Environmentally-Benign Dimethyl Carbonate-Mediated Production of Chemicals and Biofuels from Renewable Bio-Oil
Abstract
:1. Introduction
2. Physico-Chemical Properties of DMC
3. DMC Production Process
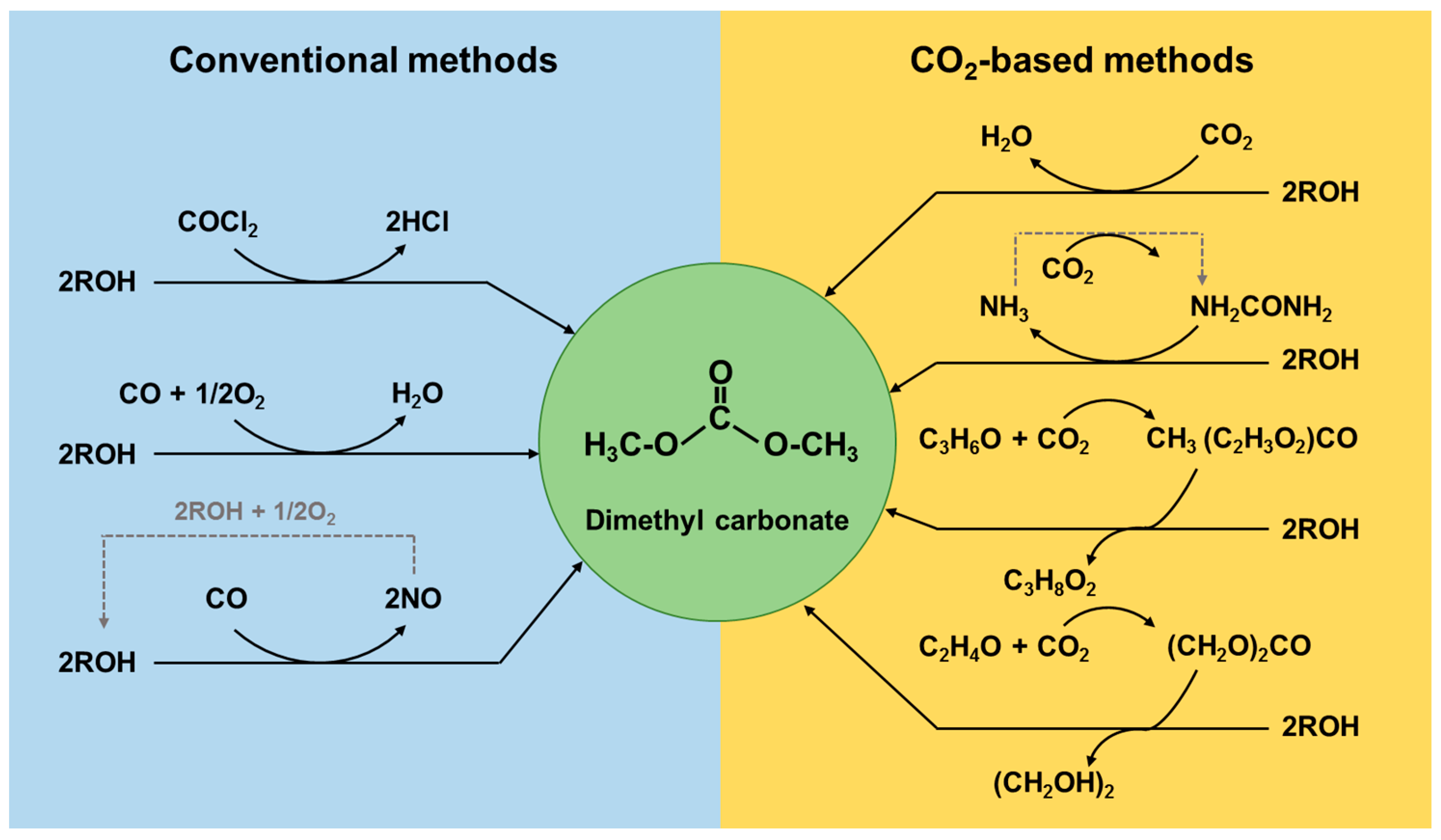
3.1. Conventional Process of DMC Production
3.2. DMC Production from Carbon Dioxide
4. DMC-Mediated Production of Fuels and Chemicals
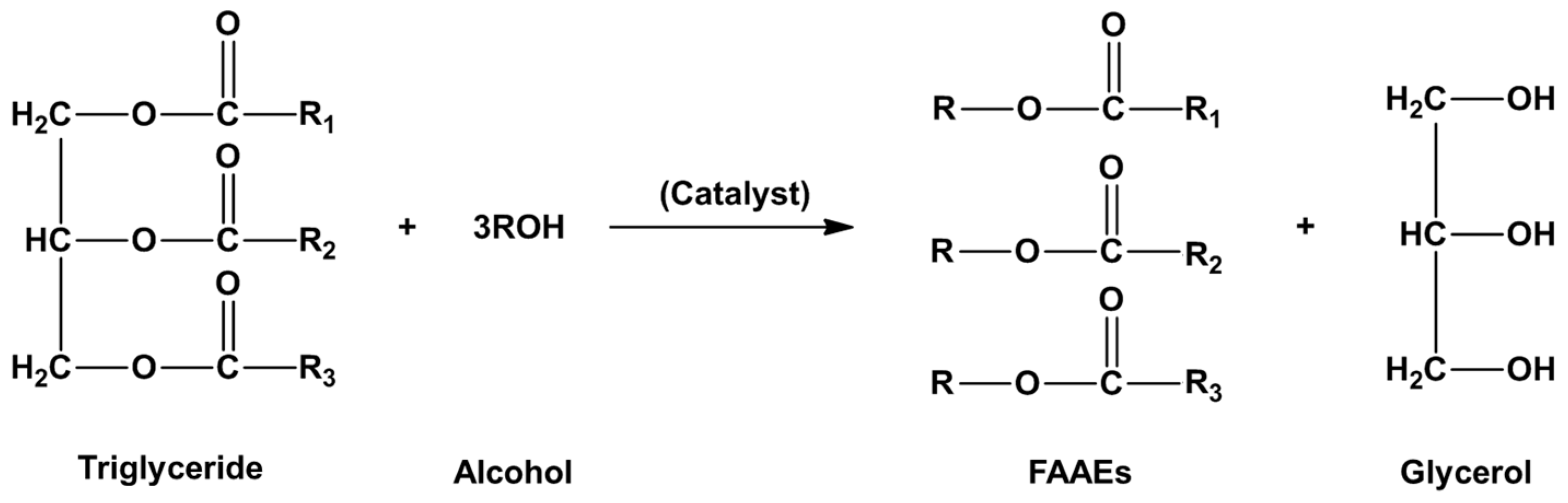
4.1. DMC-Mediated Process for Biodiesel Production
4.1.1. Chemical Process for DMC-Based Biodiesel Production
4.1.2. Enzymatic Process for DMC-Based Biodiesel Production
4.1.3. Fuel Properties of DMC-Based Biodiesel
4.1.4. Techno-Economic and Life Cycle Analysis of Enzymatic Biodiesel Production
4.2. DMC-Mediated Process for Chemicals Production
4.2.1. Chemical Process for DMC-Based Chemicals Production
4.2.2. Enzymatic Process for DMC-Based Chemicals Production
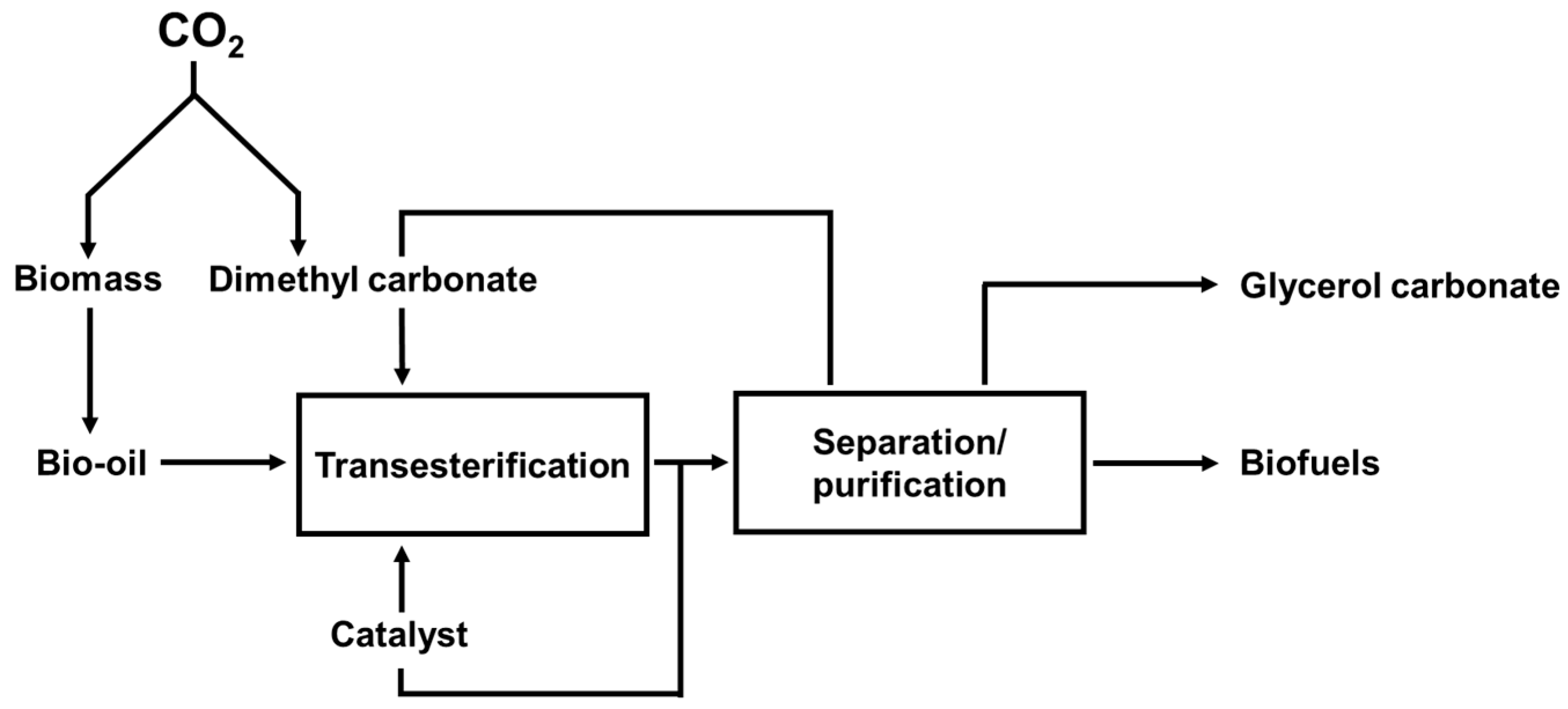
4.3. Assessment of DMC-Mediated Co-Production of Biofuels and Chemicals from Renewable Bio-Oil
5. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Impact of alcohol on biodiesel production and properties. Renew. Sustain. Energy Rev. 2016, 56, 319–333. [Google Scholar] [CrossRef]
- Dawodu, F.A.; Ayodele, O.O.; Xin, J.; Zhang, S. Dimethyl carbonate mediated production of biodiesel at different reaction temperatures. Renew. Energy 2014, 68, 581–587. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Volpato, G.; Wada, K.; Ayub, M.A.Z. Enzymatic synthesis of biodiesel from transesterification reactions of vegetable oils and short chain alcohols. J. Am. Oil Chem. Soc. 2008, 85, 925–930. [Google Scholar] [CrossRef]
- Abigor, R.D.; Uadia, P.O.; Foglia, T.A.; Haas, M.J.; Jones, K.C.; Okpefa, E.; Obliuzor, J.U.; Bafor, M.E. Lipase-catalysed production of biodiesel fuel from some Nigerian lauric oils. Biochem. Soc. Trans. 2000, 28, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Sanli, H.; Canakci, M. Effects of different alcohol and catalyst usage on biodiesel production from different vegetable oils. Energy Fuels 2008, 22, 2713–2719. [Google Scholar] [CrossRef]
- Johnson, D.T.; Taconi, K.A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. Sustain. Energy 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Poirier, M.A.; Chunbao, X. Purification of crude glycerol using acidification: Effects of acid types and product characterization. Austin J. Chem. Eng. 2014, 1, 1–7. [Google Scholar]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Glycerol Market by Source (Biodiesel, Fatty Acids, Fatty Alcohols), by Application (Personal Care, Alkyd Resins, Polyether Polyols), Downstream Opportunities (Propylene Glycol, Epichlorohydrin, 1,3-Propanediol) and Segment Forecasts to 2020. Available online: http://www.grandviewresearch.com/press-release/global-glycerol-market (accessed on 7 August 2017).
- Vivek, N.; Sindhu, R.; Madhavan, A.; Anju, A.J.; Castro, E.; Faraco, V.; Pandey, A.; Binod, P. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate–Metabolic aspects, challenges and possibilities: An overview. Bioresour. Technol. 2017, 239, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.D.; Reddy, H.; Muppaneni, T.; Deng, S. Biodiesel fuel production from algal lipids using supercritical methyl acetate (glycerin-free) technology. Fuel 2017, 195, 201–207. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Berbel, J.; Verdugo-Escamilla, C. An overview on glycerol-free processes for the production of renewable liquid biofuels, applicable in diesel engines. Renew. Sustain. Energy Rev. 2015, 42, 1437–1452. [Google Scholar] [CrossRef]
- Delledonne, D.; Rivetti, F.; Romano, U. Developments in the production and application of dimethylcarbonate. Appl. Catal. A Gen. 2001, 221, 241–251. [Google Scholar] [CrossRef]
- Huang, S.; Yan, B.; Wang, S.; Ma, X. Recent advances in dialkyl carbonates synthesis and applications. Chem. Soc. Rev. 2015, 44, 3079–3116. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.H.; Park, J.H.; Chang, T.S.; Hatti-Kaul, R. Dimethyl carbonate as a Green Chemical. Curr. Opin. Green Sustain. Chem. 2017, 5, 61–66. [Google Scholar] [CrossRef]
- Kongpanna, P.; Pavarajarn, V.; Gani, R.; Assabumrungrat, S. Techno-economic evaluation of different CO2-based processes for dimethyl carbonate production. Chem. Eng. Res. Des. 2015, 93, 496–510. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The chemistry of dimethyl carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y. Catalysis in the production and reactions of dimethyl carbonate, an environmentally benign building block. Appl. Catal. A Gen. 1997, 155, 133–166. [Google Scholar] [CrossRef]
- Aricò, F.; Tundo, P. Dimethyl carbonate: A modern green reagent and solvent. Russ. Chem. Rev. 2010, 79, 479–489. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Fujita, S.I.; Ikushima, Y.; Arai, M. Synthesis of dimethyl carbonate and glycols from carbon dioxide, epoxides, and methanol using heterogeneous basic metal oxide catalysts with high activity and selectivity. Appl. Catal. A Gen. 2001, 219, 259–266. [Google Scholar] [CrossRef]
- Kricsfalussy, Z.; Steude, H.; Waldmann, H.; Hallenberger, K.; Wagner, W.; Traenckner, H.J. Process for Preparing Dimethyl Carbonate. U.S. Patent 5,523,452, 4 June 1996. [Google Scholar]
- Han, M.S.; Lee, B.G.; Suh, I.; Kim, H.S.; Ahn, B.S.; Hong, S.I. Synthesis of dimethyl carbonate by vapor phase oxidative carbonylation of methanol over Cu-based catalysts. J. Mol. Catal. A Chem. 2001, 170, 225–234. [Google Scholar] [CrossRef]
- Itoh, H.; Watanabe, Y.; Mori, K.; Umino, H. Synthesis of dimethyl carbonate by vapor phase oxidative carbonylation of methanol. Green Chem. 2003, 5, 558–562. [Google Scholar] [CrossRef]
- Sato, Y.; Kagotani, M.; Souma, Y. A new type of supportbipyridine containing aromatic polyamide’ to CuCl2 for synthesis of dimethyl carbonate (DMC) by oxidative carbonylation of methanol. J. Mol. Catal. A Chem. 2000, 151, 79–85. [Google Scholar] [CrossRef]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Tsang, S.C.E. Recent advances in CO2 capture and utilization. ChemSusChem 2008, 1, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Han, Y.; Sun, Y. Novel reaction route for dimethyl carbonate synthesis from CO2 and methanol. Fuel Process. Technol. 2000, 62, 187–194. [Google Scholar] [CrossRef]
- Guo, X.C.; Qin, Z.F.; Wang, G.F.; Wang, J.G. Critical temperatures and pressures of reacting mixture in synthesis of dimethyl carbonate with methanol and carbon dioxide. Chin. Chem. Lett. 2008, 19, 249–252. [Google Scholar] [CrossRef]
- Jiang, C.; Guo, Y.; Wang, C.; Hu, C.; Wu, Y.; Wang, E. Synthesis of dimethyl carbonate from methanol and carbon dioxide in the presence of polyoxometalates under mild conditions. Appl. Catal. A Gen. 2003, 256, 203–212. [Google Scholar] [CrossRef]
- Peng, W.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. Recent progress in phosgene-free methods for synthesis of dimethyl carbonate. Pure Appl. Chem. 2011, 84, 603–620. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Yuvaraj, D.; Jayamuthunagai, J.; Kumar, R.P.; Palani, S. Biodiesel production using chemical and biological methods—A review of process, catalyst, acyl acceptor, source and process variables. Renew. Sustain. Energy Rev. 2014, 38, 368–382. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, B.; Xin, Z.; Liu, Q.; Sun, S. Kinetics of transesterification of palm oil and dimethyl carbonate for biodiesel production at the catalysis of heterogeneous base catalyst. Bioresour. Technol. 2010, 101, 8144–8150. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Lee, E.Y. Lipase-catalyzed simultaneous biosynthesis of biodiesel and glycerol carbonate from corn oil in dimethyl carbonate. Biotechnol. Lett. 2011, 33, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.A.; Marshall, C.L. Review of dimethyl carbonate (DMC) manufacture and its characteristics as a fuel additive. Energy Fuels 1997, 11, 2–29. [Google Scholar] [CrossRef]
- Su, E.; You, P.; Wei, D. In situ lipase-catalyzed reactive extraction of oilseeds with short-chained dialkyl carbonates for biodiesel production. Bioresour. Technol. 2009, 100, 5813–5817. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, D.; Bevoni, V.; Notari, M.; Rivetti, F. Properties of a potential biofuel obtained from soybean oil by transmethylation with dimethyl carbonate. Fuel 2007, 86, 690–697. [Google Scholar] [CrossRef]
- Islam, M.R.; Kurle, Y.M.; Gossage, J.L.; Benson, T.J. Kinetics of triazabicyclodecene-catalyzed canola oil conversion to glycerol-free biofuel using dimethyl carbonate. Energy Fuels 2013, 27, 1564–1569. [Google Scholar] [CrossRef]
- Kurle, Y.M.; Islam, M.R.; Benson, T.J. Process development and simulation of glycerol-free biofuel from canola oil and dimethyl carbonate. Fuel Process. Technol. 2013, 114, 49–57. [Google Scholar] [CrossRef]
- Panchal, B.M.; Dhoot, S.B.; Deshmukh, S.A.; Sharma, M.R.; Kachole, M.S. Production of DMC-BioD from Pongamia pinnata seed oil using dimethyl carbonate. Fuel 2013, 109, 201–205. [Google Scholar] [CrossRef]
- Kai, T.; Mak, G.L.; Wada, S.; Nakazato, T.; Takanashi, H.; Uemura, Y. Production of biodiesel fuel from canola oil with dimethyl carbonate using an active sodium methoxide catalyst prepared by crystallization. Bioresour. Technol. 2014, 163, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Tyagi, S.; Newalkar, B.; Badoni, R.P. Jatropha and Karanja oil derived DMC—Biodiesel synthesis: A kinetics study. Fuel 2015, 140, 597–608. [Google Scholar] [CrossRef]
- Schuchardt, U.; Vargas, R.M.; Gelbard, G. Alkylguanidines as catalysts for the transesterification of rapeseed oil. J. Mol. Catal. A Chem. 1995, 99, 65–70. [Google Scholar] [CrossRef]
- Ang, G.T.; Tan, K.T.; Lee, K.T. Recent development and economic analysis of glycerol-free processes via supercritical fluid transesterification for biodiesel production. Renew. Sustain. Energy Rev. 2014, 31, 61–70. [Google Scholar] [CrossRef]
- Su, E.Z.; Zhang, M.J.; Zhang, J.G.; Gao, J.F.; Wei, D.Z. Lipase-catalyzed irreversible transesterification of vegetable oils for fatty acid methyl esters production with dimethyl carbonate as the acyl acceptor. Biochem. Eng. J. 2007, 36, 167–173. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, S.; Xin, Z.; Sheng, B.; Liu, Q. Synthesis and component confirmation of biodiesel from palm oil and dimethyl carbonate catalyzed by immobilized-lipase in solvent-free system. Fuel 2010, 89, 3960–3965. [Google Scholar] [CrossRef]
- Seong, P.J.; Jeon, B.W.; Lee, M.; Cho, D.H.; Kim, D.K.; Jung, K.S.; Kim, S.W.; Han, S.O.; Kim, Y.H.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil and dimethyl carbonate. Enzyme Microb. Technol. 2011, 48, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Gharat, N.; Rathod, V.K. Ultrasound assisted enzyme catalyzed transesterification of waste cooking oil with dimethyl carbonate. Ultrason. Sonochem. 2013, 20, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.K.; Kim, Y.H.; Na, J.G.; Oh, Y.K.; Lee, E.Y. Highly efficient extraction and lipase-catalyzed transesterification of triglycerides from Chlorella sp. KR-1 for production of biodiesel. Bioresour. Technol. 2013, 147, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Lee, O.K.; Lee, E.Y. Dimethyl carbonate-mediated lipid extraction and lipase-catalyzed in situ transesterification for simultaneous preparation of fatty acid methyl esters and glycerol carbonate from Chlorella sp. KR-1 biomass. Bioresour. Technol. 2014, 158, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, O.K.; Kim, C.H.; Seo, J.W.; Oh, B.R.; Lee, E.Y. Lipase-catalyzed in-situ biosynthesis of glycerol-free biodiesel from heterotrophic microalgae, Aurantiochytrium sp. KRS101 biomass. Bioresour. Technol. 2016, 211, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Marx, S. Glycerol-free biodiesel production through transesterification: A review. Fuel Process. Technol. 2016, 151, 139–147. [Google Scholar] [CrossRef]
- Del Pilar Rodriguez, M.; Brzezinski, R.; Faucheux, N.; Heitz, M. Enzymatic transesterification of lipids from microalgae into biodiesel: A review. AIMS Energy 2016, 4, 817–855. [Google Scholar] [CrossRef]
- Go, A.R.; Lee, Y.; Kim, Y.H.; Park, S.; Choi, J.; Lee, J.; Han, O.S.; Kim, S.W.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil in solvent-free system. Enzyme Microb. Technol. 2013, 53, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Demirbas, M.F. Importance of algae oil as a source of biodiesel. Energy Convers. Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Lee, O.K.; Seong, D.H.; Lee, C.G.; Lee, E.Y. Sustainable production of liquid biofuels from renewable microalgae biomass. J. Ind. Eng. Chem. 2015, 29, 24–31. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Estevez, C.; Bayarri, N. Properties of fatty acid glycerol formal ester (FAGE) for use as a component in blends for diesel engines. Biomass Bioenergy 2015, 76, 130–140. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Eng-Seng, C.; Ravindra, P. Economic assessment of biodiesel production: Comparison of alkali and biocatalyst processes. Renew. Sustain. Energy Rev. 2011, 15, 745–751. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- Sotoft, L.F.; Rong, B.G.; Christensen, K.V.; Norddahl, B. Process simulation and economical evaluation of enzymatic biodiesel production plant. Bioresour. Technol. 2010, 101, 5266–5274. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.G.; Dennis, J.S.; Von Blottnitz, H.; Harrison, S.T.L. A life-cycle comparison between inorganic and biological catalysis for the production of biodiesel. J. Clean. Prod. 2008, 16, 1368–1378. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Vanessa, F.W.T.; Ravindra, P. Life cycle assessment of biodiesel production using alkali, soluble and immobilized enzyme catalyst processes. Biomass Bioenergy 2011, 35, 4221–4229. [Google Scholar]
- Selva, M. Green approaches to highly selective processes: Reactions of dimethyl carbonate over both zeolites and base catalysts. Pure Appl. Chem. 2007, 79, 1855–1867. [Google Scholar] [CrossRef]
- Jin, S.; Hunt, A.J.; Clark, J.H.; McElroy, C.R. Acid-catalysed carboxymethylation, methylation and dehydration of alcohols and phenols with dimethyl carbonate under mild conditions. Green Chem. 2016, 18, 5839–5844. [Google Scholar] [CrossRef]
- Deshmukh, K.M.; Qureshi, Z.S.; Dhake, K.P.; Bhanage, B.M. Transesterification of dimethyl carbonate with phenol using Brønsted and Lewis acidic ionic liquids. Catal. Commun. 2010, 12, 207–211. [Google Scholar] [CrossRef]
- Aricò, F.; Evaristo, S.; Tundo, P. Synthesis of five-and six-membered heterocycles by dimethyl carbonate with catalytic amounts of nitrogen bicyclic bases. Green Chem. 2015, 17, 1176–1185. [Google Scholar] [CrossRef]
- Selva, M.; Caretto, A.; Noè, M.; Perosa, A. Carbonate phosphonium salts as catalysts for the transesterification of dialkyl carbonates with diols. The competition between cyclic carbonates and linear dicarbonate products. Org. Biomol. Chem. 2014, 12, 4143–4155. [Google Scholar] [CrossRef] [PubMed]
- Pyo, S.H.; Hatti-Kaul, R. Chlorine-Free Synthesis of Organic Alkyl Carbonates and Five-and Six-Membered Cyclic Carbonates. Adv. Synth. Catal. 2016, 358, 834–839. [Google Scholar] [CrossRef]
- Pokharkar, V.; Sivaram, S. Poly (alkylene carbonate) s by the carbonate interchange reaction of aliphatic diols with dimethyl carbonate: Synthesis and characterization. Polymer 1995, 6, 4851–4854. [Google Scholar] [CrossRef]
- Feng, Y.X.; Yin, N.; Li, Q.F.; Wang, J.W.; Kang, M.Q.; Wang, X.K. Environmentally Benign Route for the Synthesis of Polycarbonate Diols (PCDLs)—Calcined MgAl Hydrotalcites as Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2008, 47, 2140–2145. [Google Scholar] [CrossRef]
- Foy, E.; Farrell, J.B.; Higginbotham, C.L. Synthesis of linear aliphatic polycarbonate macroglycols using dimethylcarbonate. J. Appl. Polym. Sci. 2009, 111, 217–227. [Google Scholar] [CrossRef]
- Zhu, W.; Huang, X.; Li, C.; Xiao, Y.; Zhang, D.; Guan, G. High-molecular-weight aliphatic polycarbonates by melt polycondensation of dimethyl carbonate and aliphatic diols: Synthesis and characterization. Polym. Int. 2011, 60, 1060–1067. [Google Scholar] [CrossRef]
- Park, J.H.; Jeon, J.Y.; Lee, J.J.; Jang, Y.; Varghese, J.K.; Lee, B.Y. Preparation of high-molecular-weight aliphatic polycarbonates by condensation polymerization of diols and dimethyl carbonate. Macromolecules 2013, 46, 3301–3308. [Google Scholar] [CrossRef]
- López-Garzón, C.S.; van der Wielen, L.A.; Straathof, A.J. Green upgrading of succinate using dimethyl carbonate for a better integration with fermentative production. Chem. Eng. J. 2014, 235, 52–60. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Chimentão, R.J.; Figueras, F.; Medina, F. Tunable basic and textural properties of hydrotalcite derived materials for transesterification of glycerol. Appl. Clay Sci. 2012, 58, 16–24. [Google Scholar] [CrossRef]
- Ochoa-Gómez, J.R.; Gómez-Jiménez-Aberasturi, O.; Maestro-Madurga, B.; Pesquera-Rodríguez, A.; Ramírez-López, C.; Lorenzo-Ibarreta, L.; Torrecilla-Soria, J.; Villarán-Velasco, M.C. Synthesis of glycerol carbonate from glycerol and dimethyl carbonate by transesterification: Catalyst screening and reaction optimization. Appl. Catal. A Gen. 2009, 366, 315–324. [Google Scholar] [CrossRef]
- Li, J.; Wang, T. Coupling reaction and azeotropic distillation for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate. Chem. Eng. Process. 2010, 49, 530–535. [Google Scholar] [CrossRef]
- Li, J.; Wang, T. Chemical equilibrium of glycerol carbonate synthesis from glycerol. J. Chem. Thermodyn. 2011, 43, 731–736. [Google Scholar] [CrossRef]
- Algoufi, Y.T.; Hameed, B.H. Synthesis of glycerol carbonate by transesterification of glycerol with dimethyl carbonate over K-zeolite derived from coal fly ash. Fuel Process. Technol. 2014, 126, 5–11. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, C.; Xie, W.; Gross, R.A. Controlled lipase-catalyzed synthesis of poly (hexamethylene carbonate). Macromolecules 2007, 40, 7934–7943. [Google Scholar] [CrossRef]
- Tasaki, H.; Toshima, K.; Matsumura, S. Enzymatic synthesis and polymerization of cyclic trimethylene carbonate monomer with/without methyl substituent. Macromol. Biosci. 2003, 3, 436–441. [Google Scholar] [CrossRef]
- Pyo, S.H.; Persson, P.; Lundmark, S.; Hatti-Kaul, R. Solvent-free lipase-mediated synthesis of six-membered cyclic carbonates from trimethylolpropane and dialkyl carbonates. Green Chem. 2011, 13, 976–982. [Google Scholar] [CrossRef]
- Pyo, S.H.; Hatti-Kaul, R. Selective, Green Synthesis of Six-Membered Cyclic Carbonates by Lipase-Catalyzed Chemospecific Transesterification of Diols with Dimethyl Carbonate. Adv. Synth. Catal. 2012, 354, 797–802. [Google Scholar] [CrossRef]
- Bornadel, A.; Ismail, M.; Sayed, M.; Hatti-Kaul, R.; Pyo, S.H. Six-membered cyclic carbonates from trimethylolpropane: Lipase-mediated synthesis in a flow reactor and in silico evaluation of the reaction. Biotechnol. Prog. 2017, 33, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kim, Y.H.; Lee, H.; Song, B.K. Lipase-catalyzed synthesis of glycerol carbonate from renewable glycerol and dimethyl carbonate through transesterification. J. Mol. Catal. B Enzym. 2007, 49, 75–78. [Google Scholar] [CrossRef]
- Lee, K.H.; Park, C.H.; Lee, E.Y. Biosynthesis of glycerol carbonate from glycerol by lipase in dimethyl carbonate as the solvent. Bioprocess Biosyst. Eng. 2010, 33, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Tudorache, M.; Protesescu, L.; Coman, S.; Parvulescu, V.I. Efficient bio-conversion of glycerol to glycerol carbonate catalyzed by lipase extracted from Aspergillus niger. Green Chem. 2012, 14, 478–482. [Google Scholar] [CrossRef]
- Tudorache, M.; Negoi, A.; Tudora, B.; Parvulescu, V.I. Environmental-friendly strategy for biocatalytic conversion of waste glycerol to glycerol carbonate. Appl. Catal. B 2014, 146, 274–278. [Google Scholar] [CrossRef]
- Zhu, L. Biorefinery as a promising approach to promote microalgae industry: An innovative framework. Renew. Sustain. Energy Rev. 2015, 41, 1376–1384. [Google Scholar] [CrossRef]
- Rafiq, M.; Lv, Y.Z.; Zhou, Y.; Ma, K.B.; Wang, W.; Li, C.R.; Wang, Q. Use of vegetable oils as transformer oils—A review. Renew. Sustain. Energy Rev. 2015, 52, 308–324. [Google Scholar] [CrossRef]

| Properties | DMC | DMS | Phosgene |
|---|---|---|---|
| Oral acute toxicity (rats) | LD50 13.8 g/kg | LD50 0.44 g/kg | – |
| Acute toxicity per contact (cavy) | LD50 > 2.5 g/kg | – | – |
| Acute toxicity per inhalation (rats) | LD50 140 mg/L; (4 h) | LD50 1.5 mg/L; (4 h) | LD50 0.016 mg/L; (75 min) |
| Mutagenic properties | None | Mutagenic | – |
| Irritating properties (rabbits, eyes, skin) | None | – | Corrosive |
| Biodegradability | >90% (28 days) | Rapid hydrolysis | Rapid hydrolysis |
| Feedstock | Catalyst | Catalyst Amount (%) | Molar Ratio (Oil:DMC) | Reaction Conditions | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| Soybean oil | KOCH3 | 6.5 | 1:9 | 200 °C, 10 bar, 1 h | 95.8 | [5] |
| Canola oil | NaOCH3 | 2 | 1:3 | 65 °C, 2 h | >96.0 | [42] |
| Palm oil | KOH | 8.5 | 1:9 | 65–75 °C, 8 h | 96.2 | [34] |
| Pongamia pinnata seed oil | KOH | 4 | 1:3 (w/w) | 90 °C, 6 h | 96.0 | [41] |
| Karanja oil | KOH | 9 | 1:10 | 80 °C, 8 h | >96.0 | [43] |
| Soybean oil | TBD a | 5 | 1:3 | 90 °C, 5 h | >99.5 | [38] |
| Canola oil | TBD a | 1.5 | 1:6 | 60 °C, 2 h | 98.0 | [39] |
| Canola oil | TBD a | 2.5 | 1:3 | 60 °C, 1.013 bar, 6 h | 99.45 | [40] |
| Feedstock | Catalyst | Catalyst Amount (%) | Molar Ratio (Oil:DMC) | Reaction Conditions | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| Corn oil | Novozyme 435 | 10 | 1:10 | 60 °C, 24 h | 94.0 | [35] |
| Cottonseed oil | Novozyme 435 | 10 | 1:4.5 | 50 °C, 24 h | 96.4 | [45] |
| Palm oil | Novozyme 435 | 20 | 1:10 | 55 °C, 24 h | 90.5 | [47] |
| Soybean oil | Novozyme 435 | 100 g/L in t-butanol | 1:6 | 60 °C, 48 h | 84.9 | [48] |
| Soybean oil | Novozyme 435 | 20 | 1:10 | 60 °C, 48 h | 96.4 | [55] |
| Waste cooking oil | Novozyme 435 | 10 | 1:6 | 60 °C, 4 h | 86.6 | [49] |
| Chlorella sp. KR-1 | Novozyme 435 | 20 | 1:10 (w/v) | 70 °C, 24 h | 90.5 | [51] |
| Aurantiochyrium sp. KRS101 | Novozyme 435 | 30 | 1:5 (w/v) | 50 °C, 12 h | 89.5 | [52] |
| Unit | ASTM a | DMC-Based Biodiesel | |||
|---|---|---|---|---|---|
| Reference [38] | Reference [41] | Reference [43] | |||
| Cetane number | 47 | 50.1 | |||
| Kinetic viscosity (40 °C) | mm2/s | 1.9–6.0 | 4.1 | 5.2 | 5.6 |
| Density (25 °C) | kg/m3 | - | 0.88 | 0.89 | 0.885 |
| Flash point | °C | 130 | 160 | 122 | 144 |
| Pour point | °C | −15 to 10 | −3.8 | 3 | |
| Acid number | mg KOH/g | 0.0–0.50 | <0.5 | 0.28 | 0.42 |
| Phosphorus content | mg/kg | 0–10 | 5 | ||
| Product | Reaction (with Reference) | Usage |
|---|---|---|
| Diphenyl carbonate | DMC with phenol [17] DMC with alcohols [66] | Use as a solvent, plasticizer, and chemical intermediate for various organic and polymeric compounds |
| Five- and six-membered carbonates | DMC with diols [70] DMC with trimethylopropane [85] | Use for pharmaceuticals such as antimicrobial, antioxidant, antimitotic antiangiogenic |
| Five- and six-membered heterocycles | DMC with aminoalcohols [68] | Use as a chemical intermediate for engineering thermoplastics, as well as pharmaceutical applications |
| Glycerol carbonate | DMC with glycerol [78] DMC with bio-oil (triglycerides) [54] | Prominent role as the monomer during plastic synthesis Use as ingredients for cosmetics and pharmaceuticals Use as an electrolyte for Li-ion batteries |
| Dimethyl succinate | DMC with succinate by O-alkylation reaction [76] | Use as solvent and polymer additives, as well as in coating and painting applications |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H.; Lee, E.Y. Environmentally-Benign Dimethyl Carbonate-Mediated Production of Chemicals and Biofuels from Renewable Bio-Oil. Energies 2017, 10, 1790. https://doi.org/10.3390/en10111790
Kim KH, Lee EY. Environmentally-Benign Dimethyl Carbonate-Mediated Production of Chemicals and Biofuels from Renewable Bio-Oil. Energies. 2017; 10(11):1790. https://doi.org/10.3390/en10111790
Chicago/Turabian StyleKim, Keon Hee, and Eun Yeol Lee. 2017. "Environmentally-Benign Dimethyl Carbonate-Mediated Production of Chemicals and Biofuels from Renewable Bio-Oil" Energies 10, no. 11: 1790. https://doi.org/10.3390/en10111790
APA StyleKim, K. H., & Lee, E. Y. (2017). Environmentally-Benign Dimethyl Carbonate-Mediated Production of Chemicals and Biofuels from Renewable Bio-Oil. Energies, 10(11), 1790. https://doi.org/10.3390/en10111790





