Bioinspired Platelet-like Nanovector for Enhancing Cancer Therapy via P-Selectin Targeting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
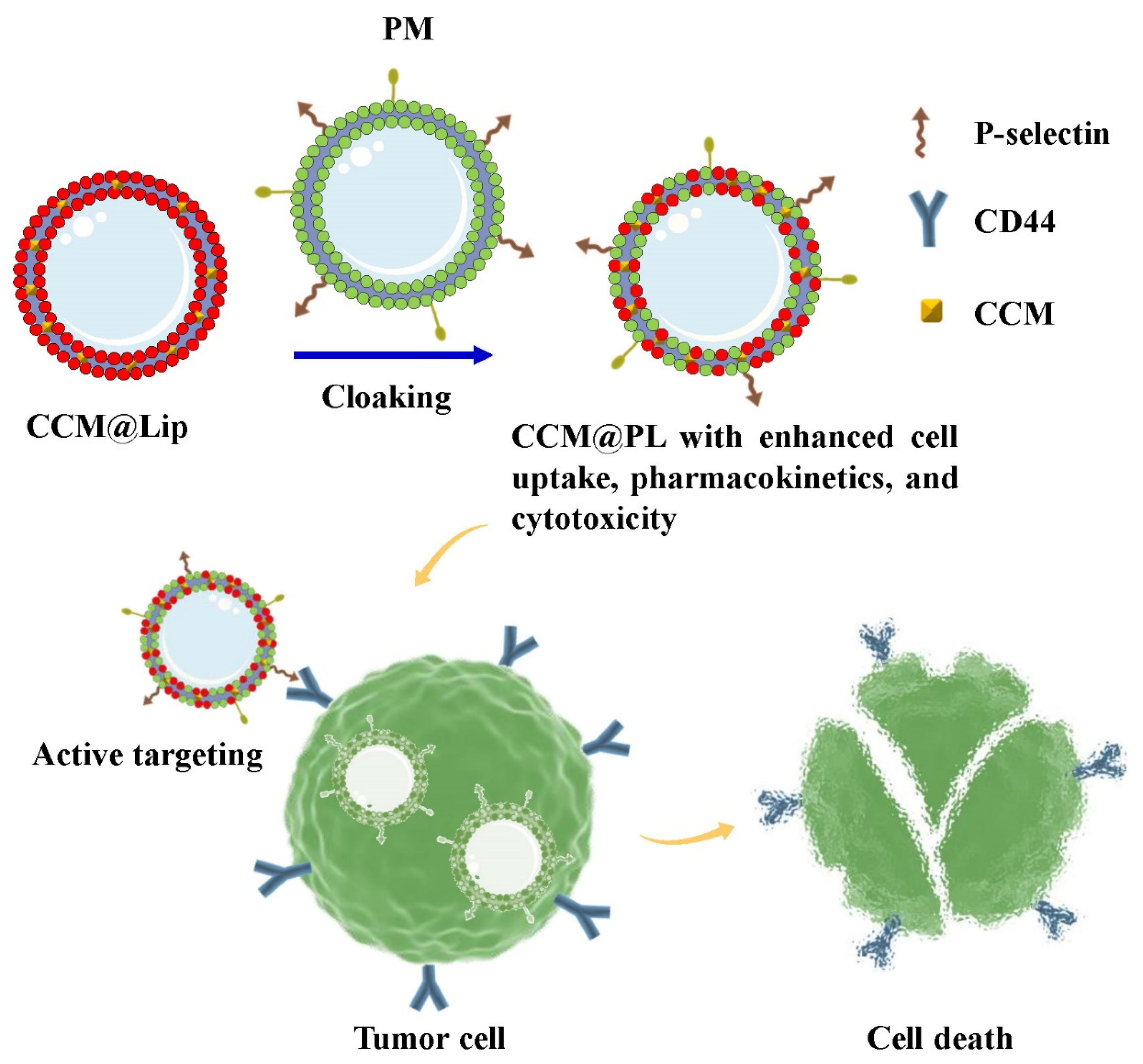
2.2. Preparation of CCM@PL
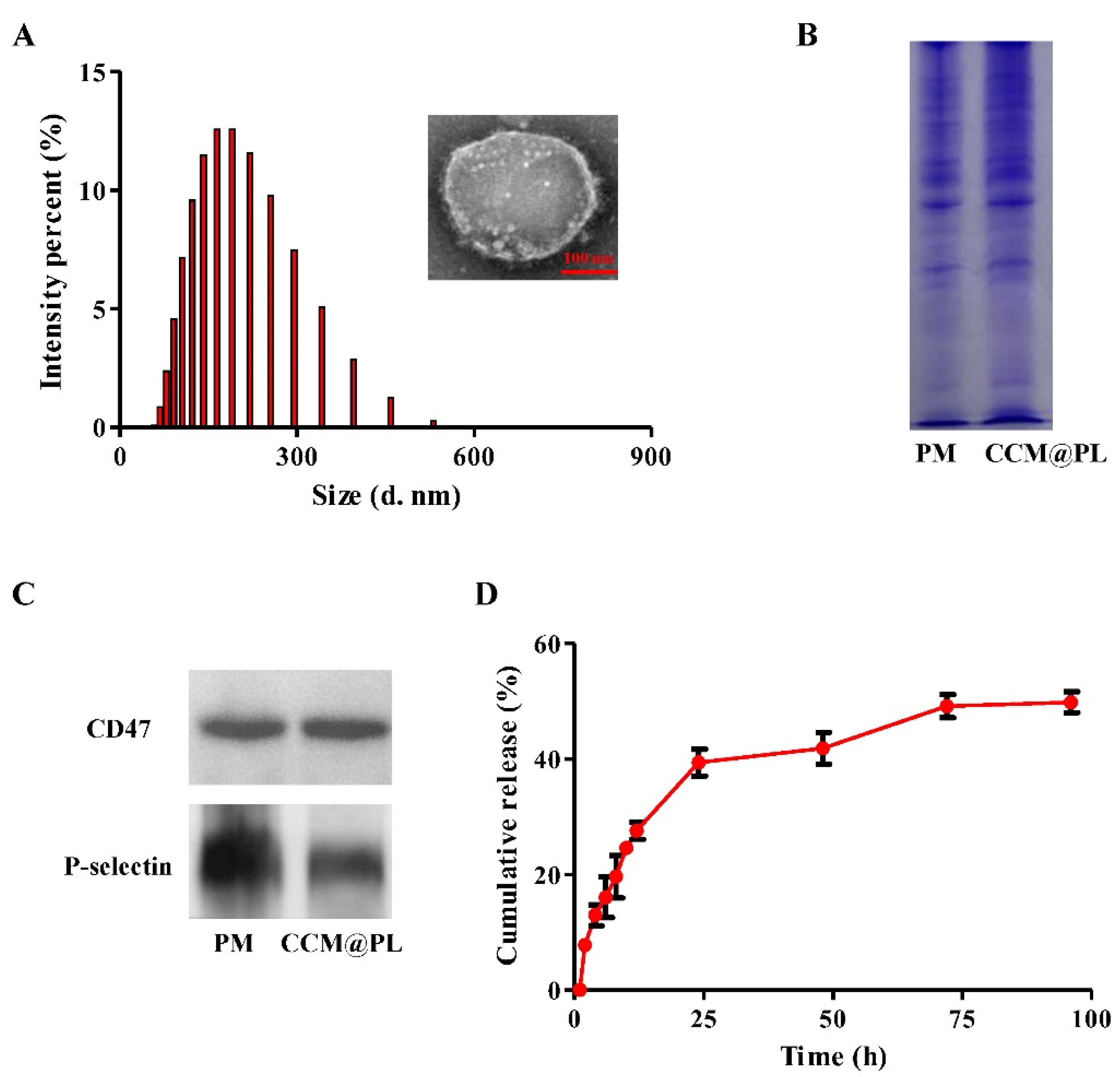
2.3. Protein Environment
2.4. Encapsulation Efficiency
2.5. In Vitro Release
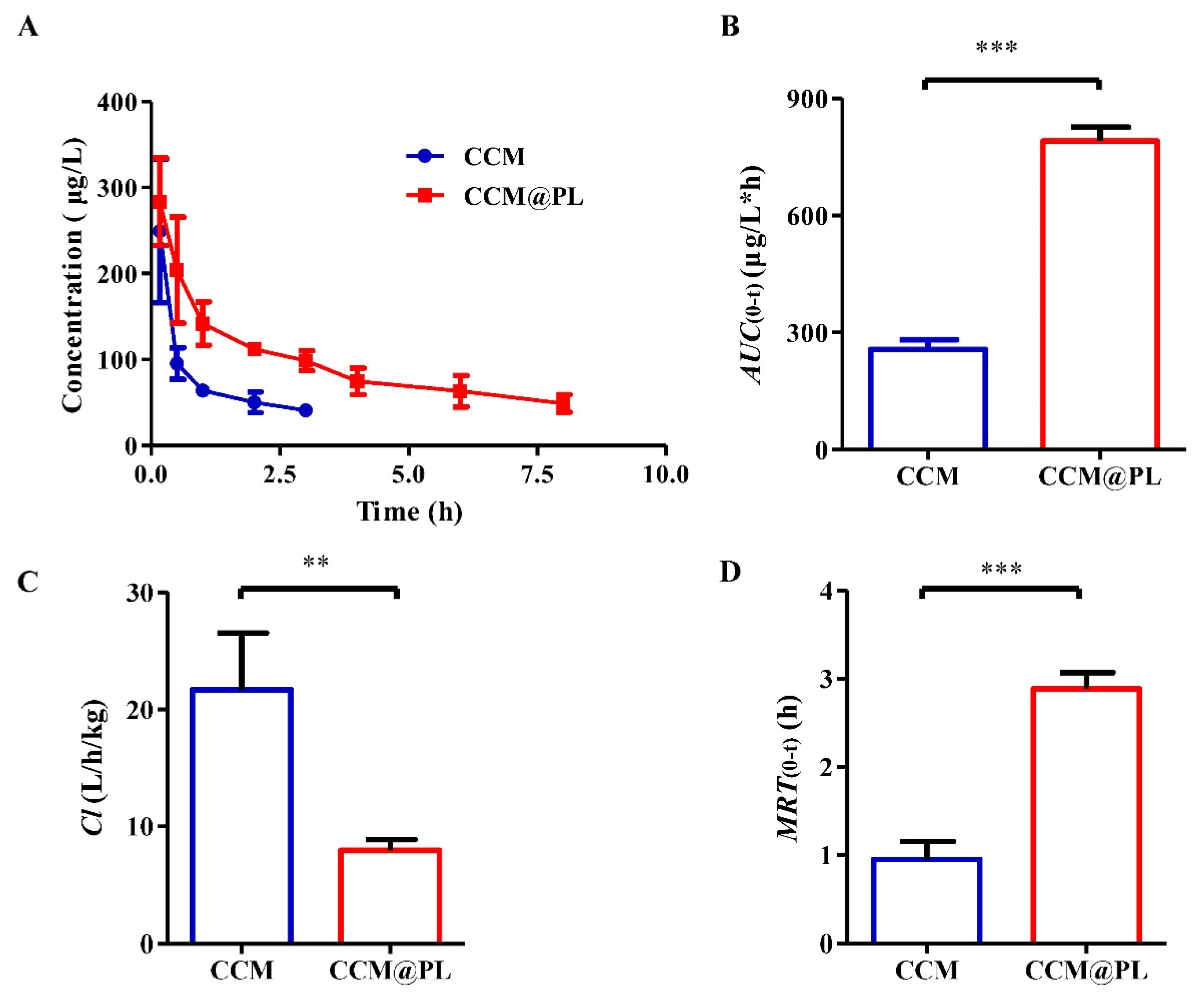
2.6. Pharmacokinetics
2.6.1. Animal Experiment
2.6.2. Sample Preparation
2.6.3. HPLC Analysis
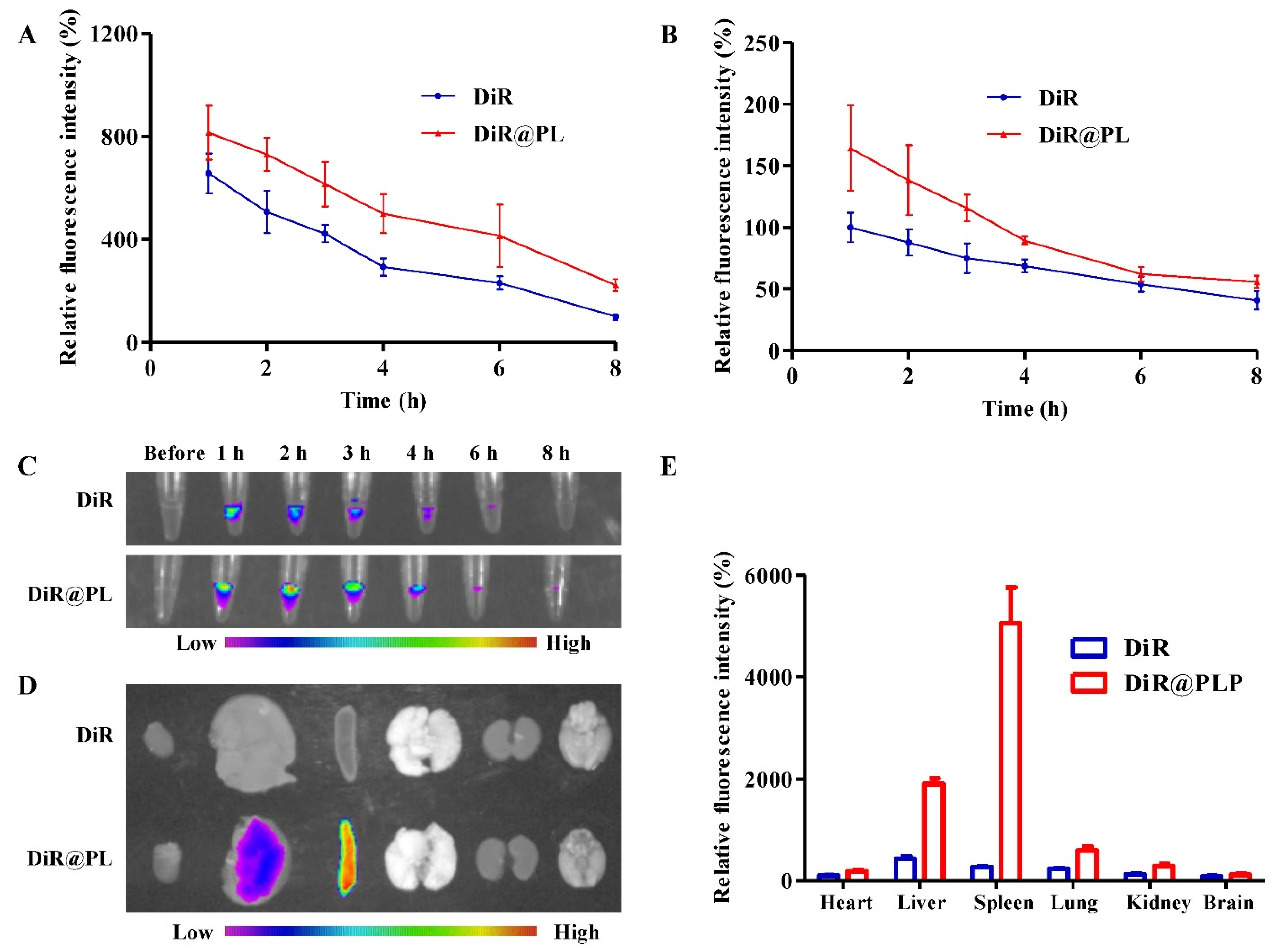
2.7. In Vivo Distribution
2.8. Cytotoxicity
2.9. Cytocompatibility
2.10. Cellular Uptake
2.11. Statistical Analysis
3. Results and Discussion
3.1. Morphology, Physicochemical Properties, and In Vitro Release
3.2. In Vivo Pharmacokinetics and Biodistribution
3.3. Enhanced Cytotoxicity and Cellular Uptake
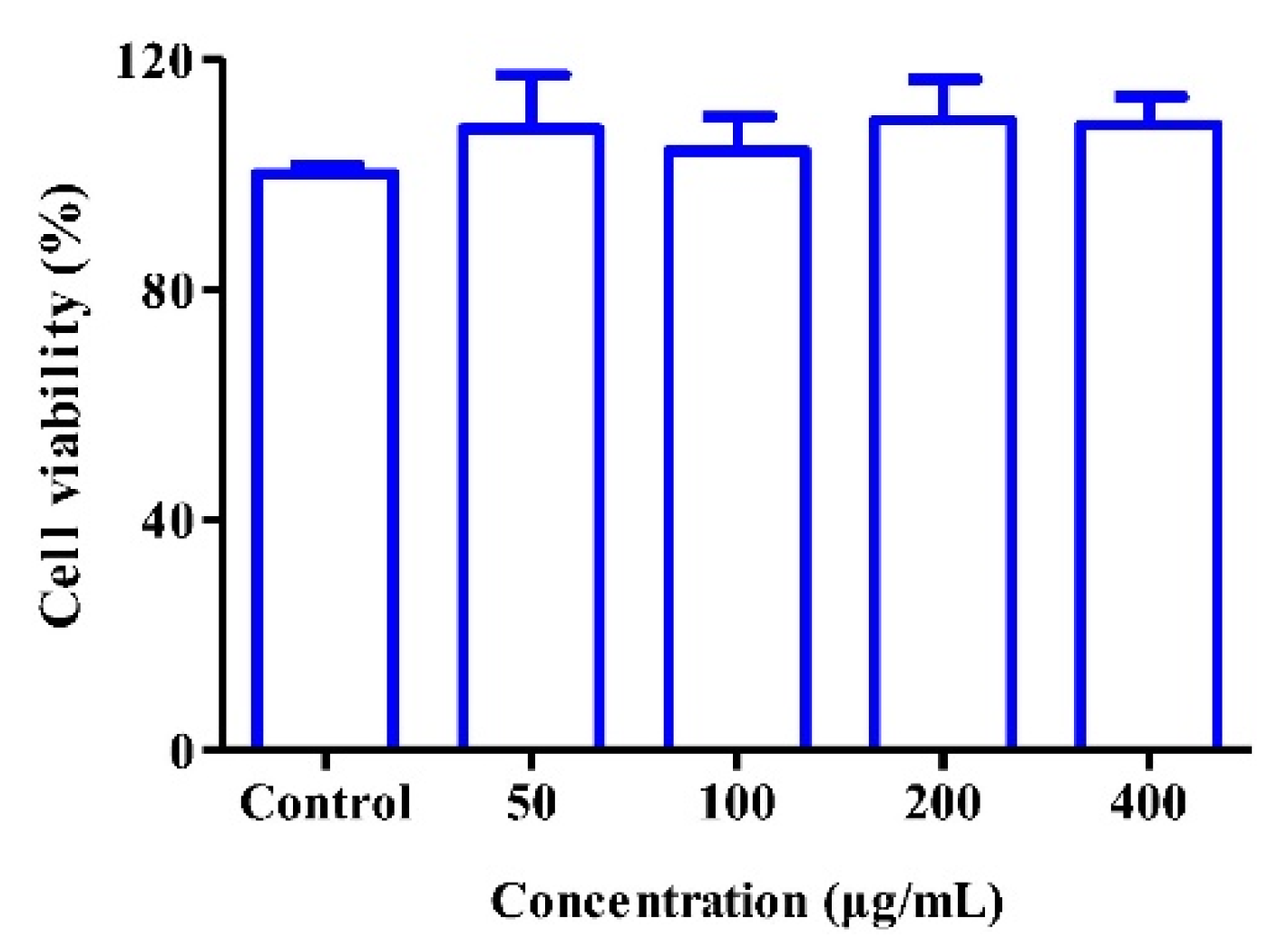
3.4. Cytocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mir, S.A.; Hamid, L.; Bader, G.N.; Shoaib, A.; Rahamathulla, M.; Alshahrani, M.Y.; Alam, P.; Shakeel, F. Role of nanotechnology in overcoming the multidrug resistance in cancer therapy: A review. Molecules 2022, 27, 6608. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; He, D.; Hu, X.; Li, K.; Yang, Q.; Fang, C.; Zhong, C.; Yang, J.; Tan, Q.; et al. Natural oral anticancer medication in small ethanol nanosomes coated with a natural alkaline polysaccharide. ACS Appl Mater. Interfaces 2020, 12, 16159–16167. [Google Scholar] [CrossRef]
- Wu, J.; Williams, G.R.; Niu, S.; Gao, F.; Tang, R.; Zhu, L.M. A multifunctional biodegradable nanocomposite for cancer theranostics. Adv. Sci. 2019, 6, 1802001. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, S.; Wu, X.; Zhang, J.; Chen, R.; Chen, M.; Wang, Y. Chinese herbal medicine-derived compounds for cancer therapy: A focus on hepatocellular carcinoma. J. Ethnopharmacol. 2013, 149, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, J.; Chen, Q.; Wang, L.; Yang, J.; Wu, A.; Jiang, N.; Liu, Y.; Chen, J.; Zou, W.; et al. A comprehensive review of genus sanguisorba: Traditional uses, chemical constituents and medical applications. Front. Pharm. 2021, 12, 750165. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, A.; Azmi, L.; Pal, S.; Alqahtani, S.S.; Rahamathulla, M.; Hani, U.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Integrating nanotechnology with naturally occurring phytochemicalsin neuropathy induced by diabetes. J. Mol. Liq. 2022, 350, 118189. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, R.; Zhang, Z.; Zhong, C.; Wang, J.; Wang, M. Curcumin-loaded liposomes with the hepatic and lysosomal dual-targeted effects for therapy of hepatocellular carcinoma. Int. J. Pharm 2021, 602, 120628. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Zhao, D.; Fang, C.; He, D.; Yang, Q.; Yang, L.; Chen, R.; Tan, Q.; Zhang, J. Oral administration of natural polyphenol-loaded natural polysaccharide-cloaked lipidic nanocarriers to improve efficacy against small-cell lung cancer. Nanomedicine 2020, 29, 102261. [Google Scholar] [CrossRef]
- Tima, S.; Okonogi, S.; Ampasavate, C.; Berkland, C.; Anuchapreeda, S. FLT3-specific curcumin micelles enhance activity of curcumin on FLT3-ITD overexpressing MV4-11 leukemic cells. Drug Dev. Ind. Pharm. 2019, 45, 498–505. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Fathi, M.; Aboutorabzade, S.M.; Sabbagh, F. Development of curcumin-loaded prunus armeniaca gum nanoparticles: Synthesis, characterization, control release behavior, and evaluation of anticancer and antimicrobial properties. Food Sci. Nutr. 2021, 9, 6109–6119. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Guo, F.; Yu, N.; Ying, S.; Lou, B.; Wu, J.; Gao, Y.; Ji, X.; Wang, H.; Li, A.; et al. A novel folic acid receptor-targeted drug delivery system based on curcumin-loaded β-cyclodextrin nanoparticles for cancer treatment. Drug Des. Dev. Ther. 2021, 15, 2843–2855. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Mansoor, S.; Rafi, Z.; Kumari, B.; Shoaib, A.; Saeed, M.; Alshehri, S.; Ghoneim, M.M.; Rahamathulla, M.; Hani, U.; et al. A review on nanotechnology: Properties, applications, and mechanistic insights of cellular uptake mechanisms. J. Mol. Liq. 2022, 348, 118008. [Google Scholar] [CrossRef]
- Alanazi, S.A.; Alanazi, F.; Haq, N.; Shakeel, F.; Badran, M.M.; Harisa, G.I. Lipoproteins-nanocarriers as a promising approach for targeting liver cancer: Present status and application prospects. Curr. Drug Deliv. 2020, 17, 826–844. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.A.; Harisa, G.I.; Badran, M.M.; Haq, N.; Radwan, A.A.; Kumar, A.; Shakeel, F.; Alanazi, F.K. Cholesterol-conjugate as a new strategy to improve the cytotoxic effect of 5-fluorouracil on liver cancer: Impact of liposomal composition. Curr. Drug Deliv. 2020, 17, 898–910. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Warsi, M.H.; Jain, G.K.; Ahmad, F.J. A combinatorial statistical design approach to optimize the nanostructured cubosomal carrier system for oral delivery of ubidecarenone for management of doxorubicin-induced cardiotoxicity: In vitro-in vivo investigations. J. Pharm. Sci. 2017, 106, 3050–3065. [Google Scholar] [CrossRef]
- Al-Joufi, F.A.; Salem-Bekhit, M.M.; Taha, E.I.; Ibrahim, M.A.; Muharram, M.M.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Enhancing ocular bioavailability of ciprofloxacin using colloidal lipid-based carrier for the management of post-surgical infection. Molecules 2022, 27, 733. [Google Scholar] [CrossRef]
- Alanazi, F.K.; Lu, D.R.; Shakeel, F.; Haq, N. Density gradient separation of carborane-containing liposome from low density lipoprotein and detection by inductively coupled plasma spectrometry. J. Liposome Res. 2014, 24, 53–58. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, X.; Zhang, Y.; Xu, J.; Xu, J.; Li, Y.; Min, H.; Shi, J.; Zhao, Y.; Wei, J.; et al. Engineering biomimetic platesomes for pH-responsive drug delivery and enhanced antitumor activity. Adv. Mater. 2019, 31, e1900795. [Google Scholar] [CrossRef]
- Javed, S.; Alshehri, S.; Shoaib, A.; Ahsan, W.; Sultan, M.H.; Alqahtani, S.S.; Kazi, M.; Shakeel, F. Chronicles of nanoerythrosomes: An erythrocyte-based biomimetic smart drug delivery system as a therapeutic and diagnostic tool in cancer therapy. Pharmaceutics 2021, 13, 368. [Google Scholar] [CrossRef]
- Pei, W.; Huang, B.; Chen, S.; Wang, L.; Xu, Y.; Niu, C. Platelet-mimicking drug delivery nanoparticles for enhanced chemo-photothermal therapy of breast cancer. Int. J. Nanomed. 2020, 15, 10151–10167. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sun, W.; Qian, C.; Wang, C.; Bomba, H.N.; Gu, Z. Anticancer platelet-mimicking nanovehicles. Adv. Mater. 2015, 27, 7043–7050. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, J.; Williams, G.R.; Fan, Q.; Niu, S.; Wu, J.; Xie, X.; Zhu, L.M. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J. Nanobiotechnology 2019, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zhang, Y.; Blum, N.T.; Lin, J.; Huang, P. Biomimetic hybrid membrane-based nanoplatforms: Synthesis, properties and biomedical applications. Nanoscale Horiz. 2020, 5, 1293–1302. [Google Scholar] [CrossRef]
- Bang, K.H.; Na, Y.G.; Huh, H.W.; Hwang, S.J.; Kim, M.S.; Kim, M.; Lee, H.K.; Cho, C.W. The delivery strategy of paclitaxel nanostructured lipid carrier coated with platelet membrane. Cancers 2019, 11, 807. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhao, G.; Wang, S.; He, Y.; Han, S.; Du, C.; Li, S.; Fan, Z.; Wang, C.; Wang, J. Platelet-membrane-camouflaged bismuth sulfide nanorods for synergistic radio-photothermal therapy against cancer. Biomater. Sci. 2019, 7, 3450–3459. [Google Scholar] [CrossRef]
- Luo, J.; Schmaus, J.; Cui, M.; Hörterer, E.; Wilk, U.; Höhn, M.; Däther, M.; Berger, S.; Benli-Hoppe, T.; Peng, L.; et al. Hyaluronate sirna nanoparticles with positive charge display rapid attachment to tumor endothelium and penetration into tumors. J. Control. Release 2021, 329, 919–933. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, Y.J. High paclitaxel-loaded and tumor cell-targeting hyaluronan-coated nanoemulsions. Colloids Surf. B Biointerfaces 2017, 150, 362–372. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Wang, M.; Liu, R.; Song, H.; Li, N.; Lu, Q.; Zhang, W.; Du, Y.; Yang, W.; et al. Bioinspired nanoplatelets for chemo-photothermal therapy of breast cancer metastasis inhibition. Biomaterials 2019, 206, 1–12. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Yang, P.; Liu, Q.; Hu, J.; Yang, W.; Liu, P.; He, F.; Bai, Y.; Gai, S.; et al. GPC3-targeted and curcumin-loaded phospholipid microbubbles for sono-photodynamic therapy in liver cancer cells. Colloids Surf. B Biointerfaces 2021, 197, 111358. [Google Scholar] [CrossRef]
- Pi, C.; Zhao, W.; Zeng, M.; Yuan, J.; Shen, H.; Li, K.; Su, Z.; Liu, Z.; Wen, J.; Song, X.; et al. Anti-lung cancer effect of paclitaxel solid lipid nanoparticles delivery system with curcumin as co-loading partner in vitro and in vivo. Drug Deliv. 2022, 29, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Qiao, Y.; Cao, J.; Ta, L.; Ci, T.; Ke, X. Biomimetic doxorubicin/ginsenoside co-loading nanosystem for chemoimmunotherapy of acute myeloid leukemia. J. Nanobiotechnology 2022, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, R.; Akbari Javar, H.; Khoobi, M.; Dehghan Kelishadi, P.; Hossein Yousefi, G.; Doosti, M.; Hossien Ghahremani, M.; Shariftabrizi, A.; Imanparast, F.; Gholibeglu, E.; et al. Evaluation of a novel biocompatible magnetic nanomedicine based on beta-cyclodextrin, loaded doxorubicin-curcumin for overcoming chemoresistance in breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, J.; Duan, Y.; Zhang, Q.; Gao, W.; Li, S.; Fang, R.H.; Zhang, L. Multimodal enzyme delivery and therapy enabled by cell membrane-coated metal-organic framework nanoparticles. Nano Lett. 2020, 20, 4051–4058. [Google Scholar] [CrossRef]
- Kunde, S.S.; Wairkar, S. Platelet membrane camouflaged nanoparticles: Biomimetic architecture for targeted therapy. Int. J. Pharm. 2021, 598, 120395. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Feng, N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110298. [Google Scholar] [CrossRef]
- Hang, C.; Zou, Y.; Zhong, Y.; Zhong, Z.; Meng, F. NIR and UV-responsive degradable hyaluronic acid nanogels for CD44-targeted and remotely triggered intracellular doxorubicin delivery. Colloids Surf. B Biointerfaces 2017, 158, 547–555. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, S.; Wu, Y.; Fan, Q.; Yang, G.; Hu, H.; Tima, S.; Chiampanichayakul, S.; Anuchapreeda, S.; Wu, J. Bioinspired Platelet-like Nanovector for Enhancing Cancer Therapy via P-Selectin Targeting. Pharmaceutics 2022, 14, 2614. https://doi.org/10.3390/pharmaceutics14122614
Wan S, Wu Y, Fan Q, Yang G, Hu H, Tima S, Chiampanichayakul S, Anuchapreeda S, Wu J. Bioinspired Platelet-like Nanovector for Enhancing Cancer Therapy via P-Selectin Targeting. Pharmaceutics. 2022; 14(12):2614. https://doi.org/10.3390/pharmaceutics14122614
Chicago/Turabian StyleWan, Shengli, Yuesong Wu, Qingze Fan, Gang Yang, Haiyang Hu, Singkome Tima, Sawitree Chiampanichayakul, Songyot Anuchapreeda, and Jianming Wu. 2022. "Bioinspired Platelet-like Nanovector for Enhancing Cancer Therapy via P-Selectin Targeting" Pharmaceutics 14, no. 12: 2614. https://doi.org/10.3390/pharmaceutics14122614
APA StyleWan, S., Wu, Y., Fan, Q., Yang, G., Hu, H., Tima, S., Chiampanichayakul, S., Anuchapreeda, S., & Wu, J. (2022). Bioinspired Platelet-like Nanovector for Enhancing Cancer Therapy via P-Selectin Targeting. Pharmaceutics, 14(12), 2614. https://doi.org/10.3390/pharmaceutics14122614






