A Novel Furocoumarin Derivative, 5-((diethylamino)me-13 thyl)-3-phenyl-7H-furo [3,2-g] chromen-7-one Upregulates Melanin Synthesis via the Activation of cAMP/PKA and MAPKs Signal Pathway: In Vitro and In Vivo Study
Abstract
1. Introduction
2. Results
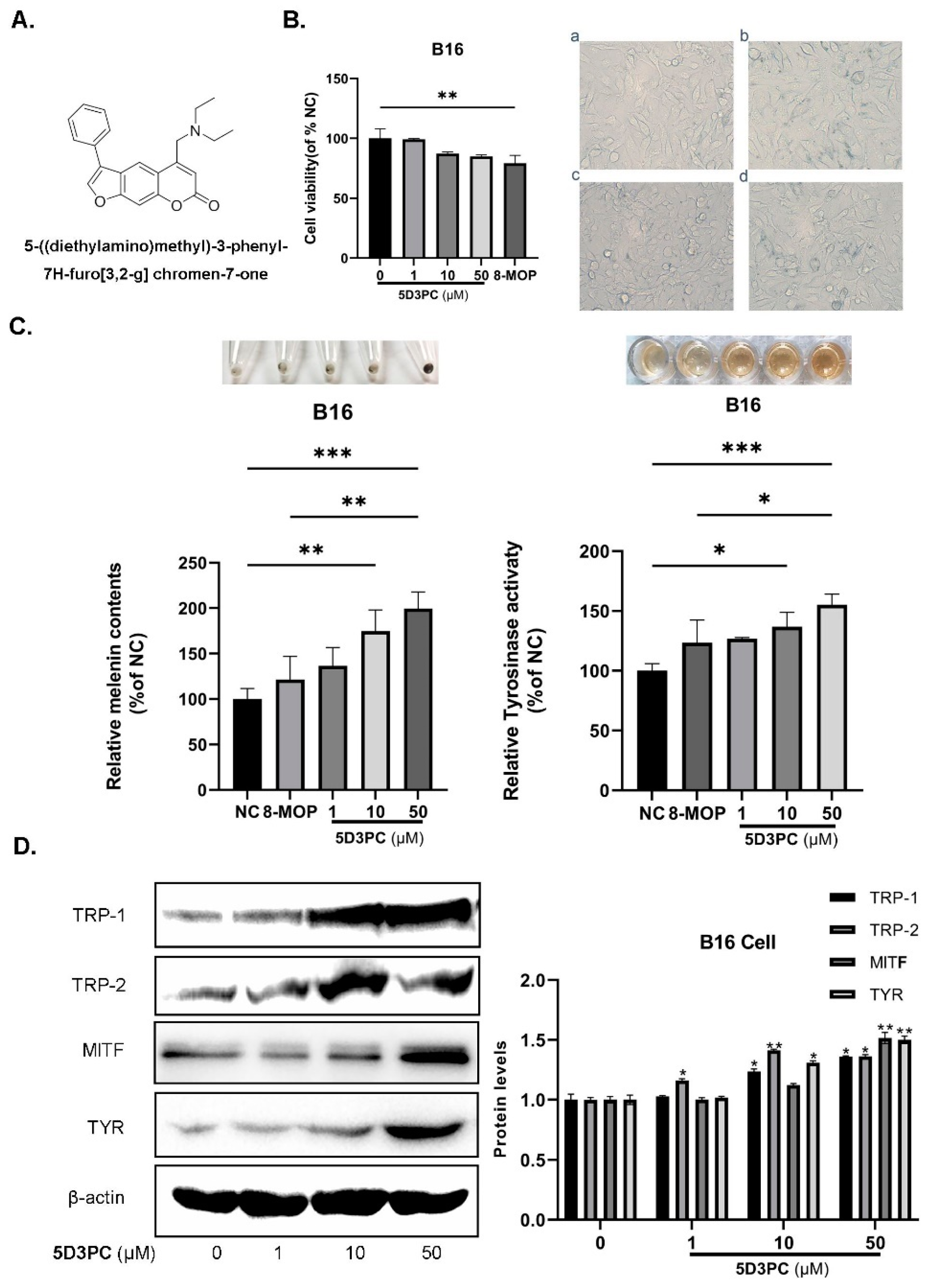
2.1. The Effect of 5D3PC on Cell Viability and the Melanogenic Effect
2.2. 5D3PC Promotes the Expression of MITF and TRPs in B16 and PIG3V Cells
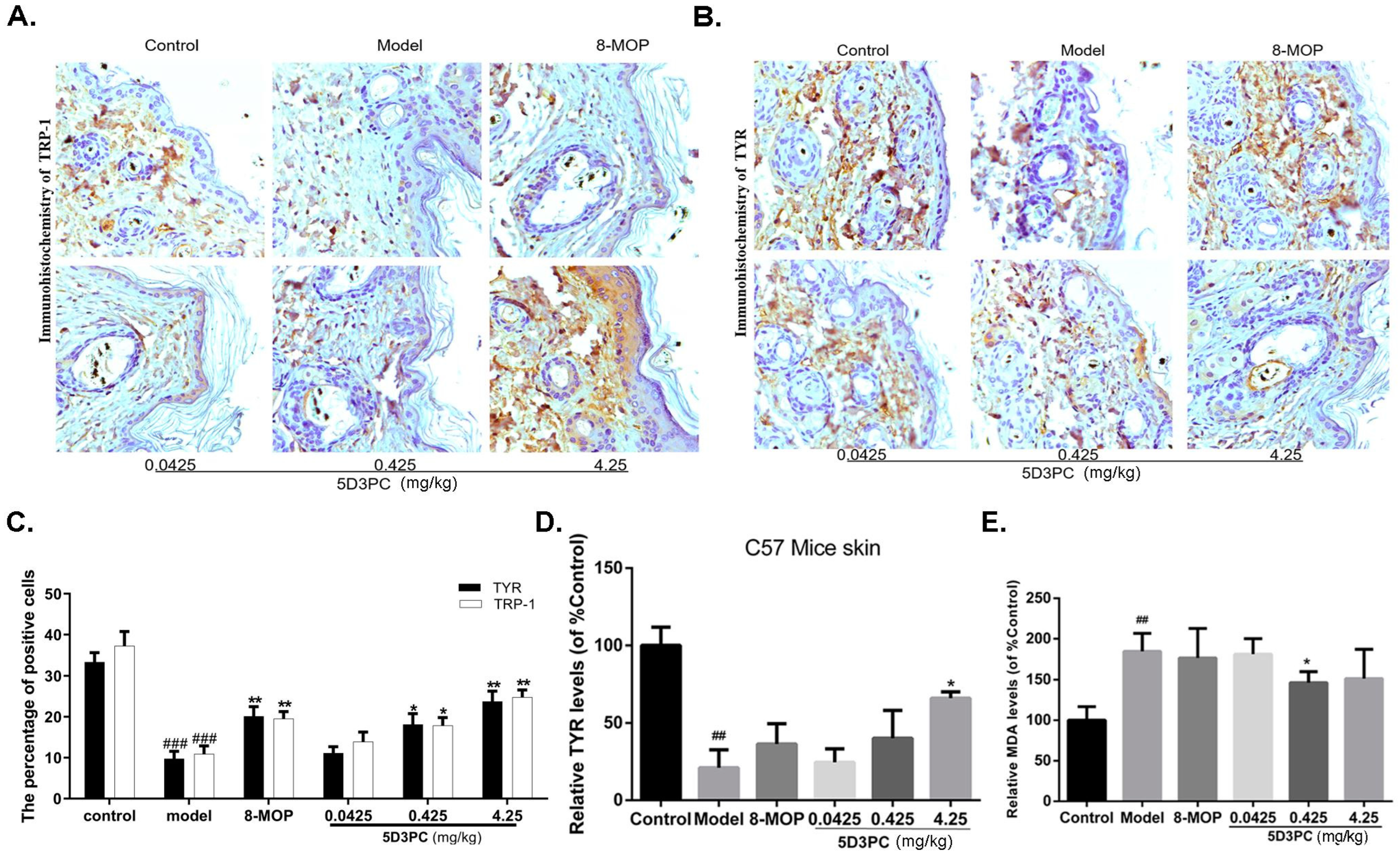
2.3. 5D3PC Ameliorates C57BL/6 Mice Model of Hydroquinone-Induced Vitiligo
2.4. The 5D3PC Administration Increases the Numbers of Melanin-Containing Hair Follicles
2.5. The 5D3PC Treatment Enhances the Expression of TYR and TRP-1 in the Skin of C57BL/6 Mice
2.6. The 5D3PC Administration Reduces the Content of MDA in Serum
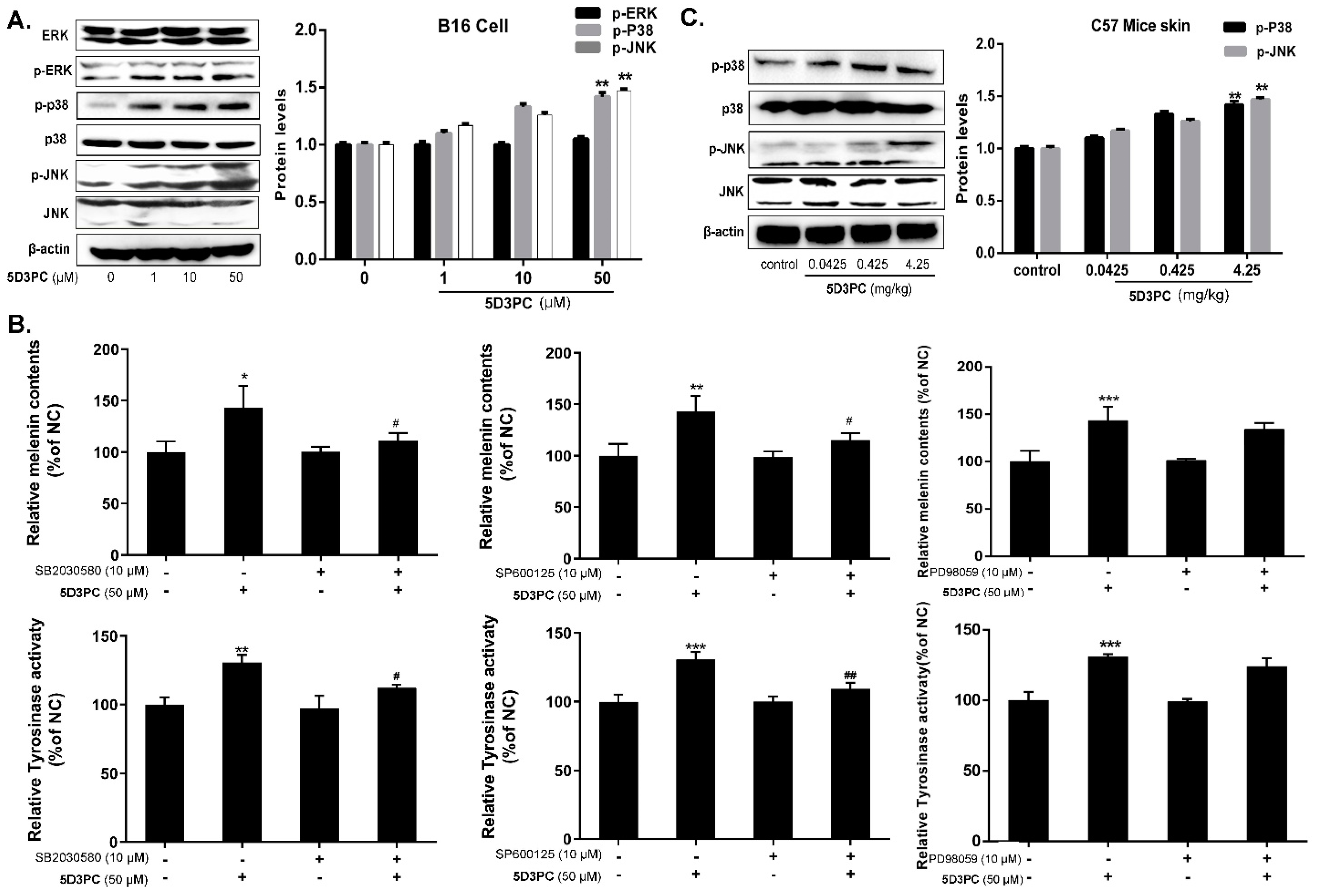
2.7. 5D3PC Induces Melanogenesis by Activating the cAMP/PKA Signal Pathway
2.8. 5D3PC Activates p38MAPK and SAPK/JNK, but Not ERK MAPK
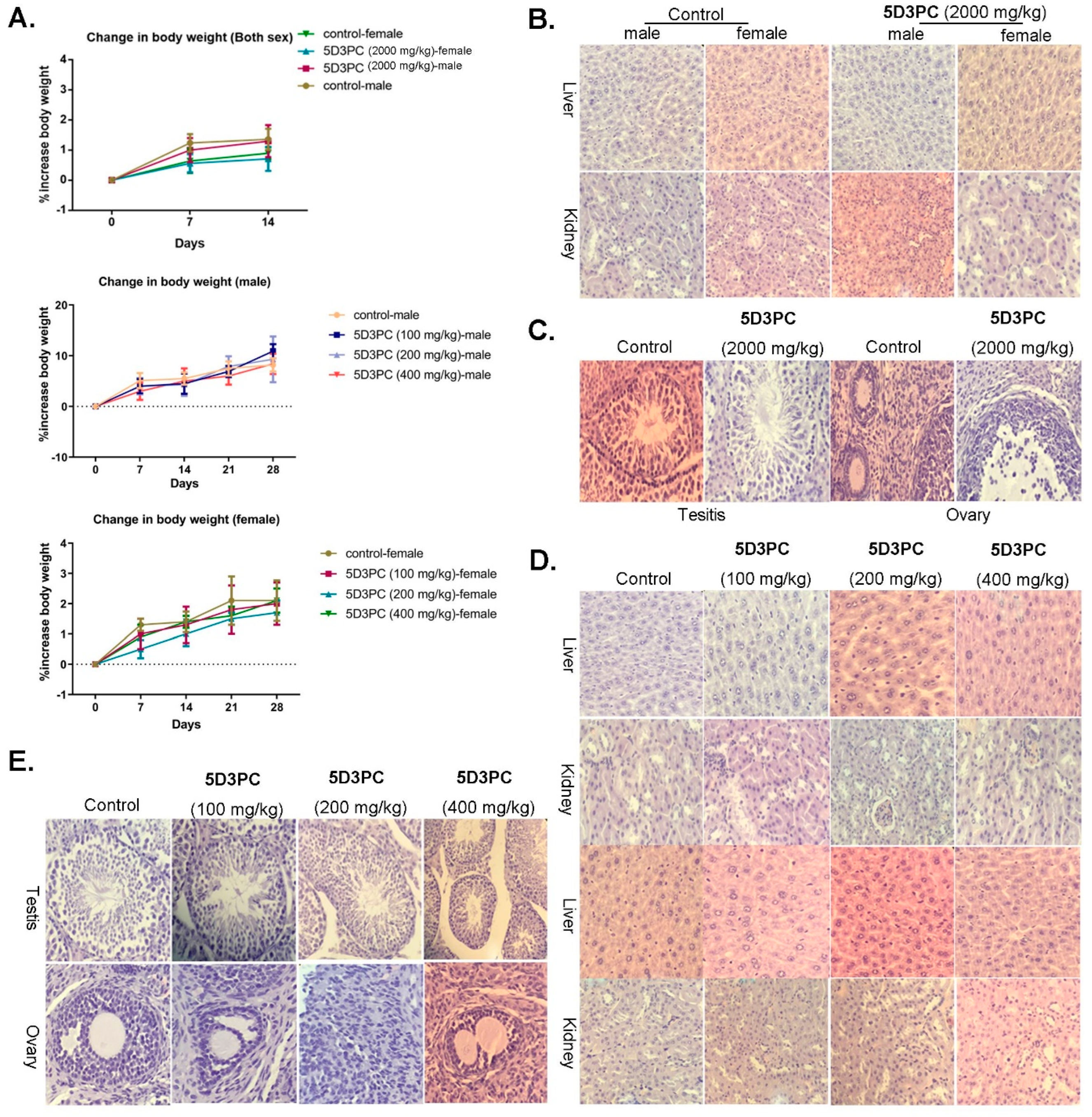
2.9. Toxicity Study of 5D3PC
3. Discussion
4. Materials and Methods
4.1. Drug and Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Tyrosinase Activity
4.5. Melanin Contents Measurement
4.6. Western Blot Analysis
4.7. cAMP Measurement in the B16 Cells
4.8. Animals
4.9. Experimental Animal Design and Drug Administration
4.10. Hematological Evaluation and Biochemical Analysis
4.11. Pathological Analysis
4.12. Immunohistochemistry Analysis
4.13. TYR, cAMP Content of Skin, and MDA Content in the Serum of the C57BL/6 Mice
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-MOP | 8-methoxypsoralan |
| TYR | Tyrosinase |
| H.Q | hydroquinone |
| MDA | malondialdehyde |
References
- Nicolaidou, E.; Katsambas, A.D. Pigmentation disorders: Hyperpigmentation and hypopigmentation. Clin. Dermatol. 2014, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Möller, K.; Sigurbjornsdottir, S.; Arnthorsson, A.O.; Pogenberg, V.; Dilshat, R.; Fock, V.; Brynjolfsdottir, S.H.; Bindesboll, C.; Bessadottir, M.; Ogmundsdottir, H.M.; et al. MITF has a central role in regulating starvation-induced autophagy in melanoma. Sci. Rep. 2019, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, E.J.; Ahn, Y.; Park, S.; Kim, S.H.; Oh, S.H. A chemical compound from fruit extract of Juglans mandshurica inhibits melanogenesis through p-ERK-associated MITF degradation. Phytomedicine 2018, 57, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Ito, S. Skin Pigmentation: Is the Control of Melanogenesis a Target within Reach? Int. J. Mol. Sci. 2018, 19, 4040. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Kim, D.-S.; Cha, S.-B.; Park, M.-C.; Park, S.-A.; Kim, H.-S.; Woo, W.-H.; Mun, Y.-J. Scopoletin Stimulates Melanogenesis via cAMP/PKA Pathway and Partially p38 Activation. Biol. Pharm. Bull. 2017, 40, 2068–2074. [Google Scholar] [CrossRef]
- Kang, M.; Park, S.H.; Park, S.J.; Oh, S.W.; Yoo, J.A.; Kwon, K.; Kim, J.; Yu, E.; Cho, J.Y.; Lee, J. p44/42 MAPK signaling is a prime target activated by phenylethyl resorcinol in its anti-melanogenic action. Phytomedicine 2019, 58, 152877. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jin, S.H.; Kang, H.Y. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch. Dermatol. Res. 2008, 300, 325–329. [Google Scholar] [CrossRef]
- Huo, S.-X.; Wang, Q.; Liu, X.-M.; Ge, C.-H.; Gao, L.; Peng, X.-M.; Yan, M. The Effect of Butin on the Vitiligo Mouse Model Induced by Hydroquinone. Phytother. Res. 2017, 31, 740–746. [Google Scholar] [CrossRef]
- Jung, S.-E.; Kang, H.Y.; Lee, E.-S.; Kim, Y.C. Changes of Epidermal Thickness in Vitiligo. Am. J. Dermatopathol. 2015, 37, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.J.; Lee, J.H.; Cho, B.R.; Seo, W.D.; Kang, H.W.; Kim, D.W.; Cho, K.J.; Lee, S.J. Dual inhibition of gamma-oryzanol on cellular melanogenesis: Inhibition of tyrosinase activity and reduction of melanogenic gene expression by a protein kinase A-dependent mechanism. J. Nat. Prod. 2012, 75, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cai, M.; Ren, Y.; Wu, H.; Liu, M.; Chen, H.; Shang, J. The different roles of 5-HT1A/2A receptors in fluoxetine ameliorated pigmentation of C57BL/6 mouse skin in response to stress. J. Dermatol. Sci. 2018, 92, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Calabro, K.; Galarneau, J.-R.; Bigio, I.; Krucker, T. Temporal Variations of Skin Pigmentation in C57Bl/6 Mice Affect Optical Bioluminescence Quantitation. Mol. Imaging Biol. 2011, 13, 1114–1123. [Google Scholar] [CrossRef]
- Kwon, S.; Sevick-Muraca, E.M. Effects of Depilation-Induced Skin Pigmentation and Diet-Induced Fluorescence on In Vivo Fluorescence Imaging. Contrast Media Mol. Imaging 2017, 2017, 7659242. [Google Scholar] [CrossRef]
- Ogbechie-Godec, O.A.; Elbuluk, N. Melasma: An Up-to-Date Comprehensive Review. Dermatol. Ther. 2017, 7, 305–318. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef]
- Na, J.-I.; Shin, J.-W.; Choi, H.-R.; Kwon, S.-H.; Park, K.-C. Resveratrol as a Multifunctional Topical Hypopigmenting Agent. Int. J. Mol. Sci. 2019, 20, 956. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, H.; Xiong, L.; Zou, L.; Dai, W.; Liu, H.; Hu, G. Protective Effects of Aqueous Extracts of Flos lonicerae Japonicae against Hydroquinone-Induced Toxicity in Hepatic L02 Cells. Oxidative Med. Cell. Longev. 2018, 2018, 4528581. [Google Scholar] [CrossRef]
- Zang, D.; Niu, C.; Aisa, H.A. Amine derivatives of furocoumarin induce melanogenesis by activating Akt/GSK-3beta/beta-catenin signal pathway. Drug Des. Dev. Ther. 2019, 13, 623–632. [Google Scholar] [CrossRef]
- Chopra, B.; Dhingra, A.K.; Dhar, K.L. Psoralea corylifolia L. (Buguchi)—folklore to modern evidence: Review. Fitoterapia 2013, 90, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Park, C.; Ikram, M.; Kang, D.; Lee, S.; Yang, J.; Park, Y.; Yoon, S.; Chun, P.; Moon, H.R. Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogues. Bioorganic Chem. 2019, 87, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J. Milestones in Melanocytes/Melanogenesis. J. Investig. Dermatol. 2011, 131, E1. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Choi, B.R.; Lee, E.K.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Song, C.H.; Lee, Y.J.; Ku, S.K. Inhibitory Effect of Dried Pomegranate Concentration Powder on Melanogenesis in B16F10 Melanoma Cells; Involvement of p38 and PKA Signaling Pathways. Int. J. Mol. Sci. 2015, 16, 24219–24242. [Google Scholar] [CrossRef]
- Shin, H.; Hong, S.D.; Roh, E.; Jung, S.H.; Cho, W.J.; Park, S.H. cAMP-dependent activation of protein kinase A as a therapeutic target of skin hyperpigmentation by diphenylmethylene hydrazinecarbothioamide. Br. J. Pharmacol. 2015, 172, 3434–3445. [Google Scholar] [CrossRef]
- Stern, R.S. The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: A 30-year prospective study. J. Am. Acad. Dermatol. 2012, 66, 553–562. [Google Scholar] [CrossRef]
- Slominski, A.; Paus, R.; Costantino, R. Differential Expression and Activity of Melanogenesis-Related Proteins During Induced Hair Growth in Mice. J. Investig. Dermatol. 1991, 96, 172–179. [Google Scholar] [CrossRef]
- Bastonini, E.; Kovacs, D.; Picardo, M. Skin Pigmentation and Pigmentary Disorders: Focus on Epidermal/Dermal Cross-Talk. Ann. Dermatol. 2016, 28, 279–289. [Google Scholar] [CrossRef]
- Kasraee, B.; Handjani, F.; Aslani, F.S. Enhancement of the depigmenting effect of hydroquinone and 4-hydroxyanisole by all-trans-retinoic acid (tretinoin): The impairment of glutathione-dependent cytoprotection? Dermatology 2003, 206, 289–291. [Google Scholar] [CrossRef]
- Bae, J.M.; Jung, H.M.; Hong, B.Y.; Lee, J.H.; Choi, W.J.; Lee, J.H.; Kim, G.M. Phototherapy for Vitiligo: A Systematic Review and Meta-analysis. JAMA Dermatol. 2017, 153, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Brondani, J.C.; Reginato, F.Z.; Brum, E.D.S.; Vencato, M.D.S.; Lhamas, C.L.; Viana, C.; da Rocha, M.I.U.M.; Bauermann, L.D.F.; Manfron, M.P. Evaluation of acute and subacute toxicity of hydroethanolic extract of Dolichandra unguis-cati L. leaves in rats. J. Ethnopharmacol. 2017, 202, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, M.; Kouchesfahani, H.M.; Shockravi, A.; Foroozanfar, M.; Parivar, K. The adverse effects of the methoxsalen and ultraviolent A radiation on spermatogenesis in mice. Iran. J. Reprod. Med. 2015, 13, 489–494. [Google Scholar] [PubMed]
- Diawaraa, M.M.; Chavez, K.J.; Simpleman, D.; Williams, D.E.; Franklin, M.R.; Hoyer, P.B. The psoralens adversely affect reproductive function in male wistar rats. Reprod. Toxicol. 2001, 15, 137–144. [Google Scholar] [CrossRef]
- Niu, C.; Zang, D.; Aisa, H.A. Design, synthesis and biological activity of novel furocoumarin derivatives as activator of melanogenesis and tyrosinase in B16 cells. Chem. Res. Chin. Univ. 2018, 34, 408–414. [Google Scholar] [CrossRef]
- Kim, A.; Yim, N.-H.; Im, M.; Jung, Y.P.; Liang, C.; Cho, W.-K.; Ma, J.Y. Ssanghwa-tang, an oriental herbal cocktail, exerts anti-melanogenic activity by suppression of the p38 MAPK and PKA signaling pathways in B16F10 cells. BMC Complement. Altern. Med. 2013, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Lee, T.H.; Lee, W.J.; Shim, W.S.; Yeo, E.J.; Kim, S.; Kim, S.Y.A. novel synthetic Piper amide derivative NED-180 inhibits hyperpigmentation by activating the PI3K and ERK pathways and by regulating Ca2+ influx via TRPM1 channels. Pigm Cell Melanoma R. 2016, 29, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.-N. Methodology for Evaluation of Melanin Content and Production of Pigment Cells in Vitro. Photochem. Photobiol. 2008, 84, 645–649. [Google Scholar] [CrossRef]
- Natale, C.A.; Duperret, E.K.; Zhang, J.; Sadeghi, R.; Dahal, A.; O’Brien, K.T.; Cookson, R.; Winkler, J.D.; Ridky, T.W. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. eLife 2016, 5, e15104. [Google Scholar] [CrossRef]
- Huo, S.-X.; Liu, X.-M.; Ge, C.-H.; Gao, L.; Peng, X.-M.; Zhao, P.-P.; Yan, M. The Effects of Galangin on a Mouse Model of Vitiligo Induced by Hydroquinone. Phytother. Res. 2014, 28, 1533–1538. [Google Scholar] [CrossRef]
- OECD. Organization for Economic Cooperation and Development. Guideline for testing of chemicals: Acute oral toxicity-acute toxic class method. Adopted 2001, 423, 1–14. [Google Scholar]
- OECD. Organisation for Economic Cooperation and Development. Guideline for the testing of chemicals: Repeated dose 28-day oral toxicity in rodents. Adopted 2008, 407, 1–13. [Google Scholar]



| Sex | Parameter | Control | 5D3PC 9609042000 mg/kg |
|---|---|---|---|
| Male | WBC (×103/µL) | 0.220 ± 0.060 | 0.160 ± 0.060 |
| Female | Lymphocytes (%) | 91.55 ± 4.450 | 69.80 ± 4.380 |
| Neutrophils (%) | 3.700 ± 0.380 | 11.00 ± 3.110 | |
| Monocytes (%) | 2.250 ± 0.070 | 16.25 ± 5.440 | |
| Eosinophils (%) | 2.500 ± 0.560 | 2.950 ± 0.210 | |
| Basophilic (%) | 0.000 ± 0.000 | 0.000 ± 0.000 | |
| RBC (×106/µL) | 3.550 ± 0.250 | 1.630 ± 0.170 | |
| HBG (g/dL) | 63.00 ± 4.450 | 29.50 ± 2.890 | |
| HCT (%) | 18.65 ± 1.320 | 7.950 ± 0.840 | |
| MCV (f L) | 52.50 ± 0.280 | 50.95 ± 3.600 | |
| MCHC (g/dL) | 338.5 ± 2.120 | 387.0 ± 43.84 | |
| RDW (%) | 14.65 ± 0.210 | 15.85 ± 0.210 | |
| AST (U/L) | 93.35 ± 4.310 | 158.2 ± 5.020 | |
| ALT(U/L) | 20.95 ± 0.210 | 45.30 ± 3.390 | |
| CRE (µmol/L) | 33.80 ± 1.270 | 10.31 ± 1.670 | |
| URE (mg/dL) | 6.770 ± 0.370 | 49.25 ± 4.030 | |
| WBC (×103/µL) | 1.950 ± 0.860 | 5.560 ± 0.320 | |
| Lymphocytes (%) | 78.05 ± 15.34 | 71.15 ± 1.760 | |
| Neutrophils (%) | 14.15 ± 11.50 | 22.90 ± 1.550 | |
| Monocytes (%) | 3.900 ± 0.390 | 3.000 ± 0.120 | |
| Eosinophils (%) | 3.550 ± 0.070 | 2.300 ± 0.110 | |
| Basophilic (%) | 0.000 ± 0.000 | 0.650 ± 0.070 | |
| RBC (×106/µL) | 7.990 ± 0.080 | 8.870 ± 0.700 | |
| HBG (g/dL) | 147.5 ± 4.300 | 168.5 ± 14.84 | |
| HCT (%) | 39.30 ± 7.350 | 45.60 ± 4.030 | |
| MCV (f L) | 49.15 ± 0.070 | 51.45 ± 0.490 | |
| MCHC (g/dL) | 372.0 ± 33.94 | 369.5 ± 0.700 | |
| RDW (%) | 14.90 ± 1.550 | 14.50 ± 0.000 | |
| AST (U/L) | 93.00 ±1.830 | 142.5 ± 2.260 | |
| ALT (U/L) | 21.35 ±1.060 | 42.80 ± 3.670 | |
| CRE (µmol/L) | 9.750 ± 0.160 | 9.030 ± 0.450 | |
| URE (mg/dL) | 7.040 ± 0.100 | 53.30 ± 3.390 |
| Sex | Parameter | Control | 5D3PC 100 mg/kg | 5D3PC 200 mg/kg | 5D3PC 400 mg/kg |
|---|---|---|---|---|---|
| Male | WBC (×103/µL) | 4.050 ± 0.280 | 2.070 ± 0.670 | 2.560 ± 1.390 | 2.660 ± 0.560 |
| Female | Lymphocytes (%) | 93.37 ± 3.940 | 80.95 ± 8.500 | 70.82 ± 3.430 | 69.05 ± 6.320 |
| Neutrophils (%) | 20.27 ± 3.900 | 13.42 ± 0.830 | 13.82 ± 1.640 | 23.70 ± 5.260 | |
| Monocytes (%) | 11.95 ± 2.640 | 15.07 ± 4.240 | 11.97 ± 2.820 | 14.80 ± 1.980 | |
| Eosinophils (%) | 1.700 ± 0.530 | 5.370 ± 1.280 | 3.220 ± 1.260 | 2.250 ± 0.370 | |
| Basophilic (%) | 0.350 ± 0.050 | 0.420 ± 0.170 | 0.220 ± 0.090 | 0.200 ± 0.140 | |
| RBC (×106/µL) | 12.15 ± 0.430 | 6.650 ± 1.200 | 9.220 ± 1.370 | 9.150 ± 1.220 | |
| HBG (g/dL) | 194.3 ± 18.00 | 130.0 ± 12.40 | 120.0 ± 10.09 | 157.7 ± 19.63 | |
| HCT (%) | 63.87 ± 4.760 | 49.55 ± 1.930 | 48.72 ± 3.760 | 52.77 ± 9.33 | |
| MCV (f L) | 49.15 ± 2.160 | 50.35 ± 2.290 | 50.42 ± 1.260 | 49.55 ± 1.840 | |
| MCHC (g/dL) | 350.8 ± 11.32 | 344.5 ± 7.930 | 344.5 ± 3.100 | 351.5 ± 3.100 | |
| RDW (%) | 14.35 ± 1.410 | 15.87 ± 1.040 | 13.57 ± 0.850 | 13.87 ± 1.140 | |
| AST (U/L) | 96.93 ± 4.860 | 109.1 ± 6.730 | 109.8 ± 7.790 | 145.2 ± 11.86 | |
| ALT (U/L) | 25.53 ± 5.290 | 43.83 ± 3.630 | 41.05 ± 5.710 | 44.03 ± 4.500 | |
| CRE (µmol/L) | 36.03 ± 3.150 | 10.79 ± 0.810 | 11.52 ± 1.660 | 11.01 ± 1.550 | |
| URE (mg/dL) | 9.060 ± 2.950 | 47.39 ± 5.560 | 52.36 ± 1.720 | 50.06 ± 1.470 | |
| WBC (×103/µL) | 4.040 ± 0.180 | 3.030 ± 1.030 | 3.390 ± 0.420 | 4.140 ± 0.330 | |
| Lymphocytes (%) | 90.45 ± 1.070 | 90.60 ± 1.070 | 83.40 ± 4.290 | 85.30 ± 5.540 | |
| Neutrophils (%) | 23.12 ± 0.830 | 18.10 ± 1.280 | 17.92 ± 1.750 | 19.92 ± 1.770 | |
| Monocytes (%) | 10.76 ± 1.070 | 19.55 ± 4.900 | 15.45 ± 4.030 | 15.62 ± 3.010 | |
| Eosinophils (%) | 3.700 ± 0.420 | 3.870 ± 0.180 | 3.620 ± 0.370 | 3.100 ± 0.720 | |
| Basophilic (%) | 0.370 ± 0.170 | 0.220 ± 0.120 | 0.260 ± 0.110 | 0.200 ± 0.140 | |
| RBC (×106/µL) | 8.520 ± 1.820 | 8.450 ± 1.170 | 7.410 ± 0.520 | 8.750 ± 0.470 | |
| HBG (g/dL) | 154.0 ± 6.450 | 125.0 ± 10.13 | 99.50 ± 4.930 | 98.50 ± 11.95 | |
| HCT (%) | 80.55 ± 2.590 | 66.37 ± 6.360 | 43.85 ± 4.860 | 44.57 ± 5.140 | |
| MCV (f L) | 52.15 ± 4.430 | 49.55 ± 1.930 | 58.60 ± 3.330 | 55.57 ± 2.250 | |
| MCHC (g/dL) | 319.0 ± 8.340 | 335.5 ± 12.04 | 340.0 ± 18.19 | 330.7 ± 11.52 | |
| RDW (%) | 16.55 ± 0.300 | 15.37 ± 1.750 | 14.45 ± 0.380 | 16.75 ± 1.260 | |
| AST (U/L) | 94.50 ± 2.760 | 105.7 ± 3.72 | 105.2 ± 4.400 | 148.9 ± 5.640 | |
| ALT(U/L) | 23.18 ± 2.190 | 42.55 ± 4.780 | 40.58 ± 2.400 | 43.35 ± 2.600 | |
| CRE (µmol/L) | 39.73 ± 3.140 | 9.960 ± 0.400 | 12.14 ± 1.480 | 15.05 ± 2.670 | |
| URE (mg/dL) | 7.810 ± 0.990 | 43.87 ± 3.070 | 52.75 ± 3.120 | 46.40 ± 5.780 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, D.; Niu, C.; Lu, X.; Aisa, H.A. A Novel Furocoumarin Derivative, 5-((diethylamino)me-13 thyl)-3-phenyl-7H-furo [3,2-g] chromen-7-one Upregulates Melanin Synthesis via the Activation of cAMP/PKA and MAPKs Signal Pathway: In Vitro and In Vivo Study. Int. J. Mol. Sci. 2022, 23, 14190. https://doi.org/10.3390/ijms232214190
Zang D, Niu C, Lu X, Aisa HA. A Novel Furocoumarin Derivative, 5-((diethylamino)me-13 thyl)-3-phenyl-7H-furo [3,2-g] chromen-7-one Upregulates Melanin Synthesis via the Activation of cAMP/PKA and MAPKs Signal Pathway: In Vitro and In Vivo Study. International Journal of Molecular Sciences. 2022; 23(22):14190. https://doi.org/10.3390/ijms232214190
Chicago/Turabian StyleZang, Deng, Chao Niu, Xueying Lu, and Haji Akber Aisa. 2022. "A Novel Furocoumarin Derivative, 5-((diethylamino)me-13 thyl)-3-phenyl-7H-furo [3,2-g] chromen-7-one Upregulates Melanin Synthesis via the Activation of cAMP/PKA and MAPKs Signal Pathway: In Vitro and In Vivo Study" International Journal of Molecular Sciences 23, no. 22: 14190. https://doi.org/10.3390/ijms232214190
APA StyleZang, D., Niu, C., Lu, X., & Aisa, H. A. (2022). A Novel Furocoumarin Derivative, 5-((diethylamino)me-13 thyl)-3-phenyl-7H-furo [3,2-g] chromen-7-one Upregulates Melanin Synthesis via the Activation of cAMP/PKA and MAPKs Signal Pathway: In Vitro and In Vivo Study. International Journal of Molecular Sciences, 23(22), 14190. https://doi.org/10.3390/ijms232214190









