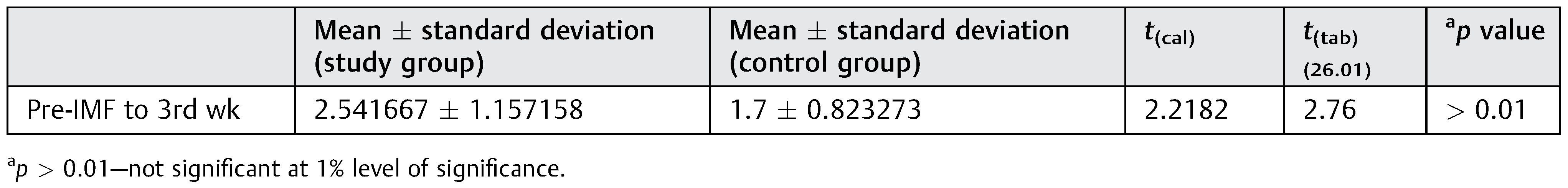The mandible is the most frequently fractured bone in maxillofacial trauma, the treatment of which consists of reduction and fixation of dislocated fragments by open or closed approach. Many techniques of direct and indirect fixation have evolved for the treatment of mandibular fractures over the years. In the 1960s and 1970s, rigid fixation with miniplates and screws replaced the wire ligation techniques which had the distinct advantage of reducing the immobilization period significantly.
Although rigid internal fixation is considered to be the best treatment option, it too carries the risks of infection, loosening and extrusion of screws and miniplates, facial deformity, pain, malunion, or even fracture of the miniplates frequently. Furthermore, it requires an operation by skilled surgeon under general anesthesia. If complications occur, then it may require a second surgery to remove hardware. In addition to the psychological trauma and economic burden, which the patient bears, this technique cannot be applied to the medically compromised patient [
1,
2,
3].
Conservative management of undisplaced or minimally displaced mandibular fracture by intermaxillary fixation (IMF) is therefore still a viable option, unfortunately it requires a prolonged period of immobilization which causes discomfort, weight loss, nutritional deficiency, and most importantly can compromise airway in case of vomiting. Thus, innovative techniques toward reducing the period of the postoperative IMF are being researched.
A relatively unknown treatment that may have an effect on fracture healing is ultrasound. Recent clinical trials have shown that low-intensity pulsed ultrasound (LIPUS) has a positive effect on bone healing.
Findings that low-intensity ultrasound reduces fracture healing time in animal and human trial, mainly in the long bones, have provided further support for this modality [
4,
5,
6,
7,
8,
9]. Very little literature is available on the effect of LIPUS on the healing of mandibular fracture. Some recent studies have shown significant effect of LIPUS on mandibular defects [
10,
11,
12].
In this study, the basic science and the use of low-intensity ultrasound on mandibular fracture were clinically evaluated, reviewed, and a hypothesis was made that nature’s process of fracture healing, while elegant, can be accelerated with respect to achieving the ability to support clinically relevant loads.
Materials and Methods
The present study was conducted on patients with isolated mandibular fractures. The inclusion criteria are as follows:
Young (15–35 years), healthy individuals without any known medical condition which could compromise healing or preclude treatment with IMF, unilateral or bilateral parasymphysis and undisplaced or minimally displaced angle fracture
Dentate patients only in whom reduction could be easily achieved with the IMF
Individuals with fresh fractures (not more than 1 week) and with no infection at site or associated soft tissue injury
Individuals with no fracture or excessive morbidity of the tooth in fracture line.
On the basis of the aforementioned criteria, the study included 28 patients with only mandibular fracture. They were randomly divided into two groups:
At the time of clinical examination, clinical mobility in case of parasymphysis or body fracture was tested by digital manipulation of the fragment and vertical or horizontal distance between two fracture fragments was measured using API’s periodontal pocket depth measuring probe by placing it at the highest occlusal point on any one fragments.
After the necessary investigations, the patient in both the groups underwent IMF using commercially available arch bar (ERICH) under 2% lignocaine with 1:80,000 adrenaline.
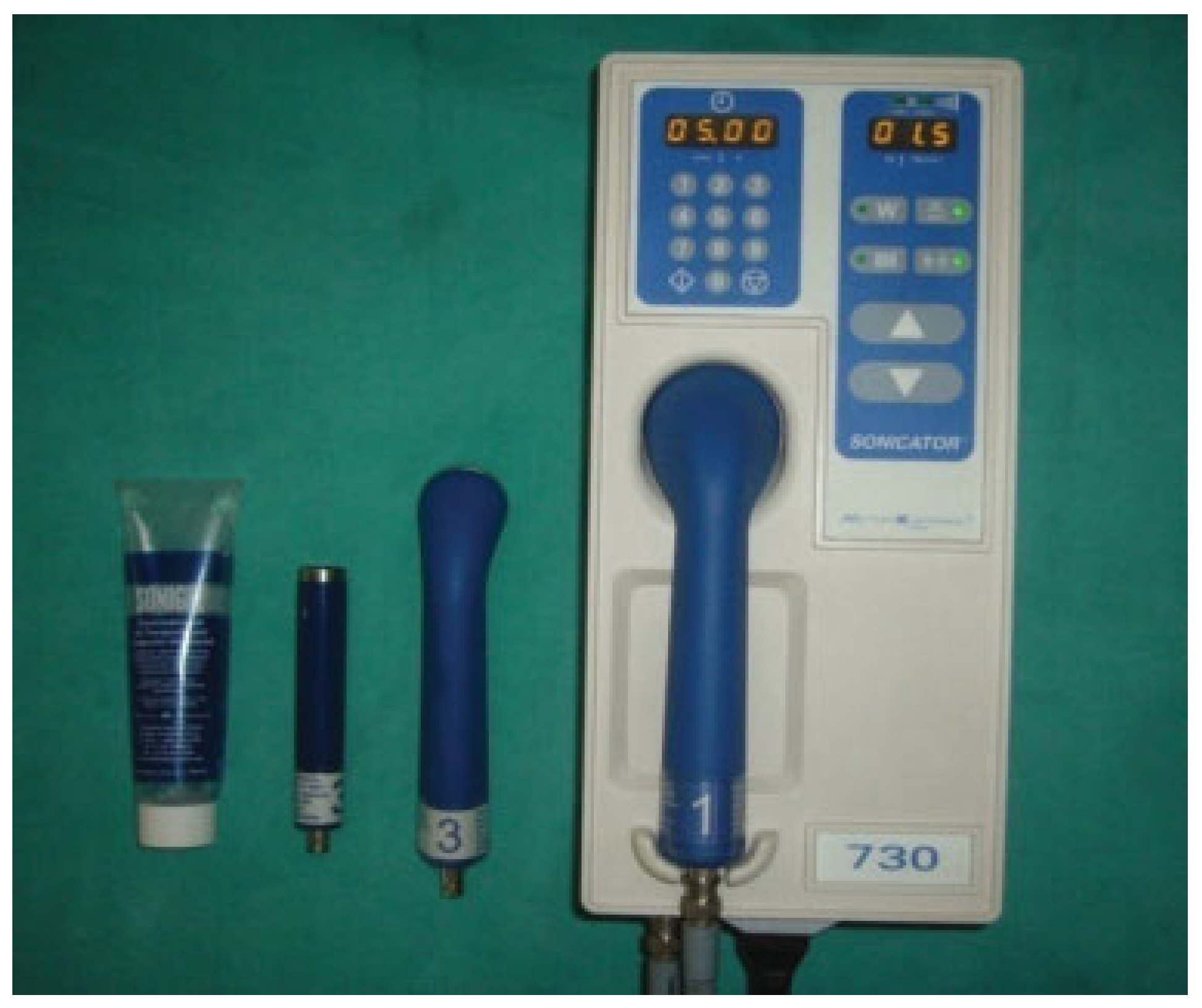
Patients in Group A received ultrasound therapy on an alternate-day basis at the fracture site with a therapeutic ultrasound device Sonicator 730 (Mettler Electronics Corp., Anaheim, CA) (
Figure 1). Approximately 12 sessions of therapy were conducted with ultrasound signal of 1 MHz frequency, with temporal and spatial intensity of 1.5 W/cm
2, and pulsed wave administered for 5 minutes at every alternate day for 24 days.
Radiographic Density at the Fracture Zone
A digital orthopantomogram (OPG) was taken before and after IMF every week for the next 5 weeks in both the groups after obtaining necessary clearance from the institutional ethical committee and were exported into the Emago Image Analysis software, following which they were standardized by the following method: in the pre-IMF, orthopantomogram density of the distobuccal cusp tip of the last erupted tooth was noted and the remaining five orthopantomograms were set accordingly. Mean and standard deviation of the values were calculated and statistically analyzed. Percentage difference in the radiographic density between the pre-IMF and 3rd and 5th weeks’ orthopantomogram was calculated and statistically analyzed.
Pain intensities were evaluated by visual analogue scale with a horizontal line that ran from “no pain” (0) to “worst pain” (10). The patients recorded these measurements themselves in triplicate and average was recorded.
Mean and standard deviation of the values were calculated and statistically analyzed.
Clinical Mobility between Fracture Fragments
Measurements of clinical mobility were designated with a subscript.
As no published method of measuring clinical mobility satisfies all criteria for measuring clinical mobility, it was decided to measure clinical mobility between the fracture fragments by digital manipulation in vertical and horizontal direction within the anatomical limits, and vertical or horizontal displacement was measured with a marked API’s periodontal depth measuring probe by placing it at the highest occlusal point over the tooth adjacent to the fracture line over one fragment. The amount of displacement was assessed with the help of marking over the probe. The same operator, repeating the procedure three times on each patient, made the measurements. The average of the measurement was taken (in mm) and recorded.
Mean and standard deviation were calculated and statistically analyzed. Patients were discharged with usual postoperative instructions and requisite medications. Group A subjects were recalled on every alternate day for ultrasound therapy for 12 sessions and patients in Group B were recalled on post-IMF 1st to 5th weeks. IMF was released after 3rd and 5th post-IMF weeks in Group A and Group B, respectively, for evaluation of healing.
Result
A total of 28 patients were included in this randomized controlled study. These patients were randomly allocated to either of two groups—Group A (the study group) and Group B (the control group). All data relevant to each parameter, that is, radiographic density, pain, and clinical mobility were collected and analyzed statistically using unpaired “t” test. Since swelling and paresthesia were minimal or absent in all patients in both the groups, they were excluded from the statistical analysis.
Analysis of Radiographic Density
The difference between radiographic density at each of the five points at 3rd and 5th weeks was calculated by subtracting the pre-IMF values from the post–3rd- and 5th-week values on digital OPGs for both the groups (n ¼ 28).
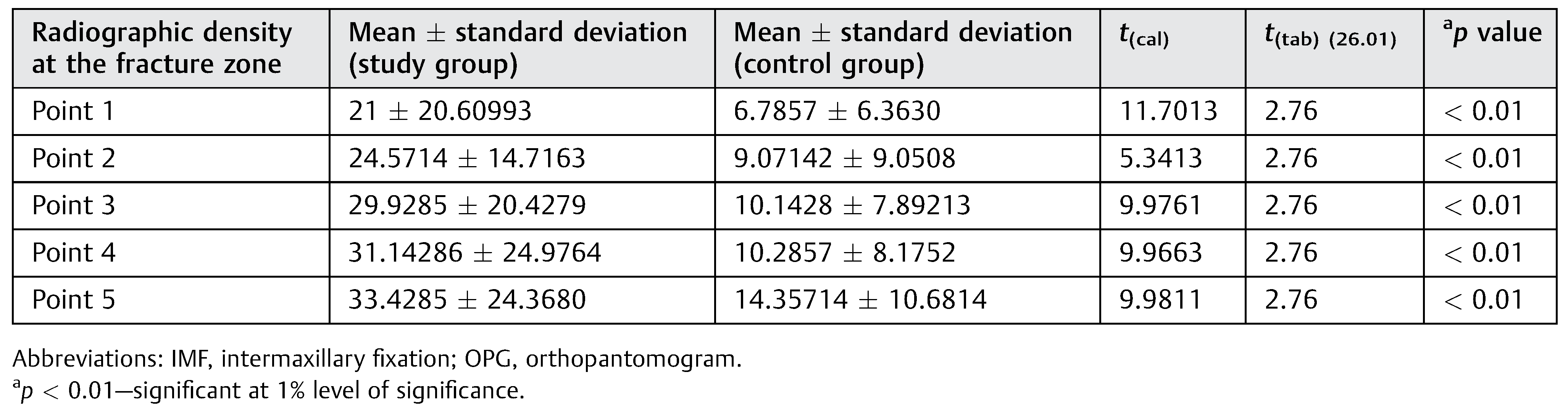
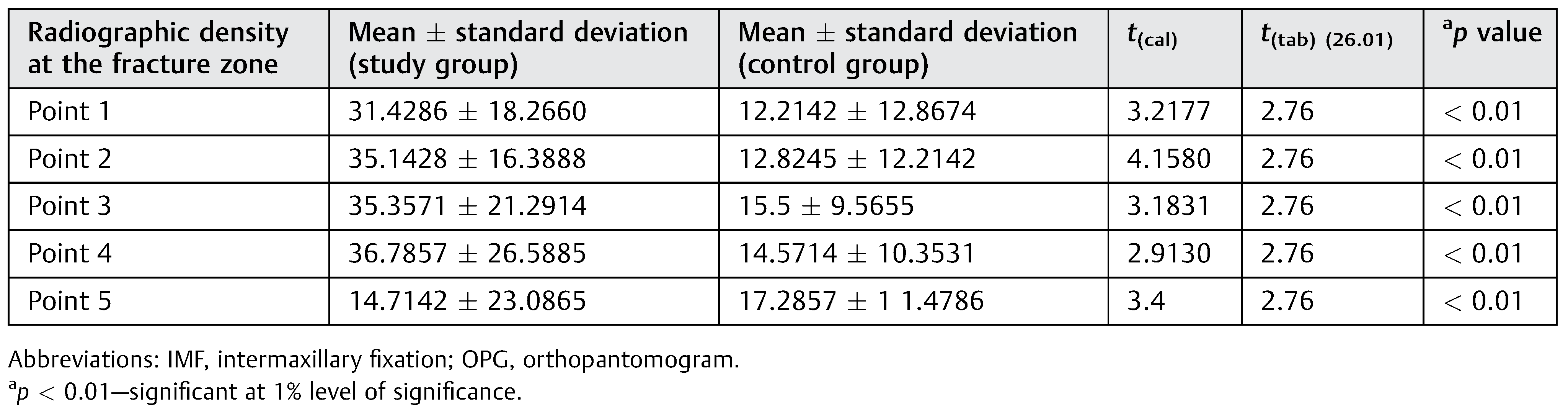
Statistically significant difference was observed for the radiographic density at the fracture zone between the two groups in all the five points studied at 3rd week as well as 5th week at 1% level of significance (
Table 1 and
Table 2). Depicts the radiographic density at the different points in the fracture zone between both the groups.
We can thus conclude that there is higher improvement in radiographic density in Group A compared with Group B at 3rd week and also at 5th-week post-IMF.
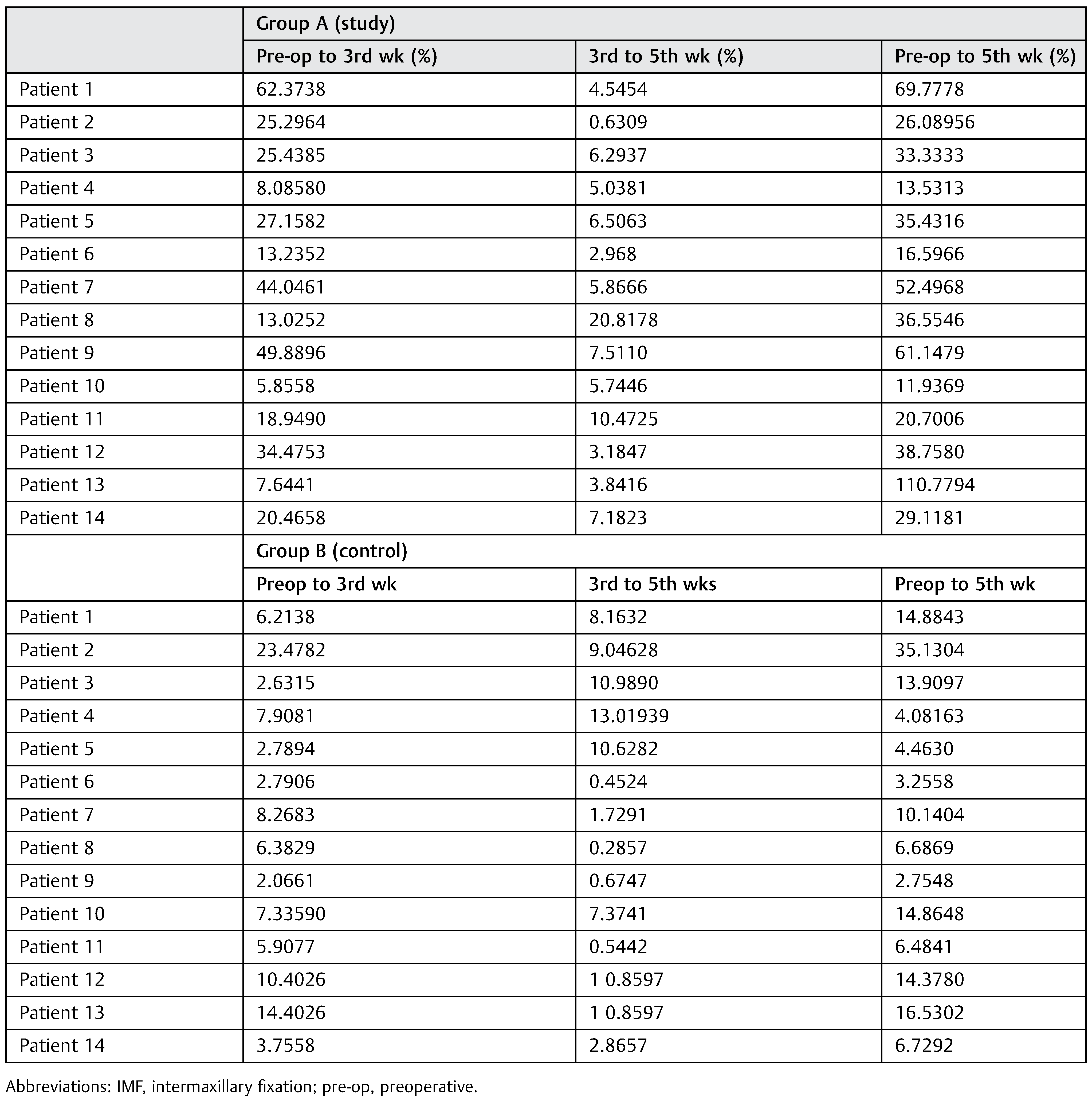
The percentage improvement from pre-IMF radiograph to the 3rd week radiograph, 3rd to 5th weeks radiograph, and from pre-IMF radiograph to 5th week was also calculated as shown in
Table 3. After checking the radiographic density at the above-mentioned points, it can be observed that there is a high percentage of improvement from pre-IMF radiograph to the subsequent radiograph in experimental group compared with the control group.
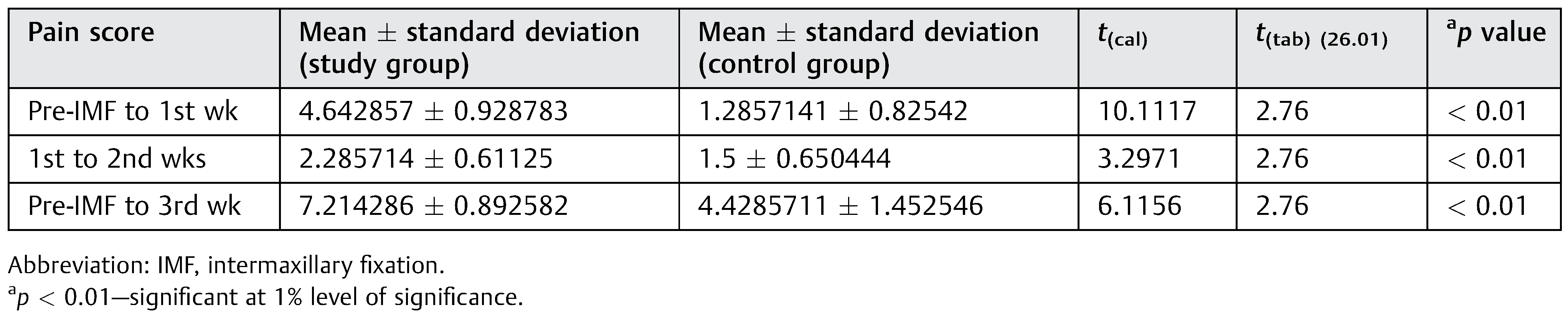
Analysis of Pain Scoring
Pain scores for both the groups were recorded at weekly intervals until the end of 3 weeks. The difference in the scores at the end of each week from the previous week was charted for both groups (
Table 4). There was a statistically significant difference between the two groups at 1% of significance as depicted in
Table 3, suggesting a marked improvement in pain perception in study group compared with the control group at the end of each post-IMF week.
Clinical Mobility
Clinical mobility was evaluated pre-IMF and only after 3 weeks of IMF. If mobility in the fracture fragments was elicited at this stage then re-IMF was done for another 2 to 3 weeks. The difference between the readings between the pre-IMF and after 3 weeks was recorded. However, no significant difference was found in clinical mobility between the control group and study group at 3 weeks (
Table 5). This suggests that at 3 weeks in undisplaced mandibular fractures, the clinical mobility decreases to the same extent irrespective of whether LIPUS has been administered or not.
Complications
Out of 28 patients, only 3 patients reported with post-IMF complications. Two of them were from Group A, in which one patient was young female who had subperiosteal bone formation after 5 weeks of post-IMF at right angle fracture involving the developing tooth germ which was the LIPUS administered site. Patient was under observation for another 1 month without any active intervention and the condition resolved itself. Another male patient, who reported with an infection at the left angle also involving tooth in the fracture line which was LIPUS administered site, was operated for the same under cover of antibiotics. One patient in Group B who had a parasymphysis fracture associated with right condylar fracture reported after 1 year of treatment with reduced mouth opening which was diagnosed as a case of fibrous ankylosis and was lost to further follow-up.
Discussion
Fracture healing is a highly complex regenerative process that is essentially a replay of developmental events. These events include the action of many different cell types, a myriad of proteins, and active gene expression that in majority of cases ultimately will restore bone’s natural integrity [
13].
In 1950, the first study was published in which the relationship between ultrasound and bone healing was investigated. This marked a turning point in research arena because it focused on the possible stimulatory effect of ultrasound on bone, rather than on its harmful effects [
14].
Duarte first reported that LIPUS stimulated the growth of bone healing of fresh fracture in experimental cortical defects and fibular osteotomy in rabbits and nonunions in humans [
4]. In 1992, Harris did an observational study using therapeutic ultrasound with intensity of 1,000 mW/cm
2 with pulsed wave in 24 patients with mandibular osteoradionecrosis and found that 48% of patients were spared surgery [
10]. In 1994, doubleblind randomized controlled trial on 67 patients having fresh tibial fracture using LIPUS with intensity of 30; 150 mW/cm
2 with pulsed wave found 38% reduction in healing time [
14]. In 1997, Kristiansen et al. conducted double-blind randomized controlled trial on 61 patients having fresh distal radial fractures using LIPUS with intensity of 30; 150 mW/cm
2 with pulsed wave found 38% reduction in healing time [
5]. In 1997, Cook et al. done double-blind randomized controlled trial on 67 patients having fresh tibial fractures and fresh distal radial fractures using LIPUS with intensity of 30; 150 mW/cm
2 with pulsed wave found 41% reduction in healing time [
15].
In 2006, Erdogan et al. conducted animal study in 30 rabbits in whom osteotomy was performed followed by LIPUS administration for 20 minutes daily, for 20 days in which ultrasound signal consisted of 1.5 MHz pressure wave administered in pulses of 200 µs with an average temporal and spatial intensity of 30 mW/cm
2 and found significant improvement in bone healing in those animals in whom LIPUS was administered [
12].
On the basis of these investigations, the US Food and Drug Administration approved the marketing of therapeutic ultrasound in the treatment of fresh fractures in certain patients [
7].
Previously, therapeutic ultrasound was considered an absolute contraindication for fractures on the basis of early animal studies that have found that therapeutic ultrasound delays healing and damages healing bone, despite some contradictory findings, although it can be used with minimal risk to the patients. Recent work, however, has shown that the effect of therapeutic ultrasound on healing bone is determined by its intensity.
Ultrasound can be defined as sound wave or pressure wave with a frequency above the limit of the human hearing range (16–20 kHz).The unit of ultrasound is Hertz or cycle per second. Being a propagating pressure wave, ultrasound is capable of transferring mechanical energy into the tissues. The energy of the ultrasound signal is transferred, propagated, or reflected depending on its frequency. Ultrasound can be divided into three types: (1) Diagnostic ultrasound uses a frequency between 3 and 5 MHz and low intensity (1–50 mW/cm2) to avoid tissue heating; (2) disruptive ultrasound, such as those used in ultrasonic cleaning devices, uses a very low frequency (20–60 kHz), and high intensity above 8 W/cm2; and (3) therapeutic ultrasound, as used in medicine and physiotherapy, usually uses frequencies between 1 and 3 MHz and intensities of 0.1 to 2.0 W/cm2.
In case of ultrasound therapy, the mechanical stimulation inherent to ultrasound waves translates into a biological response.
Wide ranging studies at both the in vitro and in vivo levels have been used to probe the biological mechanism responsible for the observed influence of ultrasound on fracture healing.
In one of the first such study, Chapman et al. reported that ultrasound induced a change in the rates of influx and efflux of potassium ion in rat thymocytes [
16]. Schortinghuis et al. later reported that low-intensity ultrasound increased calcium incorporation in both differentiating cartilage and bone cell cultures, reflecting a change in cell metabolism [
14]. Fyfe et al. showed reduction in experimental edema in rats [
22]. Dinno et al. reported cellular membrane changes in the form of increased in the intracellular concentration of the calcium [
23]. It is generally believed that greater blood flow serves as a principal factor in acceleration of the fracture healing and indeed, one of the main biological goal of inflammatory response is to re-establish the blood supply to the injured area so in the view of this, Young et al. reported increased angiogenesis in the form of more number of new blood vessel formation after application of the LIPUS [
24].
While these experiments demonstrate the ability of ultrasound to influence the cell activity, if signal is ultimately going to influence the rate of healing then ultrasound must be shown to effect the expression of genes involved in the inflammation and remodeling stages of the fracture repair. In support of this critical point, exposure of the cultured chondrocytes to low-intensity ultrasound stimulates an upregulation of aggrecan gene expression which occur earlier in the fracture healing process [
14]. This might explain the role of ultrasound in augmenting endochondral ossification and thus increasing the mechanical strength and overall repair of the fractured bone. It would likely to be misleading to overemphasize the impact of single gene.
This in vivo prospective study investigates the efficacy of the therapeutic ultrasound on reduction in the time of fracture-healing process, pain, and clinical mobility following the earliest IMF after an injury to the mandible.
Assessment of the radiographic density, pain, and clinical mobility showed improvement with LIPUS. The effect of the ultrasound in increase in radiographic density, reduction in pain, and immobility of the fracture fragment was more pronounced after 3 weeks of post-IMF. These effects became less obvious toward the 4th and 5th weeks post-IMF, when data collected from the study group were similar to the control group.
We gave minimal dose of the ultrasound (1.5 W/cm2) to minimize the overheating of the tissue and possible cavitation formation in the bone. This dose does not overheat the tissue and therefore poses no risk to the patient. We had selected this dose as per the manufacture’s guidelines in the application of the ultrasound in the head and neck region.
The head region contains delicate tissue, such as the tissues of senses and the brain. Therefore, care should be taken when ultrasound is applied to this region. Reported adverse effects in the maxillofacial region following the treatment of soft tissue and temporomandibular disorder were associated with the use of high intensities of ultrasound in the order of magnitude of several watts per square centimeter. Hemorrhages in the muscle, dizziness, nausea, and headache have been reported. However, the low intensity currently used to stimulate bone healing has not been shown to cause any harmful effect [
14].
For more than 50 years, low-intensity therapeutic ultrasound is being investigated for its effect on reducing the duration of fracture healing and its use in case of malunion and nonunions. Several studies have been reported in the literature on this subject. Most studies have reported that LIPUS significantly reduced the time of fracture healing in long bones, while a few studies have shown no benefit from the administration of ultrasound [
11]. These studies are difficult to compare because they have used a different dose of therapeutic ultrasound and followed a different study design for the assessment of the healing period. Till date, none of the studies has published the effect of LIPUS on mandibular fracture.
However, it is clear that dose, duration, and frequency of the ultrasound application may have significant impact on the efficacy of the ultrasound. In addition to this expert guidance and patient’s compliance, requirement of such device is mandatory for this treatment modality.
Reluctance for not using therapeutic ultrasound as one of the treatment modality in fracture healing among the current practitioners lie in the supposed harmful effect, cost, lack of evidence, limited efficacy, and lack of availability.
Radio densitometry or radiographic density has been used for assessment of bone healing in several studies [
5,
11,
17,
18]. The digital radiography technique provides standardization of the radiographic image by eliminating the difference caused by the development process used in conventional radiography. By the aid of image analysis software, it is possible to quantitative data regarding the radiological images (Emago, image analysis software). In a well-controlled trial with subjects serving their own control, Erdogan et al. reported improvement in radio density followed by application of the LIPUS. Kristiansen et al. reported increase in bone mineral content in distal radial fracture followed by application of LIPUS. We assessed radiographic density of five different points at the fracture zone in six radiographs taken at weekly interval. Significant improvement was observed after 3rd and 5th post-IMF in Group A compared with the Group B (
p < 0.01), Similar pattern was observed in the 4th and 5th weeks after IMF. These measurements showed that radiographic density in the LIPUS treated group is 21.64% more improvement in radiographic density in Group A compared with the Group B after 3 weeks of post-IMF.
These finding coincides with the survey conducted on time of fracture healing by Busse and Bhandari that healing rate is usually faster at the 2nd, 3rd, and 4th weeks after an injury then it becomes slower [
19]. These findings support the views of previous research work that therapeutic ultrasound shortens the inflammatory phase and speed up the reparative phase.
Acute posttraumatic pain following an injury is predominantly a consequence of inflammation. The role of therapeutic ultrasound is proven in musculoskeletal disorders, which are being practiced in the field of physiotherapy as an effective therapeutic modality. It has also been used in case of official pain dysfunction syndrome and in temporomandibular joint disorders. In our study, we found significant difference in pain reduction in study group compared with the control group after 1st, 2nd, and 3rd weeks, which is in absolute agreement with previous study regarding the assessment of pain following ultrasound therapy [
20]. Pain reduction was more rapid in LIPUS treated group compared with the control group after 2 weeks of ultrasound therapy.
Mobility of fracture fragments is attributed to the discontinuity of the fractured bone, which gets consolidated and immobile after the adequate healing have occurred. In our study, mobility of the fractured fragment was measured clinically before an IMF and after 3 weeks, an observations in our study were statistically not significant (
p > 0.01) that suggests that in both groups, adequate clinical immobility was achieved irrespective of whether LIPUS was administered or not. Our results regarding the clinical mobility did not correlate with the previous study in which mechanical strength and stiffness of the bone were found to be increased in ultrasound-treated subjects [
9,
12,
21]. This may be true because we have applied LIPUS in undisplaced fractures or minimally displaced fractures, which tend to consolidate earlier displaced fractures even without any stimulation of healing process.
Conclusion
The use of LIPUS therapy to accelerate fracture healing is widely documented and highly recommended in case of long bones as well as in controlled animal trials. In maxillofacial trauma, usage of this treatment modality is rare due to the perceived lack of evidence and unavailability of therapeutic device. The therapy can be used for select cases, that is, undisplaced or minimally displaced mandibular fracture, medically compromised patient, in patient who cannot afford the expensive treatment options such as fixation under general anesthesia or when the patient is judged to be at higher risk of postoperative infection fracture site, etc.
We analyzed the effect of administration of LIPUS in undisplaced or minimally displaced mandibular fracture comparing the treatment results to control group. Our treatment results revealed significant improvement in healing rate of fractured mandible in LIPUS administered group and this was supported by radiographic density findings which indicates higher amount of woven bone in experimental group compared with the control group. Likewise, significant reduction in pain perception rapidly in LIPUS administered group raised patient’s compliance and their positive view toward treatment results too. A consolidation of fracture fragment in terms of clinical mobility also found clinically significant although there was no difference at 3 weeks. An observation of therapy result inspired us to reduce the IMF period to 3 weeks instead of 5 weeks.
In conclusion, results of our study suggest that the application of LIPUS to the mandibular fracture may have considerable contributions to bone healing in healthy individuals.
Although limited evidence is available to support the susceptibility of maxillofacial bone to the ultrasound signal, ultrasound may be of value in the treatment of delayed unions, in callus maturation after distraction, and in the treatment of osteoradionecrosis. Given the success in the stimulation of fracture healing in other parts of body, it seems that additional research in maxillofacial region may lead to promising result and will determine the feasibility and potential of ultrasound treatment in this field. As our sample size was small, further studies are required to collaborate the results of this study.