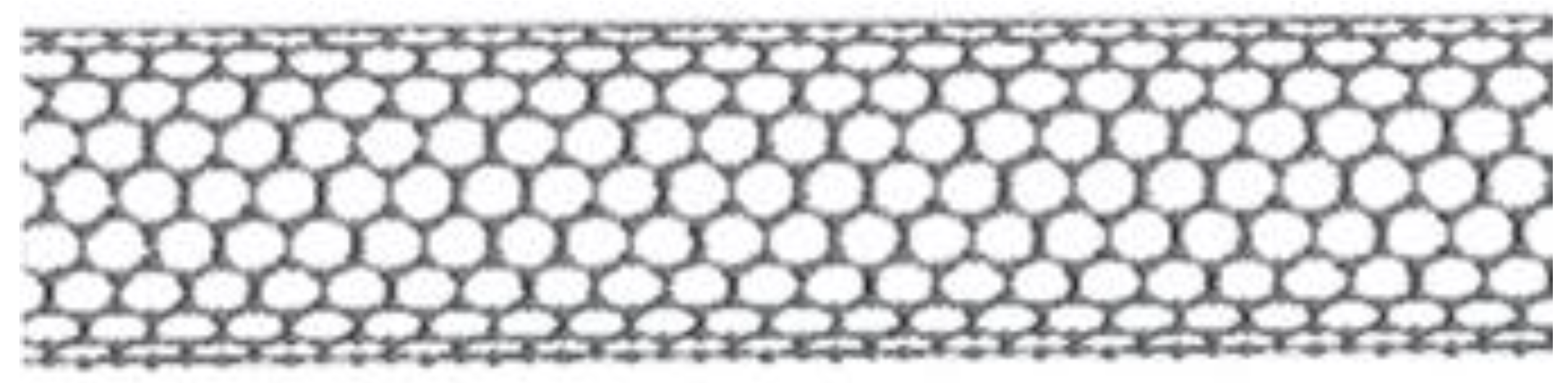
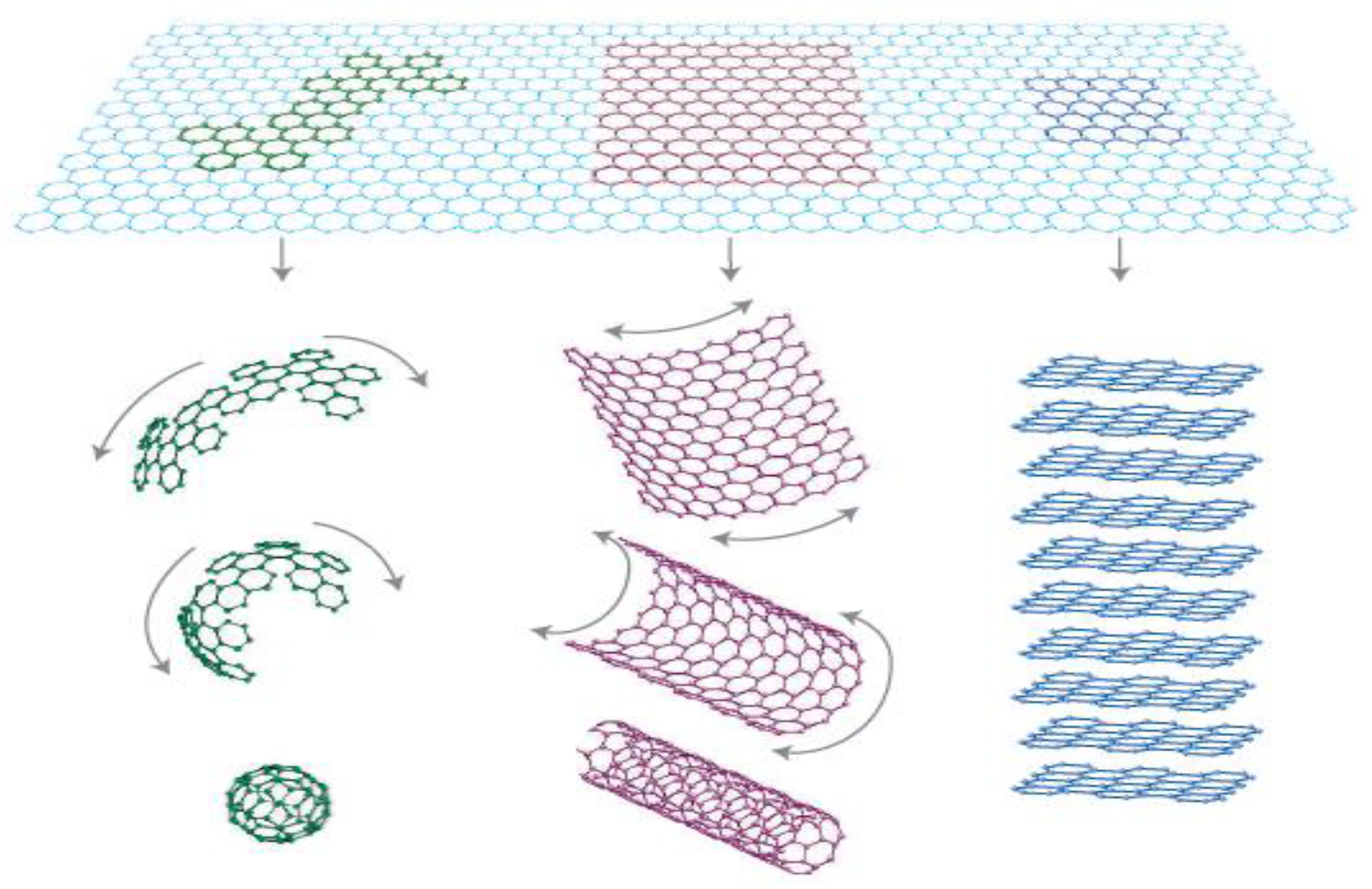
4.1.1. Structure, Properties and Synthesis of CNTs
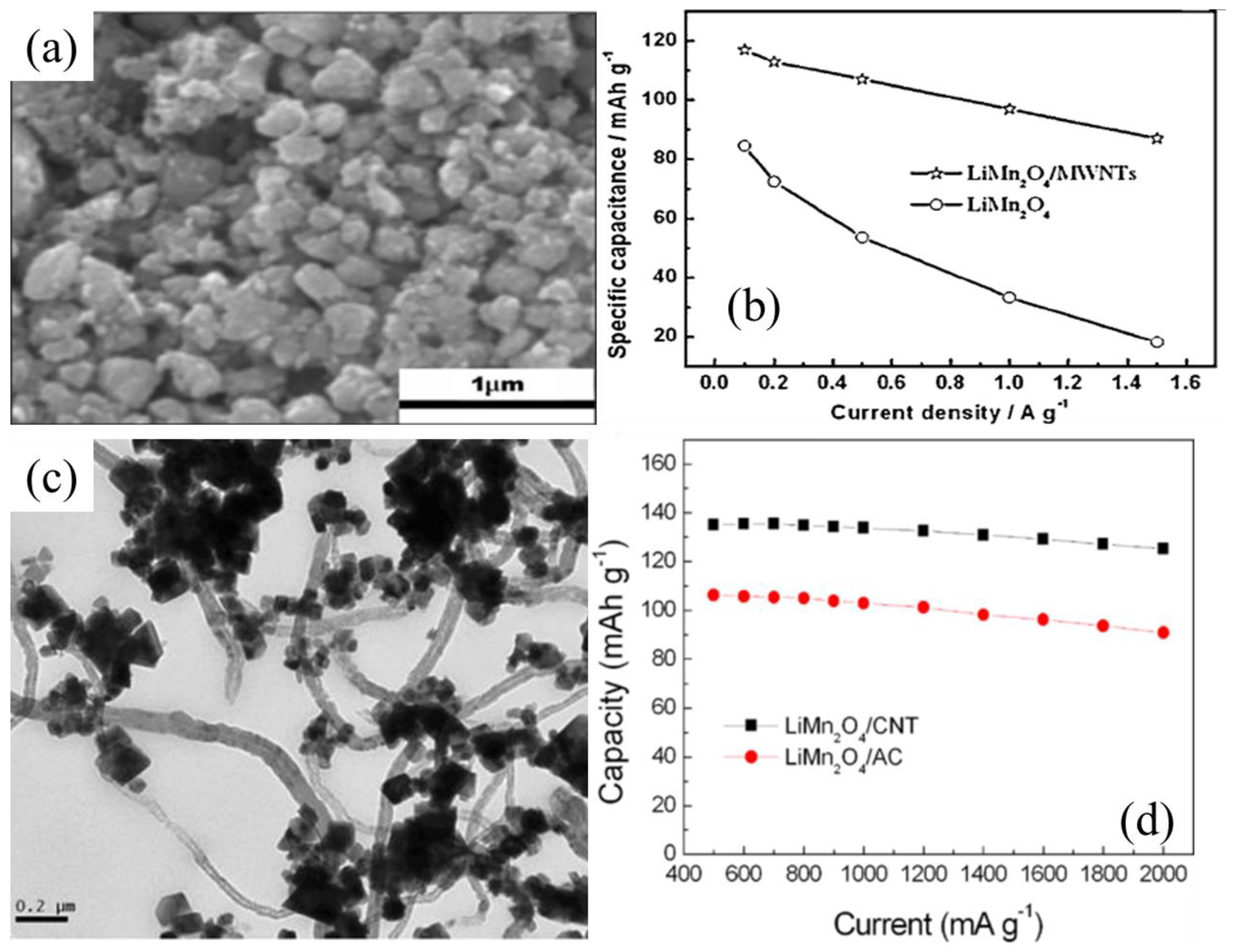
4.1.2. LiMn2O4/CNT Nanocomposites
4.2. Increase the Electrical Conductivity of LiMn2O4 Cathode by Adding Graphene as Conductive Additive
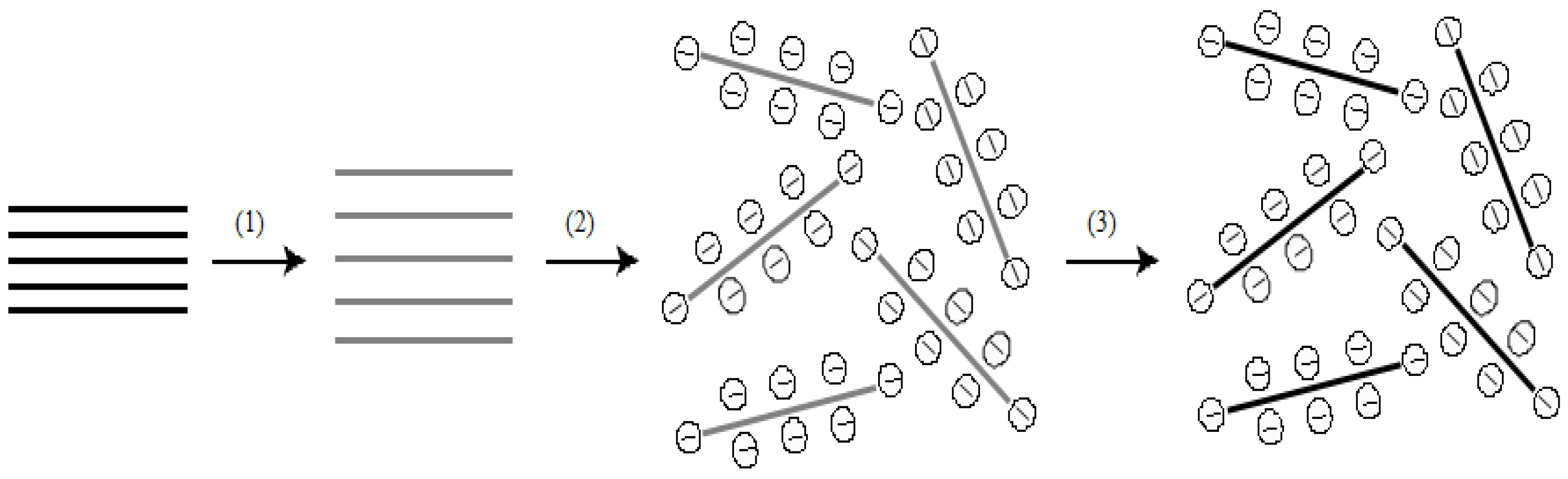
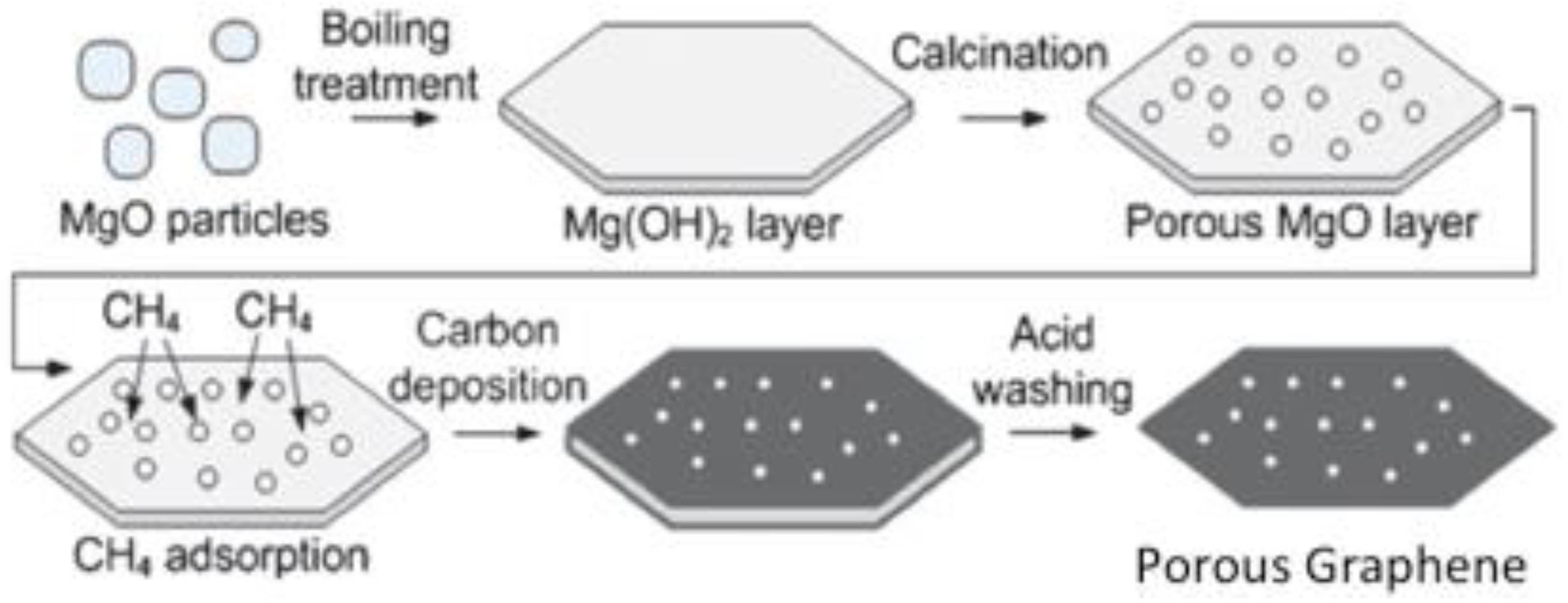
4.2.1. Structure, Properties and Synthesis of Graphene
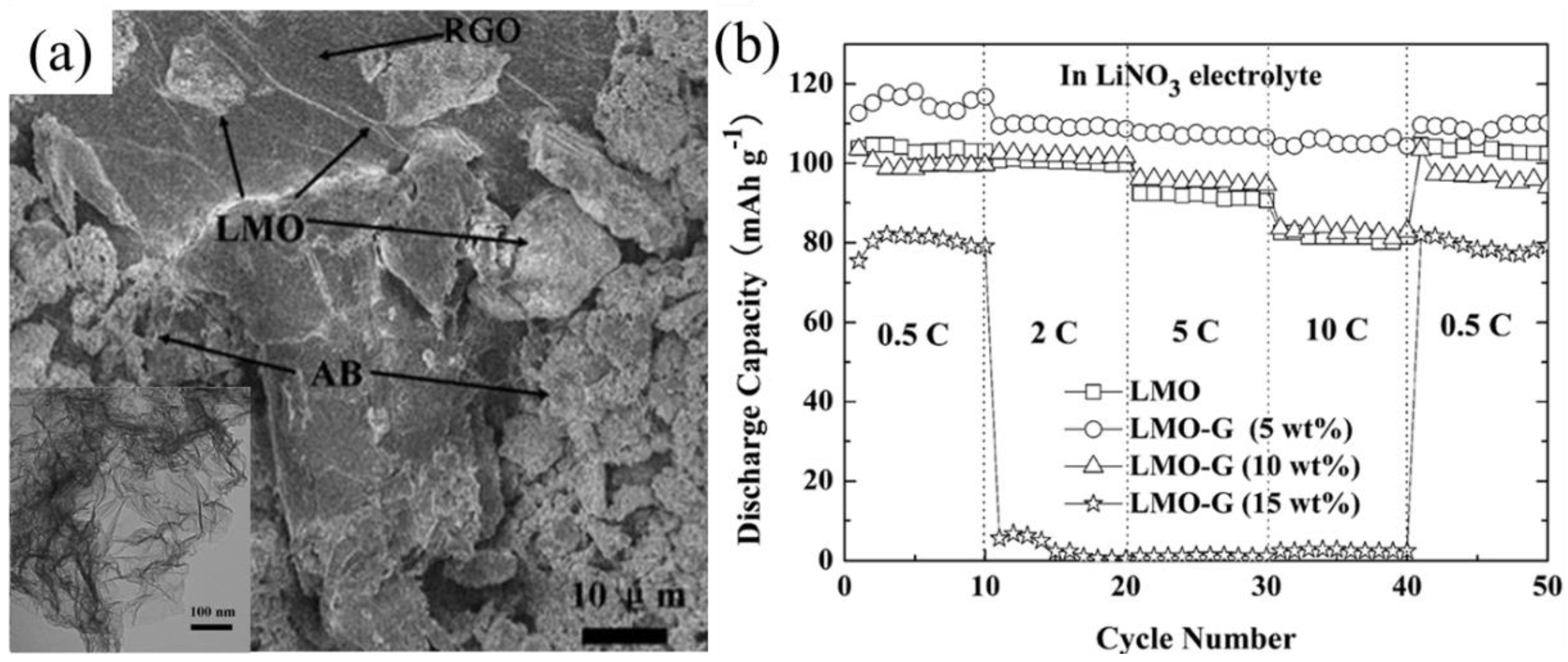
4.2.2. LiMn2O4/Graphene Nanocomposites
4.3. Comparison of Different Conductive Additives in LiMn2O4 Cathodes in Aqueous Batteries
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Soloveichik, G.L. Battery technologies for large-scale stationary energy storage. Annu. Rev. Chem. Biomol. 2011, 2, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiao, S.; Hou, Y.; Hu, C.; Liu, L.; Wu, Y. Electrode materials for aqueous asymmetric supercapacitors. RSC Adv. 2013, 3, 13059. [Google Scholar] [CrossRef]
- Alias, N.; Mohamad, A.A. Advances of aqueous rechargeable lithium-ion battery: A review. J. Power Sources 2015, 274, 237–251. [Google Scholar] [CrossRef]
- Shen, Y.; Kordesch, K. The mechanism of capacity fade of rechargeable alkaline manganese dioxide zinc cells. J. Power Sources 2000, 87, 162–166. [Google Scholar] [CrossRef]
- Shukla, A.; Venugopalan, S.; Hariprakash, B. Nickel-based rechargeable batteries. J. Power Sources 2001, 100, 125–148. [Google Scholar] [CrossRef]
- Köhler, U.; Antonius, C.; Bäuerlein, P. Advances in alkaline batteries. J. Power Sources 2004, 127, 45–52. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Gong, M.; Li, Y.; Chang, W.; Mefford, T.; Zhou, J.; Wang, J.; Regier, T.; Wei, F. An ultrafast nickel-iron battery from strongly coupled inorganic nanoparticle/nanocarbon hybrid materials. Nat. Commun. 2012, 3, 917. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.P.; Yao, S.M.; Yan, T.Y.; Zhou, Z. Alkaline rechargeable Ni/Co batteries: Cobalt hydroxides as negative electrode materials. Energy Environ. Sci. 2009, 2, 502–505. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.; Wainwright, D. Rechargeable lithium batteries with aqueous electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fu, L.; Zhao, N.; Yang, L.; Wu, Y.; Wu, H. An aqueous rechargeable lithium battery with good cycling performance. Angew. Chem. Int. Ed. 2007, 119, 299–301. [Google Scholar] [CrossRef]
- Wang, H.; Huang, K.; Zeng, Y.; Yang, S.; Chen, L. Electrochemical properties of TiP2O7 and LiTi2(PO4)3 as anode material for lithium ion battery with aqueous solution electrolyte. Electrochim. Acta 2007, 52, 3280–3285. [Google Scholar] [CrossRef]
- Luo, J.Y.; Cui, W.J.; He, P.; Xia, Y.Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hou, Y.; Zhu, Y.; Wu, Y.; Holze, R. An aqueous rechargeable lithium battery using coated Li metal as anode. Sci. Rep. 2013, 3, 1401. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chem. Int. Ed. 2012, 124, 957–959. [Google Scholar] [CrossRef]
- Whitacre, J.; Tevar, A.; Sharma, S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem. Commun. 2010, 12, 463–466. [Google Scholar] [CrossRef]
- Park, S.I.; Gocheva, I.; Okada, S.; Yamaki, J.I. Electrochemical properties of NaTi2(PO4)3 anode for rechargeable aqueous sodium-ion batteries. J. Electrochem. Soc. 2011, 158, A1067–A1070. [Google Scholar] [CrossRef]
- Wu, W.; Mohamed, A.; Whitacre, J.F. Microwave synthesized NaTi2(PO4)3 anode materials for rechargeable aqueous electrolyte sodium-ion battery. In Meeting Abstracts; Electrochemical Society (ECS): Pennington, NJ, USA, 2012; Volume 15, p. 1859. [Google Scholar]
- Li, Z.; Young, D.; Xiang, K.; Carter, W.C.; Chiang, Y.M. Towards high power high energy aqueous sodium ion batteries: The NaTi2(PO4)3/Na0.44MnO2 System. Adv. Energy Mater. 2013, 3, 290–294. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, Q.; Zhou, X.; Lee, S.; Xia, Y.; Liu, Z. New-concept batteries based on aqueous Li+/Na+ mixed-ion electrolytes. Sci. Rep. 2013, 3, 1946. [Google Scholar] [CrossRef] [PubMed]
- Wessells, C.D.; Peddada, S.V.; McDowell, M.T.; Huggins, R.A.; Cui, Y. The effect of insertion species on nanostructured open framework hexacyanoferrate battery electrodes. J. Electrochem. Soc. 2012, 159, A98. [Google Scholar] [CrossRef]
- Minakshi, M.; Singh, P.; Thurgate, S.; Prince, K. Electrochemical behavior of olivine-type LiMnPO4 in aqueous solutions. Electrochem. Solid-State Lett. 2006, 9, A471–A474. [Google Scholar] [CrossRef]
- Yan, J.; Wang, J.; Liu, H.; Bakenov, Z.; Gosselink, D.; Chen, P. Rechargeable hybrid aqueous batteries. J. Power Sources 2012, 216, 222–226. [Google Scholar] [CrossRef]
- USABC 12V Start-Stop Battery Goals. Available online: http://www.uscar.org/guest/article_view.php?articles_id=85 (accessed on 27 September 2017).
- Hoang, T.K.A.; Doan, T.N.L.; Cho, J.H.; Ying, J.; Su, J.; Lee, C.; Lu, C.; Chen, P. Sustainable gel electrolyte containing pyrazole as corrosion Inhibitor and dendrite suppressor for aqueous Zn/LiMn2O4 battery. ChemSusChem 2017, 10, 2816–2822. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.K.A.; Doan, T.N.L.; Lu, C.; Ghaznavi, M.; Zhao, H.; Chen, P. Performance of thixotropic gel electrolytes in the rechargeable aqueous Zn/LiMn2O4 battery. ACS Sustain. Chem. Eng. 2017, 5, 1804–1811. [Google Scholar] [CrossRef]
- Sun, K.E.K.; Hoang, T.K.A.; Doan, T.N.L.; Yu, Y.; Zhu, X.; Tian, Y.; Chen, P. Suppression of dendrite formation and corrosion on zinc anode of secondary aqueous batteries. ACS Appl. Mater. Interfaces 2017, 9, 9681–9687. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.K.A.; Doan, T.N.L.; Sun, K.E.K.; Chen, P. Corrosion chemistry and protection of zinc & zinc alloys by polymer-containing materials for potential use in rechargeable aqueous batteries. RSC Adv. 2015, 5, 41677–41691. [Google Scholar] [CrossRef]
- Wang, G.; Qu, Q.; Wang, B.; Shi, Y.; Tian, S.; Wu, Y.; Holze, R. Electrochemical behavior of LiCoO2 in a saturated aqueous Li2SO4 solution. Electrochim. Acta 2009, 54, 1199–1203. [Google Scholar] [CrossRef]
- Wang, G.; Yang, L.; Qu, Q.; Wang, B.; Wu, Y.; Holze, R. An aqueous rechargeable lithium battery based on doping and intercalation mechanisms. J. Solid State Electrochem. 2010, 14, 865–869. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novak, P. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A. Solid-state redox reactions of LiCoO2 (R3̅m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 1994, 141, 2972–2977. [Google Scholar] [CrossRef]
- Manickam, M.; Singh, P.; Thurgate, S.; Prince, K. Redox behavior and surface characterization of LiFePO4 in lithium hydroxide electrolyte. J. Power Sources 2006, 158, 646–649. [Google Scholar] [CrossRef]
- Qu, Q.; Shi, Y.; Tian, S.; Chen, Y.; Wu, Y.; Holze, R. A new cheap asymmetric aqueous supercapacitor: Activated carbon//NaMnO2. J. Power Sources 2009, 194, 1222–1225. [Google Scholar] [CrossRef]
- Qu, Q.; Li, L.; Tian, S.; Guo, W.; Wu, Y.; Holze, R. A cheap asymmetric supercapacitor with high energy at high power: Activated carbon//K0.27MnO2·0.6H2O. J. Power Sources 2010, 195, 2789–2794. [Google Scholar] [CrossRef]
- Qu, Q.; Fu, L.; Zhan, X.; Samuelis, D.; Maier, J.; Li, L.; Tian, S.; Li, Z.; Wu, Y. Porous LiMn2O4 as cathode material with high power and excellent cycling for aqueous rechargeable lithium batteries. Energy Environ. Sci. 2011, 4, 3985–3990. [Google Scholar] [CrossRef]
- Shaju, K.M.; Bruce, P.G. A stoichiometric nano-LiMn2O4 spinel electrode exhibiting high power and stable cycling. Chem. Mater. 2008, 20, 5557–5562. [Google Scholar] [CrossRef]
- Jiao, F.; Bao, J.; Hill, A.H.; Bruce, P.G. Synthesis of ordered mesoporous Li-Mn-O spinel as a positive electrode for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 9711–9716. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Luo, Z.; Xie, J. Nanostructured LiMn2O4 and their composites as high-performance cathodes for lithium-ion batteries. Prog. Nat. Sci. 2012, 22, 572–584. [Google Scholar] [CrossRef]
- Wang, G.; Qu, Q.; Wang, B.; Shi, Y.; Tian, S.; Wu, Y. An aqueous electrochemical energy storage system based on doping and intercalation: Ppy//LiMn2O4. ChemPhysChem 2008, 9, 2299–2301. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Cho, J. PVP-assisted ZrO2 coating on LiMn2O4 spinel cathode nanoparticles prepared by MnO2 nanowire templates. Electrochem. Commun. 2008, 10, 1478–1481. [Google Scholar] [CrossRef]
- Wang, X.; Nakamura, H.; Yoshio, M. Capacity fading mechanism for oxygen defect spinel as a 4 V cathode material in Li-ion batteries. J. Power Sources 2002, 110, 19–26. [Google Scholar] [CrossRef]
- Hernán, L.; Morales, J.; Sánchez, L.; Castellón, E.R.; Aranda, M.A.G. Synthesis, characterization and comparative study of the electrochemical properties of doped lithium manganese spinels as cathodes for high voltage lithium batteries. J. Mater. Chem. 2002, 12, 734–741. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Pol, V.G.; Gedanken, A.; Aurbach, D. Improving the high-temperature performance of LiMn2O4 spinel electrodes by coating the active mass with MgO via a sonochemical method. Electrochem. Commun. 2003, 5, 940–945. [Google Scholar] [CrossRef]
- Arumugam, D.; Kalaignan, G.P. Synthesis and electrochemical characterizations of nano-La2O3-coated nanostructure LiMn2O4 cathode materials for rechargeable lithium batteries. Mater. Res. Bull. 2010, 45, 1825–1831. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Park, J.S.; Chang, S.K.; Choi, S.; Ryu, J.H.; Song, H.K. The current move of lithium ion batteries towards the next phase. Adv. Energy Mater. 2012, 2, 860–872. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Jia, X.; Wei, F. Advances in production and applications of carbon nanotubes. Top. Curr. Chem. 2017, 375, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Huang, Z.D.; Oh, S.W.; Zhang, B.; Ma, P.C.; Yuen, M.M.F.; Kima, J.K. Carbon nanotube (CNT)-based composites as electrode material for rechargeable Li-ion batteries: A review. Compos. Sci. Technol. 2012, 72, 121–144. [Google Scholar] [CrossRef]
- Journet, C.; Maser, W.K.; Bernier, P.; Loiseau, A.; de la Chapelle, M.L.; Lefrant, S.; Deniard, P.; Lee, R.; Fischer, J.E. Large scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 1997, 388, 756–758. [Google Scholar] [CrossRef]
- Rinzler, A.G.; Liu, J.; Dai, H.; Nikolaev, P.; Huffman, C.B.; Rodríguez-Macías, F.J.; Boul, P.J.; Lu, A.H.; Heymann, D.; Colbert, D.T.; et al. Large-scale purification of single-wall carbon nanotubes: Process, product, and characterization. Appl. Phys. A 1998, 67, 29–37. [Google Scholar] [CrossRef]
- Pavel, N.; Bronikowski, M.J.; Bradley, R.K.; Rohmund, F.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem. Phys. Lett. 1999, 313, 91–97. [Google Scholar] [CrossRef]
- Ren, Z.F. Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science 1998, 282, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, J.Q.; Qian, W.Z.; Zhang, Y.Y.; Wei, F. The road for nanomaterials industry: A review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small 2013, 8, 1237–1265. [Google Scholar] [CrossRef] [PubMed]
- Guler, M.O.; Akbulut, A.; Cetinkaya, T.; Akbulut, H. The effect of MWCNT reinforcing on the electrochemical performance of LiMn2O4/MWCNT nanocomposite cathodes. Int. J. Energy Res. 2014, 38, 509–517. [Google Scholar] [CrossRef]
- Zhao, X.; Hayner, C.M.; Kung, H.H. Self-assembled lithium manganese oxide nanoparticles on carbon nanotube or graphene as high-performance cathode material for lithium-ion batteries. J. Mater. Chem. 2011, 21, 17297–17303. [Google Scholar] [CrossRef]
- Ding, Y.; Li, J.; Zhao, Y.; Guan, L. Direct growth of LiMn2O4 on carbon nanotubes as cathode materials for lithium ion batteries. Mater. Lett. 2012, 68, 197–200. [Google Scholar] [CrossRef]
- Tang, M.; Yuan, A.; Zhao, H.; Xu, J. High-performance LiMn2O4 with enwrapped segmented carbon nanotubes as cathode material for energy storage. J. Power Sources 2013, 235, 5–13. [Google Scholar] [CrossRef]
- Xia, H.; Ragavendran, K.R.; Xie, J.; Lu, L. Ultrafine LiMn2O4/carbon nanotube nanocomposite with excellent rate capability and cycling stability for lithium-ion batteries. J. Power Sources 2012, 212, 28–34. [Google Scholar] [CrossRef]
- Jia, X.; Yan, C.; Chen, Z.; Wang, R.; Zhang, Q.; Guo, L.; Wei, F.; Lu, Y. Direct growth of flexible LiMn2O4/CNT lithium-ion cathodes. Chem. Commun. 2011, 47, 9669–9671. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.B.; Nam, K.W.; Yoon, W.S.; Bak, S.M.; Yang, X.Q.; Cho, B.W.; Kim, K.B. Nano-sized lithium manganese oxide dispersed on carbon nanotubes for energy storage applications. Electrochem. Commun. 2009, 11, 1575–1578. [Google Scholar] [CrossRef]
- Hong, H.P.; Kim, M.S.; Lee, Y.H.; Yu, J.S.; Lee, C.J.; Min, N.K. Spray deposition of LiMn2O4 nanoparticle-decorated multiwalled carbon nanotube films as cathode material for lithium-ion batteries. Thin Sol. Films 2013, 547, 68–71. [Google Scholar] [CrossRef]
- Chen, S.; Mi, C.; Su, L.; Gao, B.; Fu, Q.; Zhang, X. Improved performances of mechanical-activated LiMn2O4/MWNTs cathode for aqueous rechargeable lithium batteries. J. Appl. Electrochem. 2009, 39, 1943–1948. [Google Scholar] [CrossRef]
- Sun, K.; Juarez, D.A.; Huang, H.; Jung, E.; Dillon, S.J. Aqueous lithium ion batteries on paper substrates. J. Power Sources 2014, 248, 582–587. [Google Scholar] [CrossRef]
- Zhu, X.; Doan, T.N.L.; Yu, Y.; Tian, Y.; Sun, K.E.K.; Zhao, H.; Chen, P. Enhancing rate performance of LiMn2O4 cathode in rechargeable hybrid aqueous battery by hierarchical carbon nanotube/acetylene black conductive pathways. Ionics 2016, 22, 71–76. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, X.; Doan, T.N.L.; Tian, Y.; Zhao, H.; Chen, P. Binder-free flexible LiMn2O4/carbon nanotube network as high power cathode for rechargeable hybrid aqueous battery. J. Power Sources 2016, 326, 498–504. [Google Scholar] [CrossRef]
- Cheng, Q.; Song, Z.; Ma, T.; Smith, B.B.; Tang, R.; Yu, H.; Jiang, H.; Chan, C.K. Folding paper-based lithium-ion batteries for higher areal energy densities. Nano Lett. 2013, 13, 4969–4974. [Google Scholar] [CrossRef] [PubMed]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, H.; Choi, J.; Lee, H.; Kang, T.; Kim, B.; Kim, S. Temperature dependent structural changes of graphene layers on 6H-SiC (0001) surfaces. Condens. Matter Phys. 2008, 20, 225017. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.; Stormer, H.; Zeitler, U.; Maan, J.; Boebinger, G.; Kim, P.; Geim, A. Room-temperature quantum Hall effect in graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; Xu, R.; Luu, N.S.; Secor, E.B.; Hamamoto, K.; Li, Q.; Kim, S.; Sangwan, V.K.; Balla, I.; Guiney, L.M.; et al. Comprehensive enhancement of nanostructured lithium-ion battery cathode materials via conformal graphene dispersion. Nano Lett. 2017, 17, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Jo, G.; Choe, M.; Lee, S.; Park, W.; Kahng, Y.H.; Lee, T. The application of graphene as electrodes in electrical and optical devices. Nanotechnology 2012, 23, 112001. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.; Jiang, D.; Schedin, F.; Booth, T.; Khotkevich, V.; Morozov, S.; Geim, A. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets. Chem. Mater. 2009, 15, 3514–3520. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Ning, G.; Wei, T.; Zhi, L.; Wei, F. Porous graphene networks as high performance anode materials for lithium ion batteries. Carbon 2013, 60, 558–561. [Google Scholar] [CrossRef]
- Ning, G.; Fan, Z.; Wang, G.; Gao, J.; Qian, W.; Wei, F. Gram-scale synthesis of nanomesh graphene with high surface area and its application in supercapacitor electrodes. Chem. Commun. 2011, 47, 5976. [Google Scholar] [CrossRef] [PubMed]
- Iezhokin, I.; Offermans, P.; Brongersma, S.; Giesbers, A.; Flipse, C. High sensitive quasi freestanding epitaxial graphene gas sensor on 6H-SiC. Appl. Phys. Lett. 2013, 103, 053514. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Hu, Z.; Wang, X. Cutting and unzipping multiwalled carbon nanotubes into curved graphene nanosheets and their enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 2012, 4, 6827–6834. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Choucair, P.T.; John, A. Stride. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 2009, 4, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Mueller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Kima, H.; Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Earnshaw, A.; Harrington, T.J. The Chemistry of the Transition Elements; Oxford University Press: Oxford, UK, 1972. [Google Scholar]
- McLellan, R.B. The solubility of carbon in solid gold, copper, and silver. Scr. Metal. 1969, 3, 389–391. [Google Scholar] [CrossRef]
- López, G.A.; Mittemeijer, E.J. The solubility of C in solid Cu. Scr. Mater. 2004, 51, 1–5. [Google Scholar] [CrossRef]
- Oshima, C.; Nagashima, A. Ultra-thin epitaxial films of graphite and hexagonal boron nitride on solid surfaces. J. Phys. Condens. Matter 1997, 9, 1–20. [Google Scholar] [CrossRef]
- Baker, H. ASM International Materials, Alloy Phase Diagrams. In ASM Handbook; ASM International Materials: Geauga County, OH, USA, 1992. [Google Scholar]
- Sutter, P.; Hybertsen, M.S.; Sadowski, J.T.; Sutter, E. Electronic structure of rew-layer epitaxial graphene on Ru(0001). Nano Lett. 2009, 9, 2654. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Sharma, N.; Rao, G.; Chowdari, B. Li-storage and cyclability of urea combustion derived ZnFe2O4 as anode for Li-ion batteries. Electrochim. Acta 2008, 53, 2380–2385. [Google Scholar] [CrossRef]
- Fu, Y.; Wan, Y.; Xia, H.; Wang, X. Nickel ferrite–graphene heteroarchitectures: Toward high-performance anode materials for lithium-ion batteries. J. Power Sources 2012, 213, 338–342. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.S.; Chen, J.J.; Zhang, C. Synthesis and electrochemical performance of LiFePO4/graphene composites by solid-state reaction. Mater. Lett. 2012, 71, 54–56. [Google Scholar] [CrossRef]
- Jiang, R.; Cui, C.; Ma, H. Using graphene nanosheets as a conductive additive to enhance the rate performance of spinel LiMn2O4 cathode material. Phys. Chem. Chem. Phys. 2013, 15, 6406–6415. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, B.; Wang, Y.; Zheng, L.; Duan, X.; Wang, L.; Yang, J.; Qian, Y. Improved electrochemical performance of LiMn2O4/graphene composite as cathode material for lithium ion battery. Int. J. Electrochem. Sci. 2012, 7, 10627–10632. [Google Scholar]
- Jo, K.Y.; Han, S.Y.; Lee, J.M.; Kim, I.Y.; Nahm, S.; Choi, J.W.; Hwang, S.J. Remarkable enhancement of the electrode performance of nanocrystalline LiMn2O4 via solvothermally-assisted immobilization on reduced graphene oxide nanosheets. Electrochim. Acta 2013, 92, 188–196. [Google Scholar] [CrossRef]
- Bak, S.M.; Nam, K.W.; Lee, C.W.; Kim, K.H.; Jung, H.C.; Yang, X.Q.; Kim, K.B. Spinel LiMn2O4/reduced graphene oxide hybrid for high rate lithium ion batteries. J. Mater. Chem. 2011, 21, 17309–17315. [Google Scholar] [CrossRef]
- Yu, G.; Hu, L.; Vosgueritchian, M.; Wang, H.; Xie, X.; McDonough, J.R.; Cui, X.; Cui, Y.; Bao, Z. Solution-processed graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yan, J.; Wei, T.; Zhi, L.; Ning, G.; Li, T.; Wei, F. Asymmetric supercapacitors based on graphene/MnO2 and activated carbon nanofiber electrodes with high power and energy density. Adv. Funct. Mater. 2011, 21, 2366–2375. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Wang, D.W.; Li, F.; Liu, B.; Cheng, H.M. High-energy MnO2 nanowire/graphene and graphene asymmetric electrochemical capacitors. ACS Nano 2010, 4, 5835–5842. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. Nanocarbon-Containing High Power Cathode for Rechargeable Hybrid Aqueous Battery; UWSpace: Waterloo, ON, Canada, 2017. [Google Scholar]



| Type | Ni-Cd Battery | Ni-MH Battery | Aqueous Alkali-Metal Ion Battery | Lead-Acid Battery | Zn/LiMn2O4 Battery | |
|---|---|---|---|---|---|---|
| Index | ||||||
| Working Votage (V) | 1.2 | 1.2 | 1.5 | 1.8–2.0 | 1.8 | |
| Energy Density (Wh·kg−1) | 30–40 | 70–80 | 20–60 | 30–50 | 50–80 | |
| Power Density (W·kg−1) | 60–90 | 250–1000 | - | 250–600 | 500–800 | |
| Cycle Life | <550 | 500–800 | >1000 | 300–500 | 1000–4000 | |
| Cost ($·kW·h−1) | 300–600 | 350–650 | - | 30–50 | 30–50 | |
| Cathode | Specific Capacity at 4 C (mAh·g−1) | Specific Capacity at 10 C (mAh·g−1) | Cycling Performance | Reference |
|---|---|---|---|---|
| LiMn2O4/MWNTs | 110 | 90 | 1000 (93%) | [66] |
| LiMn2O4/CNT | 136 | 130 | 2000 (72%) | [61] |
| 3.3 wt % CNT/AB/LiMn2O4 | 125 | 105 | 300 (70%) | [68] |
| Binder-free flexible LiMn2O4/CNT | 120 | 100 | 300 (80%) | [69] |
| LiMn2O4-RGO (5 wt %) | 107 | 105 | 100 (88%) | [97] |
| 3.3 wt % PG/AB/LiMn2O4 | 110 | 94 | 300 (87%) | [104] |
| Conductive Additive | Specific Surface Area (m2·g−1) | Conducting Mode | Price (US: $·kg−1) |
|---|---|---|---|
| AB | 50–70 | Point-to-point | 30 |
| CNTs | 200–300 | Point-to-line | 100 |
| PG | 1100–1200 | Point-to-plane | 3000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Hoang, T.K.A.; Chen, P. Novel Carbon Materials in the Cathode Formulation for High Rate Rechargeable Hybrid Aqueous Batteries. Energies 2017, 10, 1844. https://doi.org/10.3390/en10111844
Zhu X, Hoang TKA, Chen P. Novel Carbon Materials in the Cathode Formulation for High Rate Rechargeable Hybrid Aqueous Batteries. Energies. 2017; 10(11):1844. https://doi.org/10.3390/en10111844
Chicago/Turabian StyleZhu, Xiao, Tuan K. A. Hoang, and Pu Chen. 2017. "Novel Carbon Materials in the Cathode Formulation for High Rate Rechargeable Hybrid Aqueous Batteries" Energies 10, no. 11: 1844. https://doi.org/10.3390/en10111844
APA StyleZhu, X., Hoang, T. K. A., & Chen, P. (2017). Novel Carbon Materials in the Cathode Formulation for High Rate Rechargeable Hybrid Aqueous Batteries. Energies, 10(11), 1844. https://doi.org/10.3390/en10111844




