Abstract
Agarwood is the most expensive non-construction wood product in the world. As a therapeutic agent, agarwood can cure some diseases, but few studies have been carried out on the antagonistic abilities of endophytic fungi associated with agarwood. Agarwood is mainly found in the genus Aquiaria. The objectives of this study are to understand the antimicrobial activities and their potential as biocontrol agents of the endophytic fungi of Aquilaria sinensis. First, fresh samples of A. sinensis were collected from Yunnan and Guangdong Provinces in 2020–2021, and the endophytic fungi were isolated and identified to genus level based on the phylogenetic analyses of the Internal Transcribed Spacer (ITS) region. In this bioassay, 47 endophytic strains were selected to check their bioactivities against three bacterial pathogens viz. Erwinia amylovora, Pseudomonas syringae, and Salmonella enterica; and three fungal pathogens viz. Alternaria alternata, Botrytis cinerea, and Penicillium digitatum. The antibiosis test was carried out by the dual culture assay (10 days), and among the 47 strains selected, 40 strains belong to 18 genera viz. Alternaria, Annulohypoxylon, Aspergillus, Botryosphaeria, Colletotrichum, Corynespora, Curvularia, Daldinia, Diaporthe, Fusarium, Lasiodiplodia, Neofusicoccum, Neopestalotiopsis, Nigrospora, Paracamarosporium, Pseudopithomyces, Trichoderma, Trichosporon and one strain belongs to Xylariaceae had antimicrobial activities. In particular, Lasiodiplodia sp. (YNA-D3) showed the inhibition of all the bacterial and fungal pathogens with a significant inhibition rate. In addition, the strains viz; Curvularia sp. (GDA-3A9), Diaporthe sp. (GDA-2A1), Lasiodiplodia sp. (YNA-D3), Neofusicoccum sp. (YNA-1C3), Nigrospora sp. (GDA-4C1), and Trichoderma sp. (YNA-1C1) showed significant antimicrobial activities and are considered worthy of further studies to identify individual fungal species and their bioactive compounds. This study enriches the diversity of endophytic fungi associated with agarwood, and their potential antagonistic effects against bacterial and fungal pathogens.
1. Introduction
Aquilaria Lam. (Thymelaeaceae Juss.) is the main genus that can produce agarwood [1,2]. Agarwood, a fragrant, dark, and resinous heartwood is the most expensive non-construction wood product in the world [3,4]. In China, agarwood is used in traditional Chinese medicine, and only A. sinensis (Lour.) Spreng. is the main agarwood tree species cultivated in Guangdong, Guangxi, Hainan, and Yunnan Provinces [4,5,6,7,8]. Current research on endophytic fungi associated with A. sinensis mainly focuses on the agarwood formation ability of the endophytic fungi [9,10], and only a few of the A. sinensis associated endophytic fungi have been studied for antimicrobial activities via dual culture assay [11]. In a previous study, 38 endophytic strains have been reported to have antimicrobial activities, for example, Botryosphaeria rhodina (Berk. & M.A. Curtis) Arx, Cladosporium edgeworthiae H. Zhang & Z.Y. Zhang, Fusarium oxysporum Schltdl., and Guignardia mangiferae A.J. Roy showed antimicrobial activities [12]; and a variety of important secondary metabolites with antibacterial and antimicrobial activities have been extracted from Nemania aquilariae Tibpromma & Zhang Lu [10]. However, the microorganisms that can be inhibited by agarwood are not clear enough, thus it is necessary to continue the research on the microbial spectrum of agarwood [13].
In this study, endophytic fungi associated with agarwood isolated from different plant tissues were used to test their antagonistic abilities against three pathogenic bacteria viz. Erwinia amylovora (Burrill) Winslow et al., Pseudomonas syringae van Hall, and Salmonella enterica (ex Kauffmann and Edwards) Le Minor and Popoff; and three pathogenic fungi viz. Alternaria alternata (Fr.) Keissl., Botrytis cinerea Pers., and Penicillium digitatum (Pers.) Sacc.
2. Materials and methods
2.1. Sample Collection and Isolation
2.1.1. Sample Collection
Fresh samples of A. sinensis were collected three times; i.e., two times in Yunnan Province (21°55′48″ N, 101°15′36″ E, in November 2020; 22°21′09″ N, 101°01′06″ E, in September 2021) and one time in Guangdong Province (21°49′48″ N, 111°40′12″ E, in December 2020). Samples from Yunnan Province are denoted YNA, while from Guangdong Province are denoted GDA. The leaves and twigs of healthy plants, and the branches and twigs with agarwood dark resin were collected. Branch cutters, knives, and saws were used to cut the samples and they were cleaned with 75% alcohol before and after use. After collection, the fresh samples were placed in a thermal insulation ice box, brought back to the laboratory, and placed in the 4 °C refrigerators until the endophytic fungi are isolated.
2.1.2. Isolation of Endophytic Fungi
Du et al. [14] with some adjustments was followed for the isolation of endophytic fungi in fresh agarwood samples. The bark of fresh samples was removed and then washed under running water, transferred to a laminar flow hood and the samples were cut into small pieces (0.5 cm × 0.5 cm) by sterilized knives and blades (sterilized with 75% alcohol). The surface disinfection steps of each sample are washed in sterile water, 75% alcohol for 30 s, 2.5% sodium hypochlorite for 1 min, and 75% alcohol for 30 s, finally, samples were washed in sterile water three times, and transferred to the sterilized filter paper to absorb the water. All tools were dipped in 95% alcohol and flamed before and after use. All the steps were done in a laminar flow hood. Five sterilized small pieces were placed in each 90 mm potato dextrose agar (PDA) plate (Ampicillin was added), and incubated at 28 °C for 14 days. During incubation, plates were checked every two days and the fresh mycelia were transferred to new 60 mm PDA plates to get pure cultures. The pure cultures were used for DNA extraction. Living pure cultures were deposited in the Zhongkai University of Agriculture and Engineering Culture Collection (ZHKUCC), China.
2.2. Endophytic Fungi Identification
2.2.1. DNA Extraction, PCR Amplification and Sequencing
Ten days old fresh mycelia were used for DNA extraction using the Biospin Fungus Genomic DNA Extraction Kit–BSC14S1 (BioFlux, Hangzhou, China), following the manufacturer’s instructions [15]. Polymerase chain reaction (PCR) was used to amplify the ITS gene (internal transcribed spacer 1, 5.8S ribosomal RNA gene and internal transcribed spacer 2), using primers ITS5/ITS4 [16]. The PCR amplification was followed Du et al. [17], and the total volume of PCR mixtures for amplifications was 25 μL, with 94 °C: 3 min, (94 °C: 30 s, 55 °C: 50 s, 72 °C: 90 s) × 35 cycles, 72 °C: 10 min, final 4 °C. Finally, PCR products were purified and sequenced by Qinke Biotech Co., Kunming, China.
2.2.2. Phylogenetic Analyses
Phylogenetic analyses are widely used in the identification of endophytic fungi, and the ITS gene is commonly used to primarily identify endophytic fungi to genus level [18,19,20,21,22]. In this study, to confirm the endophytic fungal genera, the ITS phylogenetic analyses were performed by Randomized Accelerated Maximum Likelihood (RAxML) analyses according to the parameters described in Dissanayake et al. [15]. The obtained sequences of the forward and reverse were merged in Geneious (9.1.2), and the merged sequences were subjected to BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch, accessed on 18 September 2022). Based on the BLAST search, the closest sequences were retrieved from the aNationl Center for Biotechnology Information (NCBI) (https://www.ncbi.nlm.nih.gov/, accessed on 18 September 2022). The sequences were aligned in the online website MAFFT v.7 (https://mafft.cbrc.jp/alignment/server/, accessed on 18 September 2022) [23], and automatic cutting was done in trimAl.v1.2rev59. BioEdit v. 7.0.5.2 [24] was used to manually combine the sequences, and subsequently, multiple sequence alignments were converted from FASTA to PHYLIP in ALTER (http://www.sing-group.org/ALTER/, accessed on 18 September 2022) [25]. The RAxML tree was run using the PHYLIP file, in RAxML-HPC BlackBox (8.2.12) [26,27] on the CIPRES Science Gateway platform (https://www.phylo.org/portal2/home.action, accessed on 18 September 2022) [28], with the GTR+I+G model of evolution. The final tree was visualized in FigTree v. 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/, accessed on 18 September 2022) [29], and edited in Microsoft PowerPoint 2010. The sequences generated in this study were uploaded to NCBI (https://submit.ncbi.nlm.nih.gov/subs/, accessed on 18 September 2022) to obtain the GenBank numbers (Table 1).

Table 1.
Original code, strain name, strain number, ITS GenBank accession number, class in Ascomycota, plant tissue, collection site, and date of collection of 47 fungal strains associated with Aquilaria sinensis used in this study. The contents in the table are arranged according to the genus of endophytic fungi.
2.3. Pre Dual Culture Assay for Antibiosis Test (Pretest)
The ability of endophytic fungal isolates to inhibit the growth of pathogens was evaluated by the dual culture technique [30]. The six pathogens (three bacterial pathogens viz. E. amylovora, P. syringae, and S. enterica; and three fungal pathogens viz. A. alternata, B. cinerea, and P. digitatum) used in this study were obtained from the China General Microbiological Culture Collection Center (CGMCC).
The pretest is a screening test conducted before the formal test. We used 47 strains from all isolated strains for the pretest. The 47 endophytic fungi strains and six pathogenic strains (Table 2) were incubated at 28 °C for 10 days before the test. Fungi were cultivated on PDA, while bacteria were cultivated in nutrient agar (NA). After 10 days of incubation, the fungal colonies were cut into 0.4 cm diameter discs (sterilized plastic straw) in the laminar flow cabinet, then endophytic fungi and pathogenic fungi were inoculated in the same 90 mm PDA plates, and endophytic fungi and pathogenic bacteria (bacteria scraped with 0.4 cm wide strip) were inoculated in the same 90 mm NA plates. The control was inoculated with only pathogens. All the plates were incubated at 28 °C for 10 days. After 10 days, the colony growth of the test group and the control group were checked and recorded. According to the test results, we compared the colony diameters of the test group and the control group, and then endophytic fungi that can inhibit three pathogenic fungi or three pathogenic bacteria were selected. These selected strains with antagonistic activities were used for formal testing.

Table 2.
Six pathogens were purchased from China General Microbiological Culture Collection Center (CGMCC). The PB (pathogenic bacteria) and PF (pathogenic fungi) are new codes created in this study to distinguish pathogenic bacteria and pathogenic fungi.
2.4. Dual Culture Assay for Antibiosis Test (Formal Test)
2.4.1. Methods of Dual Culture Assay
The test method is similar to the pretest. According to the results of the pretest, among 47 endophytic fungi, 25 strains were able to inhibit pathogenic bacteria, 40 strains were able to inhibit pathogenic fungi, and 18 strains were able to inhibit both pathogenic fungi and bacteria. Therefore, 47 endophytes and six pathogens were incubated at 28 °C for 10 days before the formal test. Fungi were incubated on PDA, while bacteria were incubated in NA.
The endophytic and pathogenic fungi grown on PDA plates were cut into small fungal discs (0.4 cm diam.) using a sterilized plastic straw in laminar. Then, the 25 selected endophytic fungal strains were inoculated with three pathogenic bacteria (0.4 cm wide strip) in the same NA plates, and each test was replicated three times (total of 25 × 3 × 3 = 225 plates). The 40 selected endophytic strains were inoculated with three pathogenic fungi in the same PDA plates, and each test was replicated three times (total of 40 × 3 × 3 = 360 plates). The pathogens were inoculated on the left of the petri dish, while the endophytic fungi were inoculated on the right by keeping a space of 6 cm between the pathogens and endophytes. Negative controls were set in the antibiosis tests of each pathogen. The control group used the same culture medium as the test group. The pathogen was inoculated on the left of the medium, while nothing was inoculated on the right. Controls were incubated under the same conditions as the test groups. After inoculation, petri dishes were incubated at 28 °C for 10 days. While incubating, they were observed, photographed and the diameter of the pathogens in the test group and the control group was measured every two days.
2.4.2. Calculation and Analysis of Inhibition Rate
According to the test results, the data were processed and analyzed. The antibiosis effects and the degree of endophytic fungi effect on pathogens can be expressed by calculating the inhibition rate of endophytic fungi on the growth diameter of pathogens. The inhibition rate was calculated according to the method described in Gao et al. [58] and Rajani et al. [59], and the calculation formula used is as follows:
Inhibition% = (Cd − Td)/(Cd − 0.4) × 100
Notes: Cd = radial growth of the pathogen in pure control culture, Td = radial growth of the pathogen in dual culture. The width of the original fungal discs and bacterial strip in this test is 0.4 cm.
2.4.3. Statistical Analyses
The statistical analyses of the inhibition rate were carried out in Microsoft Excel 2010. The measured data (colony diameter) were recorded in an excel table. The inhibition rate and average inhibition rate were obtained by the formula. The standard deviation (SD) reflects the dispersion degree of a data set, and the values were obtained by inserting the function (STDEV) of standard deviation into the excel table. In addition, clustered column graphs were inserted in the excel table based on the average inhibition rate and edited in Microsoft Excel 2010.
3. Results
3.1. Results of Sample Collection and Isolation
In this study, agarwood samples were collected from Guangdong and Yunnan Provinces. The fresh samples were isolated to obtain pure cultures for molecular analyses and antibiosis tests. Figure S1 shows the culture morphologies of 47 endophytic fungi strains, and in Table 1, we list the host, collection site, and other information of 47 endophytic fungi used in this study.
3.2. Single Gene Phylogenetic Analyses
The single-gene phylogenetic analyses were carried out by constructing an RAxML phylogenetic tree based on ITS. The RAxML analyses gave a final ML optimization likelihood value of −12,190.561600. The matrix had 567 distinct alignment patterns, with 19.72% of undetermined characters or gaps. Parameters for the GTR+I+G model of the ITS were as follows: estimated base frequencies A = 0.249972, C = 0.260278, G = 0.245555, T = 0.244194; substitution rates AC = 1.190651, AG = 3.363586, AT = 2.316682, CG = 1.153165, CT = 3.693909, GT = 1.000000; proportion of invariable sites I = 0.105968; and gamma distribution shape parameter α = 0.446892.
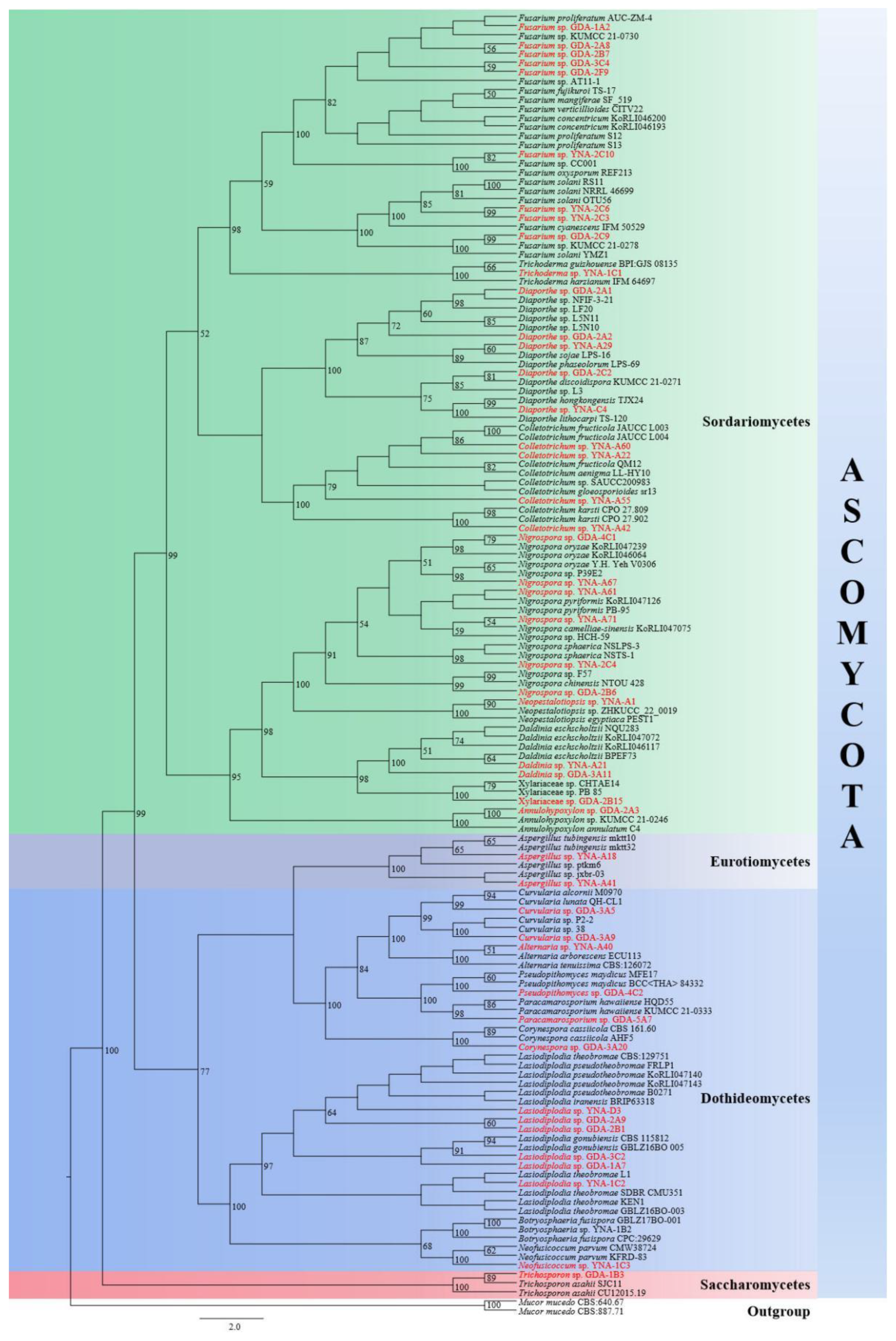
The final RAxML tree is shown in Figure 1. The 47 strains are distributed in four classes in Ascomycota, viz. Dothideomycetes, Eurotiomycetes, Saccharomycetes, and Sordariomycetes. According to the BLAST results and phylogenetic analyses, 46 strains were identified at the genus level, and they belong to 18 genera. While one of our strains (GDA-2B15) is closest to two strains of Xylariaceae viz. (CHTAE14) and (PB-85), therefore, GDA-2B15 was identified as a member of Xylariaceae in this paper.
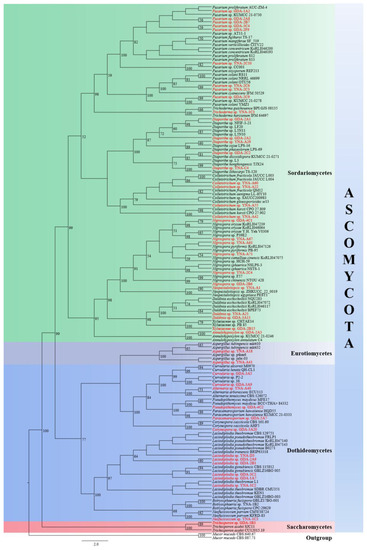
Figure 1.
A RAxML single gene phylogenetic tree of 47 endophytic fungi strains and their related sequences based on ITS. Bootstrap support values for maximum likelihood (ML) equal to or higher than 50% are indicated above the branches. The endophytic fungi with original strain numbers isolated in this study are marked with red font.
The results can be summarized as 47 endophytic fungi strains belong to Ascomycota Caval.-Sm., of which 30 strains belong to Sordariomycetes O.E. Erikss. & Winka (63.83%), 14 strains belong to Dothideomycetes O.E. Erikss. & Winka (29.79%), two strains belong to Eurotiomycetes O.E. Erikss. & Winka (4.26%), and one strain belongs to Saccharomycetes G. Winter (2.13%).
3.3. Dual Culture Assay for Antibiosis Test (Pretest)
A total of 47 endophytic fungi strains were tested on six pathogens under the same conditions. The results showed that 18 strains had inhibitory effects on all six pathogens, seven strains had inhibitory effects on all three pathogenic bacteria, and 22 strains had inhibitory effects on all three pathogenic fungi. Therefore, 25 strains had inhibitory effects on all three pathogenic bacteria, and 40 strains had inhibitory effects on all three pathogenic fungi. Therefore, 25 strains and 40 strains were used to conduct formal tests on three pathogenic bacteria and three pathogenic fungi respectively.
3.4. Dual Culture Assay for Antibiosis Test (Formal Test)
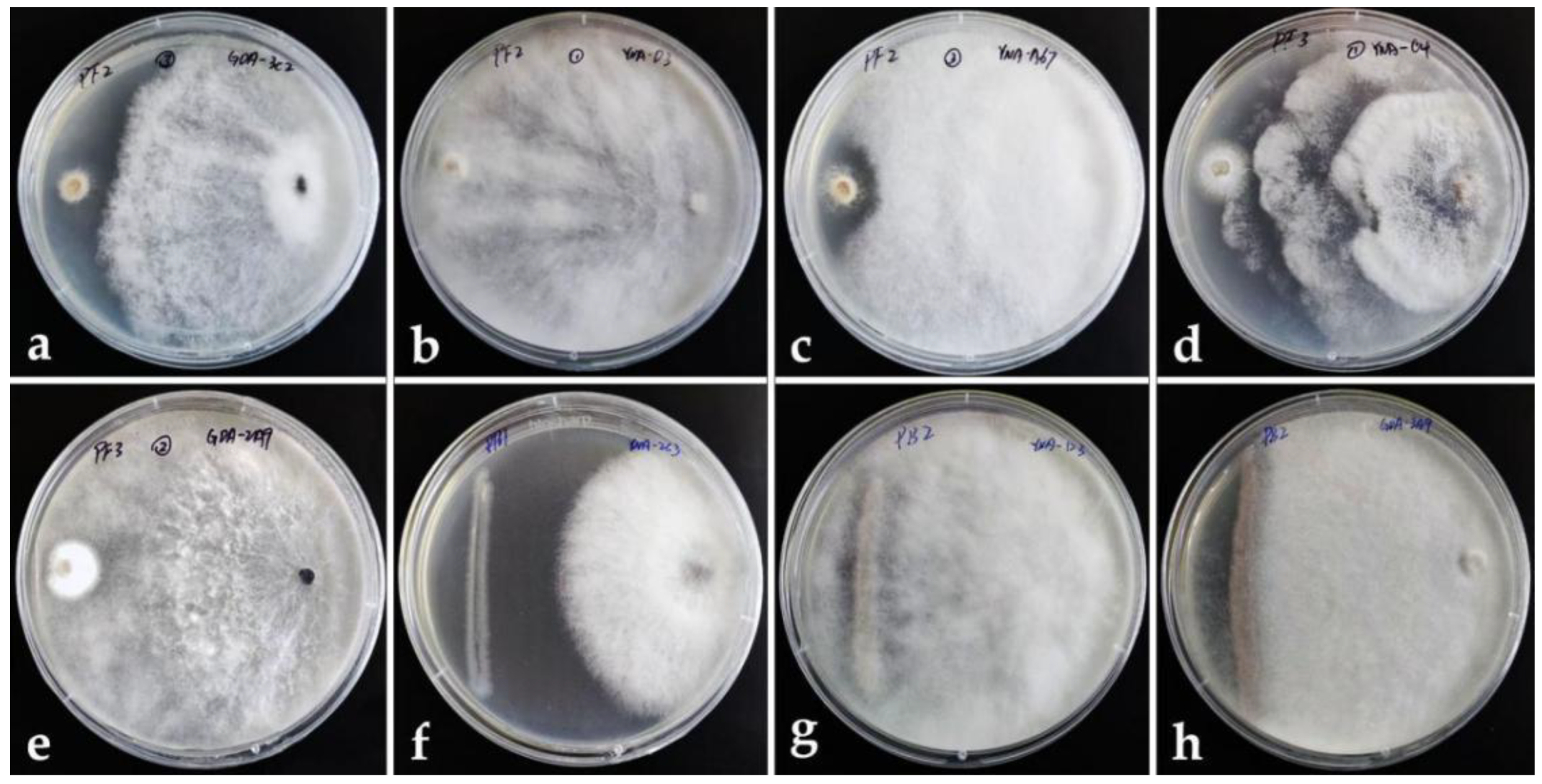
Through the results of the pretest, we carried out the formal test with the selected strains (25 endophytic fungi for pathogenic bacteria, and 40 endophytic fungi for pathogenic fungi) By calculating the inhibition rate through the formula, the strains whose inhibition rate was more than 60% were considered to have an inhibition effect, and the results recorded in Table 3 and only Lasiodiplodia sp.(YNA-D3) can inhibit all six pathogens, and its inhibition rate to pathogenic fungi is higher than bacteria pathogens (Inhibition rate: 93.30% to PF2-B. cinerea, 76.73% to PF3-P. digitatum, 75.90% to PF1-A. alternata, 74.07% to PB2-P. syringae, 63.33% to PB3-S. enterica, 63.64% to PB1-E. amylovora). Figure 2 shows the pictures of several endophytic fungi with significant inhibition rates to pathogens in the dual culture assay.

Table 3.
The results and inhibition rate percentage ± standard deviation of dual culture assay-formal test (10 days). “−” indicates that the dual culture assay-formal test of endophytic fungi against the pathogen has not been carried out. Taxa are arranged according to the alphabetical order of generic names.
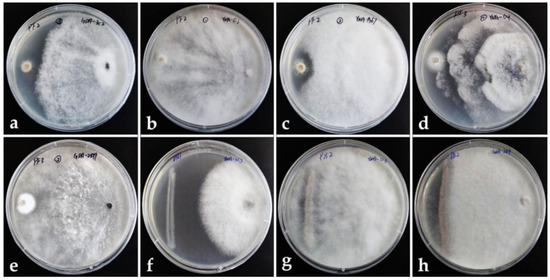
Figure 2.
Dual culture assay. Left: pathogen. Right: endophytic fungus. (a–c) Endophytic fungi dominate against the pathogenic fungus PF2. (d,e) Endophytic fungi dominate against pathogenic fungus PF3. (f) Endophytic fungi dominate against pathogenic bacterium PB1. (g,h) Endophytic fungi dominate against pathogenic bacterium PB2.
3.4.1. Inhibition of 25 Endophytic Fungi on Three Pathogenic Bacteria
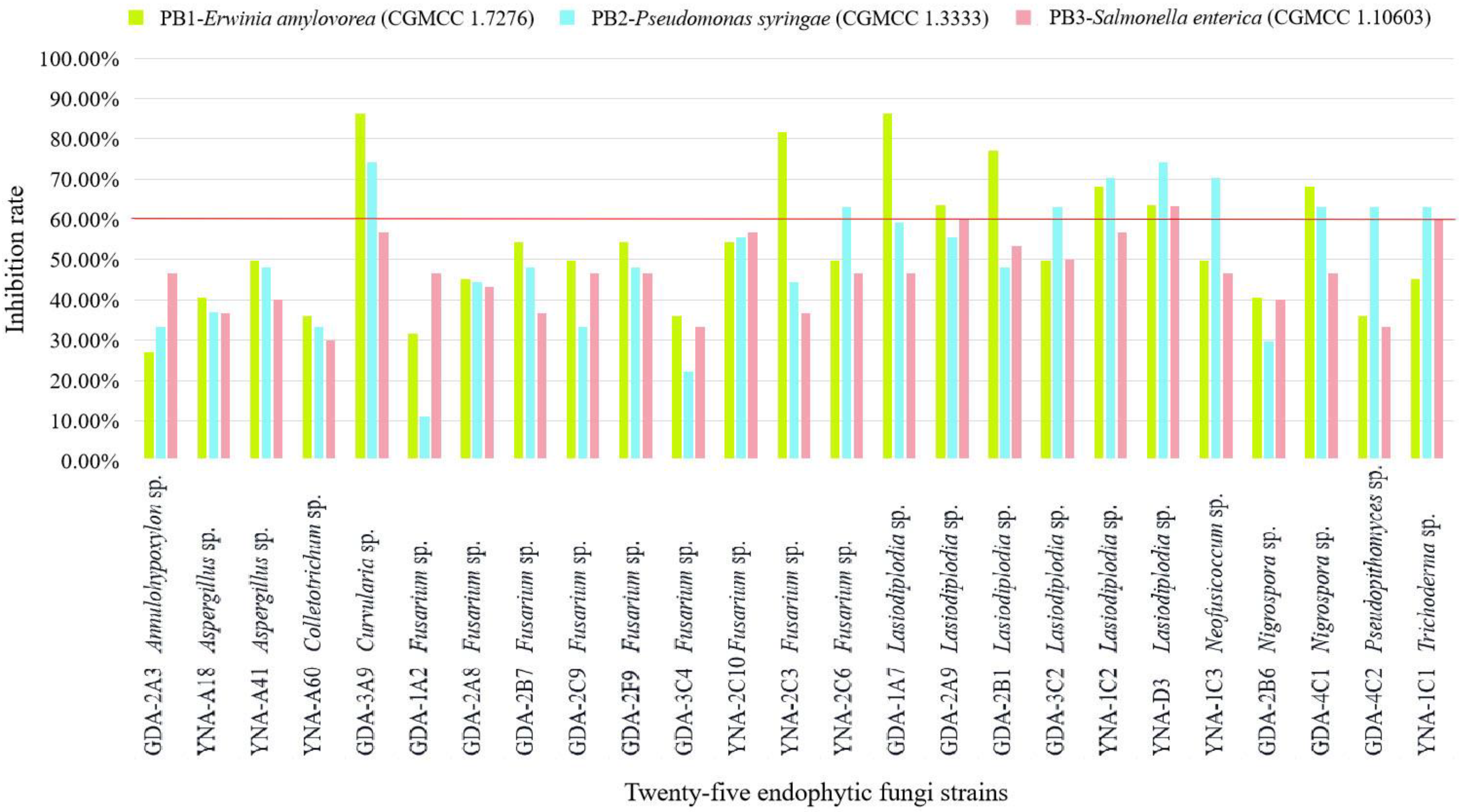
The inhibitory effect (≥60%) of 25 endophytic fungi on pathogenic bacteria is shown in Figure 3 and Table 3, and the inhibitory effect is ranked as E. amylovora (CGMCC 1.7276) > P. syringae (CGMCC 1.3333) > S. enterica (CGMCC 1.10603). For S. enterica (CGMCC 1.10603), there is almost no inhibitory effect.
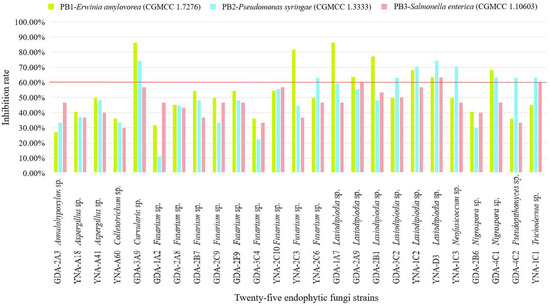
Figure 3.
Inhibition rate of 25 endophytic fungi to three pathogenic bacteria. The inhibition ≥60% is considered a good inhibition effect.
For PB1-E. amylovora (CGMCC 1.7276), eight strains showed inhibitory effects (Table 3 and Figure 3), and the three strains with the highest inhibition rate are Curvularia sp. (GDA-3A9, 86.36%), Lasiodiplodia sp. (GDA-1A7, 86.36%), and Fusarium sp. (YNA-2C3, 81.82%). Among the eight strains, the genus Lasiodiplodia Ellis & Everh. has the highest number of strains (five strains).
For PB2-P. syringae (CGMCC 1.3333), nine strains showed inhibitory effects (Table 3 and Figure 3), and the three strains with the highest inhibition rate are Curvularia sp. (GDA-3A9, 74.07%), Lasiodiplodia sp. (YNA-D3, 74.07%), and Lasiodiplodia sp. (YNA-1C2, 70.37%). Among the nine strains, the genus Lasiodiplodia has the largest number of strains (three strains).
For PB3-S. enterica (CGMCC 1.10603), three strains showed inhibitory effects (Table 3 and Figure 3), and Lasiodiplodia sp. (YNA-D3, 63.33%) had the strongest inhibitory effect, followed by Lasiodiplodia sp. (GDA-2A9, 60.00%), and Trichoderma sp. (YNA-1C1, 60.00%). Among the three strains, the genus Lasiodiplodia has the largest number of strains (two strains).
3.4.2. Inhibition of 40 Endophytic Fungi on Three Pathogenic Fungi
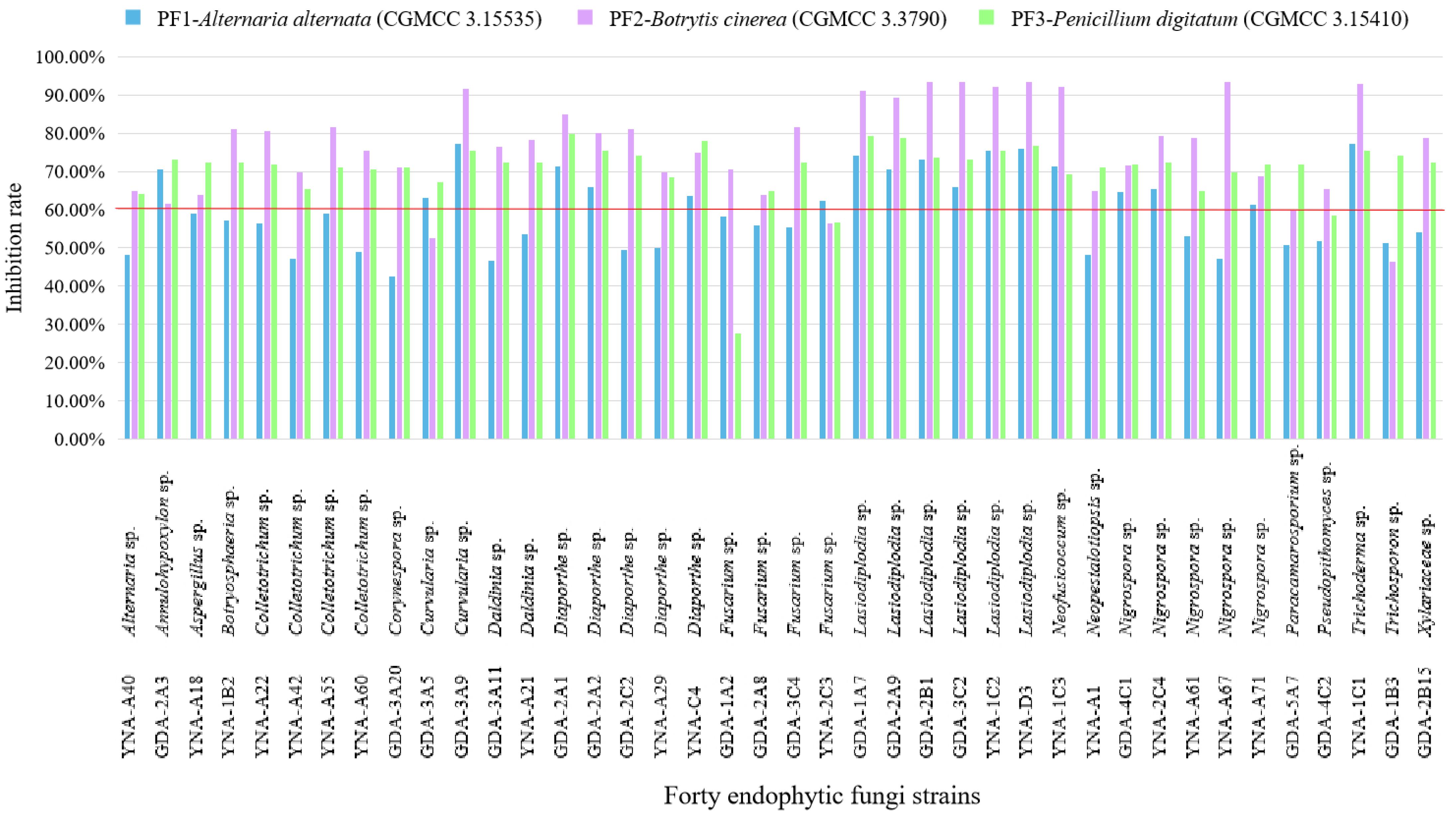
The inhibitory effect (≥60%) of 40 endophytic fungi on pathogenic fungi shows some good results in Figure 4 and Table 3, and the inhibitory effect is ranked as B. cinerea (CGMCC 3.3790) > P. digitatum (CGMCC 3.15410) > A. alternata (CGMCC 3.15535).
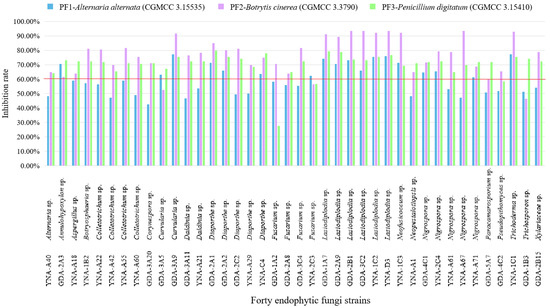
Figure 4.
Inhibition rate of 40 endophytic fungi to three pathogenic fungi. The inhibition ≥60% is considered a good inhibition effect.
For PF1-A. alternata (CGMCC 3.15535), 18 strains showed inhibitory effects (Table 3 and Figure 4), among them, the three strains with the highest inhibition rate are Curvularia sp. (GDA-3A9, 77.07%), Trichoderma sp. (YNA-1C1, 77.07%), and Lasiodiplodia sp. (YNA-D3, 75.90%). Among the 18 strains, the genus Lasiodiplodia has the largest number of strains (six strains).
For PF2-B. cinerea (CGMCC 3.3790), 36 strains showed inhibitory effects (Table 3 and Figure 4), among them, the three strains with the highest inhibition rate are Lasiodiplodia sp. (GDA-3C2, 93.30%), Lasiodiplodia sp. (YNA-D3, 93.30%), and Lasiodiplodia sp. (GDA-2B1, 93.30%). Among the 36 strains, the genus Lasiodiplodia has the largest number of strains (six strains).
For PF3-P. digitatum (CGMCC 3.15410), 38 strains showed inhibitory effects (Table 3 and Figure 4), among them, the three strains with the highest inhibition rate are Diaporthe sp. (GDA-2A1, 79.87%), Lasiodiplodia sp. (GDA-1A7, 79.25%), and Lasiodiplodia sp. (GDA-2A9, 78.62%). Among the 38 strains, the genus Lasiodiplodia has the largest number of strains (six strains).
To sum up, the endophytic fungi used in this test have a good inhibitory effect on PF2-B. cinerea (CGMCC 3.3790), which can reach a 93.30% inhibition rate, however, for PB3-S. enterica (CGMCC 1.10603), there was almost no inhibitory effect, and the highest inhibitory rate was 63.33%. Among the inhibition results of endophytic fungi on these six pathogens, it can be seen that most fungi with inhibitory effect belong to the genus Lasiodiplodia, and Lasiodiplodia sp. (YNA-D3) showed the best inhibition effect on pathogens (anti-PB1 63.47%, anti-PB2 74.07%, anti-PB3 63.33%, anti-PF1 75.90%, anti-PF2 93.30%, and anti-PF3 76.73%).
4. Discussion
The 47 endophytic fungal strains isolated from agarwood were tested against six bacterial and fungal pathogens. The reasons for selecting these six pathogens are: few studies have been carried out on the pathogens of A. sinensis trees, thus no pathogenic strains of A. sinensis are available to be used, and these six pathogens can cause severe damages, their hosts and distribution are very wide and common [42,48,53,55].
The results of the dual culture assay showed that 40 endophytic fungi strains with antimicrobial activities out of 47 strains belong to 18 genera viz. Alternaria Nees, Annulohypoxylon Y.M. Ju, J.D. Rogers & H.M. Hsieh, Aspergillus P. Micheli ex Haller, Botryosphaeria Ces. & De Not., Colletotrichum Corda, Corynespora Güssow, Curvularia Boedijn, Daldinia Ces. & De Not., Diaporthe Nitschke, Fusarium Link, Lasiodiplodia, Neofusicoccum Crous, Slippers & A.J.L. Phillips, Neopestalotiopsis Maharachch., K.D. Hyde & Crous, Nigrospora Zimm., Paracamarosporium Wijayaw. & K.D. Hyde, Pseudopithomyces Ariyaw. & K.D. Hyde, Trichoderma Pers., and Trichosporon Behrend while one strain was identified as Xylariaceae Tul. & C. Tul., while their inhibitory effects on different pathogens were identified as different (Table 3). Among them, the strains of six genera (Curvularia, Diaporthe, Lasiodiplodia, Neofusicoccum, Nigrospora, and Trichoderma) showed relatively significant inhibition effects (Table 3) and the most significant of which is Lasiodiplodia sp. (YNA-D3), which can inhibit all six pathogens.
In previous studies, some agarwood endophytic fungal strains have been shown to have antimicrobial properties that are consistent with our results viz. Botryosphaeria rhodina [12], Colletotrichum sp. [12], Diaporthe sp. [60], Fusarium equiseti (Corda) Sacc. [61], F. oxysporum [12,61], F. solani (Mart.) Sacc. [61], F. verticillioides (Sacc.) Nirenberg [62], Lasiodiplodia theobromae (Pat.) Griffon & Maubl. [61], and Xylaria mali Fromme [63].
In addition, in this study, this is the first time that 13 genera of agarwood endophytic fungi are reported for antimicrobial activities viz. Alternaria, Annulohypoxylon, Aspergillus, Corynespora, Curvularia, Daldinia, Neofusicoccum, Neopestalotiopsis, Nigrospora, Paracamarosporium, Pseudopithomyces, Trichoderma, and Trichosporon. At the same time, nine genera viz. Alternaria, Annulohypoxylon, Corynespora, Daldinia, Neofusicoccum, Neopestalotiopsis, Paracamarosporium, Pseudopithomyces, and Trichosporon were reported as endophytic fungi of agarwood for the first time.
In this study, some potential fungal strains that can be used as biocontrol agents were screened (Table 3). Botrytis cinerea (CGMCC 3.3790) is one of the most destructive pathogens with a large number of hosts [53]. This pathogen is resistant to commonly used synthetic fungicides, so it is necessary to carry out more research on biological control strategies [53,54]. In this study, strains of the five genera viz. Curvularia sp., Lasiodiplodia sp., Neofusicoccum sp., Nigrospora sp., and Trichoderma sp. with inhibition rates to B. cinerea (CGMCC 3.3790) more than 90% were identified. These strains have the potential to be developed into fungicides against B. cinerea (CGMCC 3.3790).
In conclusion, this study enriches the diversity of the endophytic fungi of agarwood and their antagonistic potential against bacterial and fungal pathogens. The most significant fungal strain is Lasiodiplodia YNA-D3 which can inhibit all pathogens and needs further studies to identify and analyze its secondary metabolites with antimicrobial effects. In addition, in-depth studies on the endophytic fungi associated with agarwood are needed to develop effective biocontrol agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8111197/s1, Figure S1: Culture morphologies of 47 endophytic fungal strains obtained in this study (after 10 days on PDA).
Author Contributions
Conceptualization, S.C.K. and S.T.; Data curation, T.-Y.D.; Formal analysis, T.-Y.D.; Funding acquisition, S.C.K., D.-Q.D., N.S., J.-C.X., A.M.E., S.A.-R. and S.T.; Methodology, T.-Y.D. and X.Z.; Software, T.-Y.D.; Supervision, S.C.K. and S.T.; Writing–original draft, T.-Y.D.; Writing–review & editing, S.C.K., X.Z., D.-Q.D., A.M., N.S., J.-C.X., S.L.S., A.M.E., S.A.-R. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number NSFC 31760013, 31950410558, 32260004, High-Level Talent Recruitment Plan of Yunnan Provinces (“Young Talents” Program). The authors extend their appreciation to project number (RSP-2021/120), King Saud University, Riyadh, Saudi Arabia and Chiang Mai University for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to Li Lu and Er-Fu Yang for their help. Tian-Ye Du thanks Mae Fah Luang University for the award of fee-less scholarship. Nakarin Suwannarach thanks Chiang Mai University, Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The IUCN Red List of Threatened Species. Version 2017-3. Available online: www.Iucnredlist.org (accessed on 10 October 2022).
- Kalra, R.; Kaushik, N. A review of chemistry, quality and analysis of infected agarwood tree (Aquilaria sp.). Phytochem. Rev. 2017, 16, 1045–1079. [Google Scholar] [CrossRef]
- Naziz, P.S.; Das, R.; Sen, S. The scent of stress: Evidence from the unique fragrance of agarwood. Front. Plant Sci. 2019, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Azren, P.D.; Lee, S.Y.; Emang, D.; Mohamed, R. History and perspectives of induction technology for agarwood production from cultivated Aquilaria in Asia: A review. J. For. Res. 2018, 30, 1–11. [Google Scholar] [CrossRef]
- Lv, F.F.; Li, S.S.; Feng, J.; Liu, P.W.; Gao, Z.H.; Yang, Y.; Xu, Y.H.; Wei, J.H. Hydrogen peroxide burst triggers accumulation of jasmonates and salicylic acid inducing sesquiterpene biosynthesis in wounded Aquilaria sinesis. J. Plant Physiol. 2019, 234–235, 167–175. [Google Scholar] [CrossRef]
- Rasool, S.; Mohamed, R. Understanding agarwood formation and its challenges. In Agarwood; Mohamed, R., Ed.; Tropical Forestry; Springer: Berlin/Heidelberg, Germany; Singapore, 2016; pp. 39–56, Chapter 3. [Google Scholar] [CrossRef]
- Cui, J.L.; Guo, S.X.; Fu, S.B.; Xiao, P.G.; Wang, M.L. Effects of inoculating fungi on agilawood formation in Aquilaria sinensis. Sci. Bull. 2013, 58, 3280–3287. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; 2015 Version; Chinese Medical Science and Technology Press: Beijing, China, 2015; Volume 1, pp. 185–186. [Google Scholar]
- Du, T.Y.; Dao, C.J.; Mapook, A.; Stephenson, S.L.; Elgorban, A.M.; Al-Rejaie, S.; Suwannarach, N.; Karunarathna, S.C.; Tibpromma, S. Diversity and biosynthetic activities of agarwood associated fungi. Diversity 2022, 14, 211. [Google Scholar] [CrossRef]
- Tibpromma, S.; Zhang, L.; Karunarathna, S.C.; Du, T.Y.; Wang, Y.H. Volatile constituents of endophytic fungi isolated from Aquilaria sinensis with descriptions of two new species of Nemania. Life 2021, 11, 363. [Google Scholar] [CrossRef]
- Hidayat, A.; Turjaman, M.; Faulina, S.A.; Ridwan, F.; Aryanto, A.; Najmulah, N.; Irawadi, T.T.; Iswanto, A.H. Antioxidant and antifungal activity of endophytic fungi associated with agarwood trees. J. Korean Wood Sci. Technol. 2019, 47, 459–471. [Google Scholar] [CrossRef]
- Gong, L.; Guo, S. Endophytic fungi from Dracaena cambodiana and Aquilaria sinensis and their antimicrobial activity. Afri. J. Biotechnol. 2009, 8, 731–736. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Yu, Z.X.; Wang, C.H.; Wu, C.M.; Guo, P.; Wei, J.H. Chemical constituents and pharmacological activity of agarwood and Aquilaria plants. Molecules 2018, 23, 342. [Google Scholar] [CrossRef]
- Du, T.Y.; Karunarathna, S.C.; Hyde, K.D.; Mapook, A.; Wariss, H.M.; Aluthwattha, S.T.; Wang, Y.H.; Mortimer, P.E.; Xu, J.C.; Tibpromma, S. The endophytic fungi of Aquilaria sinensis from southern China. Fungal Biotec 2022, 2, 1–15. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Bhunjun, C.S.; Maharachchikumbura, S.S.N.; Liu, J.K. Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere 2020, 11, 2652–2676. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J.L. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR protocols, a Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Du, T.Y.; Hyde, K.D.; Mapook, A.; Mortimer, P.E.; Xu, J.C.; Karunarathna, S.C.; Tibpromma, S. Morphology and phylogenetic analyses reveal Montagnula puerensis sp. nov. (Didymosphaeriaceae, Pleosporales) from southwest China. Phytotaxa 2021, 514, 001–025. [Google Scholar] [CrossRef]
- Ko, T.W.K.; Stephenson, S.L.; Bahkali, A.H.; Hyde, K.D. From morphology to molecular biology: Can we use sequence data to identify fungal endophytes? Fungal Divers. 2011, 50, 113–120. [Google Scholar] [CrossRef]
- Guo, L.D.; Huang, G.R.; Wang, Y.; He, W.H.; Zheng, W.H.; Hyde, K.D. Molecular identification of white morphotype strains of endophytic fungi from Pinus tabulaeformis. Mycol. Res. 2003, 107, 680–688. [Google Scholar] [CrossRef]
- Guo, L.D.; Hyde, K.D.; Liew, E.C.Y. Detection and taxonomic placement of endophytic fungi within frond tissues of Livistona chinensis based on rDNA sequences. Mol. Phylogenet. Evol. 2001, 20, 1–13. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Bhat, J.D.; Mortimer, P.E.; Xu, J.; Promputtha, I.; Doilom, M.; Yang, J.; Tang, A.M.C.; Karunarathna, S.C. Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. MycoKeys 2018, 33, 25. [Google Scholar] [CrossRef]
- Tibpromma, S.; Karunarathna, S.C.; Bhat, J.D.; Suwannarach, N.; Stephenson, S.L.; Elgorban, A.M.; Al-Rejaie, S.; Xu, J.; Mortimer, P.E. Using culture-dependent and molecular techniques to identify endophytic fungi associated with tea leaves (Camellia spp.) in Yunnan Province, China. Diversity 2022, 14, 287. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7, improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit, a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Glez-Peña, D.; Gómez-Blanco, D.; Reboiro-Jato, M.; Fdez-Riverola, F.; Posada, D. ALTER, program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 2010, 38, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8, a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop (GCE); IEEE Computer Society: New Orleans, LA, USA, 2010; pp. 1–8. [Google Scholar]
- Rambaut, A. 2012—FigTree version 1.4; University of Edinburgh: Edinburgh, Scotland, 2012. [Google Scholar]
- Rahman, M.A.; Begum, M.F.; Alam, M.F. Screening of Trichoderma isolates as a biological control agent against Ceratocystis paradoxa causing pineapple disease of sugarcane. Mycobiology 2009, 37, 277–285. [Google Scholar] [CrossRef]
- Bonn, W.G.; van der Zwet, T. Distribution and economic importance of fire blight. In Fire Blight: The Disease and Its Causative Agent, Erwinia amylovora; Vanneste, J.L., Ed.; CAB International: Wallingford, CT, USA, 2000; pp. 37–53. [Google Scholar] [CrossRef]
- Thomson, S.V. Epidemiology of fire blight. In Fire Blight: The Disease and Its Causative Agent, Erwinia amylovora; Vanneste, J.L., Ed.; CAB International: Wallingford, UK, 2000; pp. 9–36. [Google Scholar] [CrossRef]
- Vanneste, J.L. Fire blight: The disease and its causative agent, Erwinia amylovora; CABI Publishing: Oxfordshire, UK, 2000. [Google Scholar] [CrossRef]
- Oh, C.S.; Beer, S.V. Molecular genetics of Erwinia amylovora involved in the development of fire blight. FEMS Microbiol. Lett. 2005, 253, 185–192. [Google Scholar] [CrossRef]
- Van der Zwet, T.; Orolaza-Halbrendt, N.; Zeller, W. Fire Blight: History, Biology, and Management; APS Press: St. Paul, MN, USA, 2012. [Google Scholar] [CrossRef]
- Born, Y.; Fieseler, L.; Klumpp, J.; Eugster, M.R.; Zurfluh, K.; Duffy, B.; Loessner, M.J. The tail-associated depolymerase of Erwinia amylovora phage L1 mediates host cell adsorption and enzymatic capsule removal, which can enhance infection by other phage. Environ. Microbiol. 2014, 16, 2168–2180. [Google Scholar] [CrossRef]
- Piqué, N.; Miñana-Galbis, D.; Merino, S.; Tomás, J.M. Virulence factors of Erwinia amylovora: A review. Int. J. Mol. Sci. 2015, 16, 12836–12854. [Google Scholar] [CrossRef]
- Kharadi, R.R.; Schachterle, J.K.; Yuan, X.; Castiblanco, L.F.; Peng, J.; Slack, S.M.; Zeng, Q.; Sundin, G.W. Genetic dissection of the Erwinia amylovora disease cycle. Annu. Rev. Phyt. 2021, 59, 191–212. [Google Scholar] [CrossRef]
- Horst, R.K. Westcott’s Plant Disease Handbook, 5th ed.; Chapman & Hall: New York, NY, USA, 1990. [Google Scholar] [CrossRef]
- Hwang, M.S.; Morgan, R.L.; Sarkar, S.F.; Wang, P.W.; Guttman, D.S. Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae. Appl. Environ. Microb. 2005, 71, 5182–5191. [Google Scholar] [CrossRef]
- Kennelly, M.M.; Cazorla, F.M.; de Vicente, A.; Ramos, C.; Sundin, G.W. Pseudomonas syringae diseases of fruit trees: Progress toward understanding and control. Plant Dis. 2007, 91, 4–17. [Google Scholar] [CrossRef]
- Schwartz, K.J. Salmonellosis in swine. Compend Contin. Educ. Pract. Vet 1991, 13, 139–146. [Google Scholar] [CrossRef]
- Rice, D.H.; Besser, T.E.; Hancock, D.D. Epidemiology andvirulence assessment of Salmonella dublin. Vet Microbiol. 1997, 56, 111–124. [Google Scholar] [CrossRef]
- Uzzau, S.; Brown, D.J.; Wallis, T.; Rubino, S.; Leori, G.; Bernard, S.; Casadesús, J.; Platt, D.J.; Olsen, J.E. Host adapted serotypes of Salmonella enterica. Epidemiol Infect 2000, 125, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Knodler, L.A.; Elfenbein, J.R. Salmonella enterica . Trends Microbiol. 2019, 27, 964–965. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.D.; Mohakud, N.K.; Panda, R.K.; Sahu, B.R.; Suar, M. Prevalence and multidrug resistance in Salmonella enterica Typhimurium: An overview in South East Asia. World J. Microb. Biot. 2021, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Twaroch, T.E.; Curin, M.; Valenta, R.; Swoboda, I. Mold allergens in respiratory allergy: From structure to therapy. Allergy Asthma Immunol. Res. 2015, 7, 205–220. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- Gabriel, M.F.; Postigo, I.; Tomaz, C.T.; Martínez, J. Alternaria alternata allergens: Markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ. Int. 2016, 89–90, 71–80. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Rotondo, F.; Gannibal, P.B. Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycol. Prog. 2016, 15, 3. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, P.; Ge, X.; Tian, P. Overview of Alternaria alternata Membrane Proteins. Indian J. Microbiol. 2020, 60, 269–282. [Google Scholar] [CrossRef]
- Sánchez, P.; Vélez-del-Burgo, A.; Suñén, E.; Martínez, J.; Postigo, I. Fungal Allergen and Mold Allergy Diagnosis: Role and Relevance of Alternaria alternata Alt a 1 Protein Family. J. Fungi 2022, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Techn. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Garrido, C.; Collado, I.G. Endophytic microorganisms for biocontrol of the phytopathogenic fungus Botrytis cinerea. Phytochem. Rev. 2020, 19, 721–740. [Google Scholar] [CrossRef]
- Poppe, L.; Vanhoutte, S.; Höfte, M. Modes of action of Pantoea agglomerans CPA-2, an antagonist of postharvest pathogens on fruits. Eur. J. Plant Pathol. 2003, 109, 963–973. [Google Scholar] [CrossRef]
- Ghooshkhaneh, N.G.; Golzarian, M.R.; Mamarabadi, M. Detection and classification of citrus green mold caused by Penicillium digitatum using multispectral imaging. J. Sci. Food Agric. 2018, 98, 3542–3550. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.H.; Bazioli, J.M.; de Moraes Pontes, J.G.; Fill, T.P. Penicillium digitatum infection mechanisms in citrus: What do we know so far? Fungal Biol. UK 2019, 123, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, B.; Liu, H.; Han, J.; Zhang, Y. Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis cinerea. Biol. Control 2017, 105, 27–39. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Shaanker, R.U. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef] [PubMed]
- Monggoot, S.; Popluechai, S.; Gentekaki, E.; Pripdeevech, P. Fungal endophytes: An alternative source for production of Volatile compounds from agarwood oil of Aquilaria subintegra. Microb. Ecol. 2017, 74, 54–61. [Google Scholar] [CrossRef]
- Cui, J.L.; Guo, S.X.; Xiao, P.G. Antitumor and antimicrobial activities of endophytic fungi from medicinal parts of Aquilaria sinensis. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2011, 12, 385–392. [Google Scholar] [CrossRef]
- Chi, H.K.; Cuong, L.H.; Hang, T.T.N.; Luyen, N.D.; Huong, L.M. Biological characterization of fungal endophytes isolated from agarwood tree Aquilaria crassna pierre ex lecomte. Vietnam. J. Biotechnol. 2016, 14, 149–156. [Google Scholar] [CrossRef][Green Version]
- Tian, J.J.; Gao, X.X.; Zhang, W.M.; Wang, L.; Qu, L.H. Molecular identification of endophytic fungi from Aquilaria sinensis and artificial agarwood induced by pinholes-infusion technique. Afr. J. Biotechnol. 2013, 12, 3115–3131. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).