Three-Dimensional Volumetric Restoration by Structural Fat Grafting
Abstract
:Patients and Methods
Technique
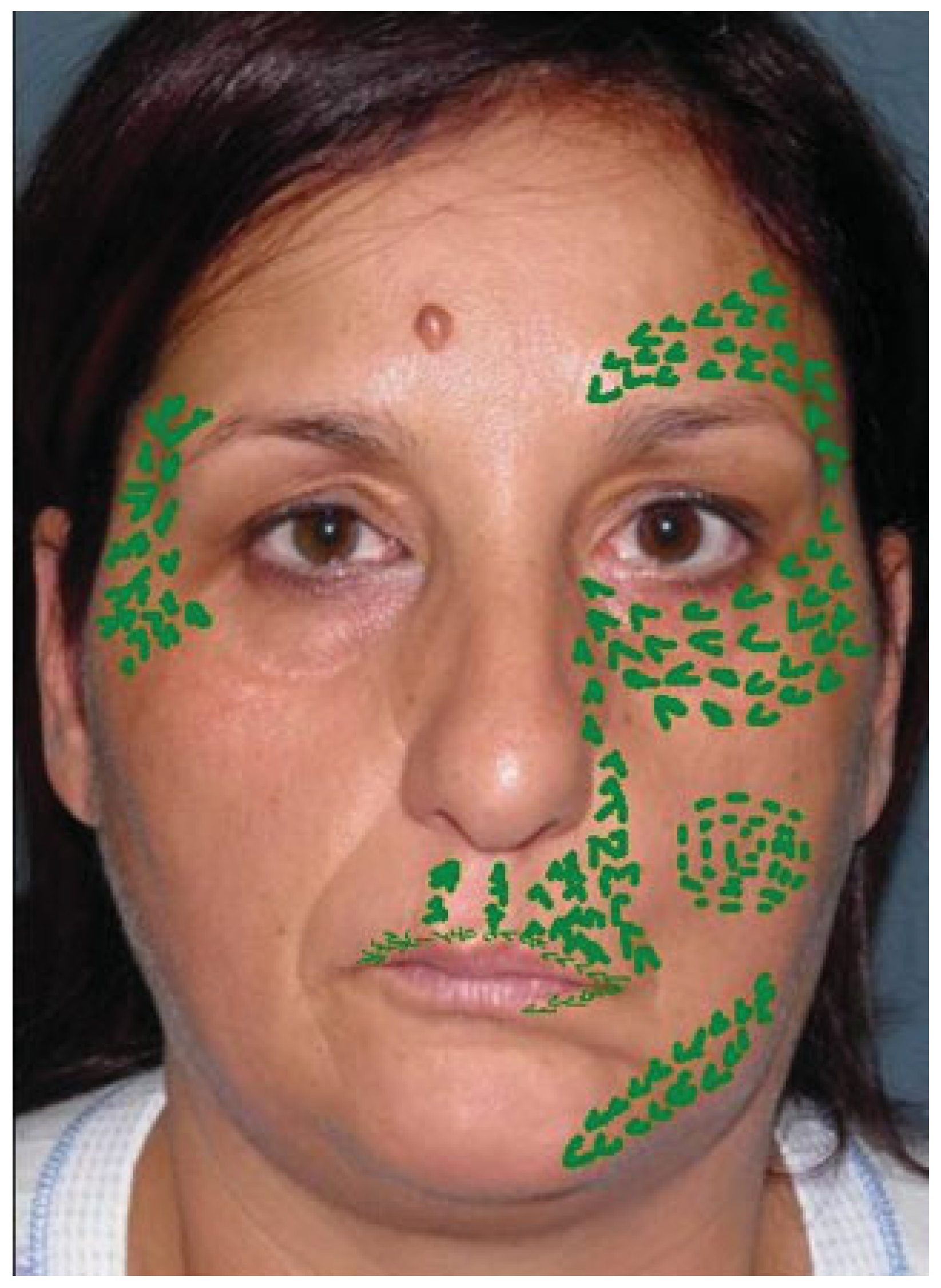
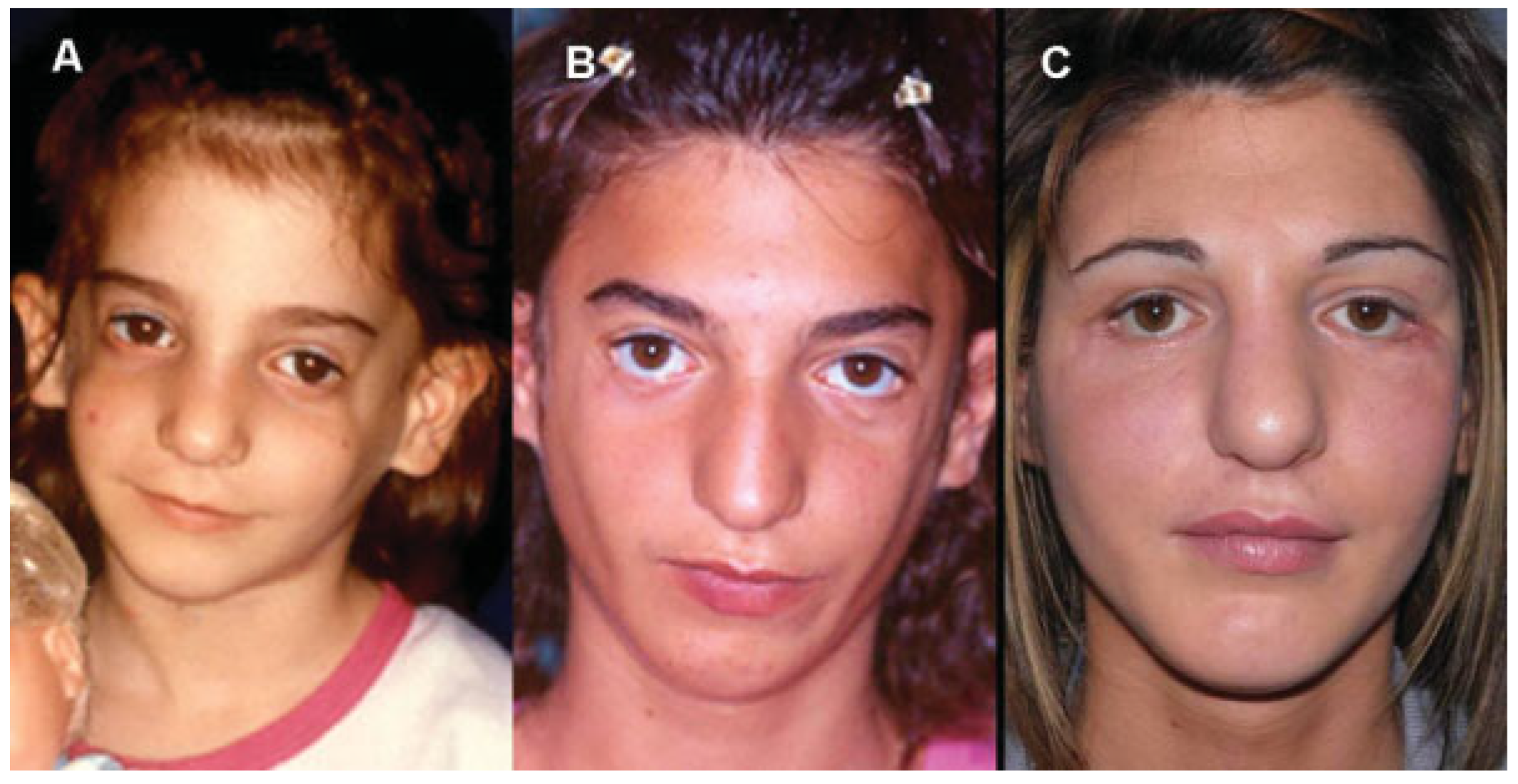
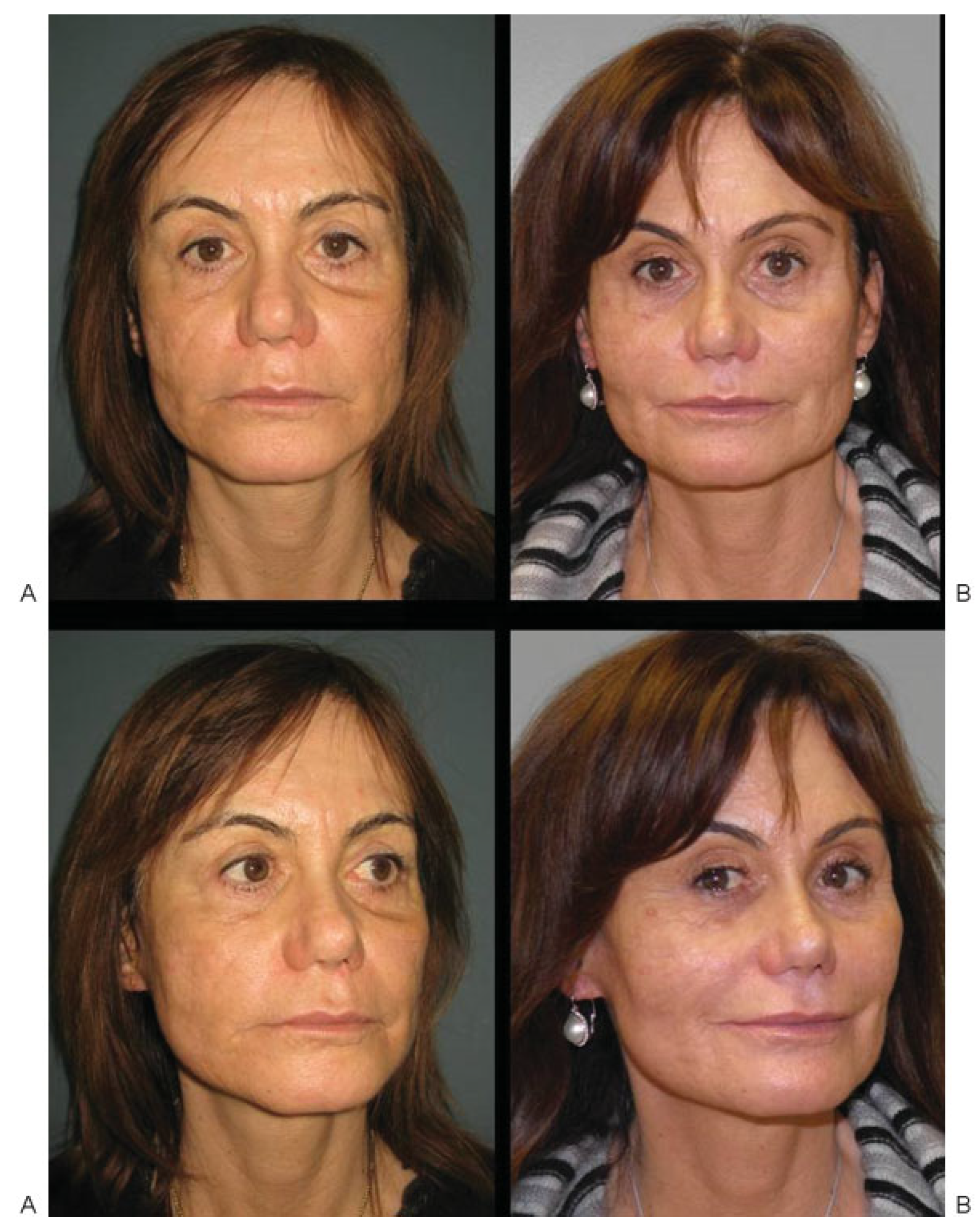
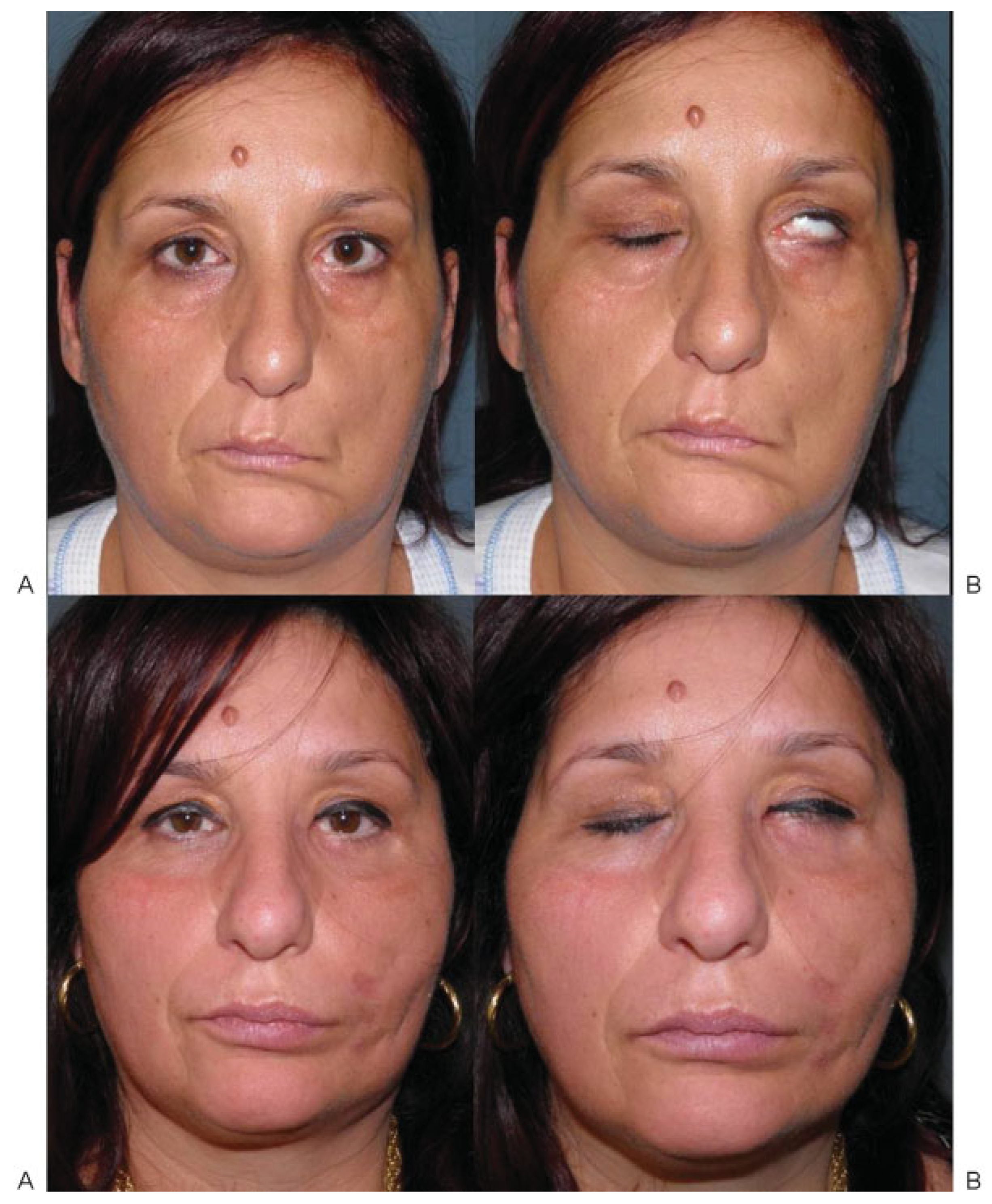
Case 1
Case 2
Case 3
Case 4
Discussion
Conclusion
References
- Coleman, S.R. Facial recontouring with lipostructure. Clin Plast Surg 1997, 24, 347–367. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural fat grafts: the ideal filler? Clin Plast Surg 2001, 28, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. (Ed.) Structural Fat Grafting; Quality Medical Publishing Inc: Saint Louis, MO, 2004. [Google Scholar]
- Rigotti, G.; Marchi, A.; Sbarbati, A. Adipose-derived mesenchymal stem cells: past, present, and future. Aesthetic Plast Surg 2009, 33, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med 2005, 54, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Moseley, T.A.; Zhu, M.; Hedrick, M.H. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg 2006, 118 (Suppl. 3), 121S–128S. [Google Scholar] [CrossRef]
- Clauser, L.C.; Tieghi, R.; Galiè, M.; Carinci, F. Structural fat grafting: facial volumetric restoration in complex reconstructive surgery. J Craniofac Surg 2011, 22, 1695–1701. [Google Scholar] [CrossRef]
- Clauser, L.; Sarti, E.; Dallera, V.; Galiè, M. Integrated reconstructive strategies for treating the anophthalmic orbit. J Craniomaxillofac Surg 2004, 32, 279–290. [Google Scholar] [CrossRef]
- Clauser, L.; Tieghi, R. Optimizing maxillofacial and craniofacial results. Presented at: American Society of Plastic Surgeons Annual Meeting, Seattle, WA, October 2009. [Google Scholar]
- Clauser, L. Optimizing maxillofacial and craniofacial results. In Fat Injection From Filling to Regeneration; Coleman, S.R., Mazzola, R.F., Eds.; Quality Medical Publishing Inc: Saint Louis, MO, 2009; pp. 475–500. [Google Scholar]
- Clauser, L.; Polito, J.; Mandrioli, S.; et al. Structural fat grafting in complex reconstructive surgery. J Craniofac Surg 2008, 19, 187–191. [Google Scholar] [CrossRef]
- Clauser, L.C.; Tieghi, R.; Consorti, G. Parry-Romberg syndrome: volumetric regeneration by structural fat grafting technique. J Craniomaxillofac Surg 2010, 38, 605–609. [Google Scholar] [CrossRef]
- Consorti, G.; Tieghi, R.; Clauser, L.C. Frontal linear scleroderma: longterm result in volumetric restoration of the fronto-orbital area by structural fat grafting. J Craniofac Surg 2012, 23, e263–e265. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, C.; Benitez, S.; Wisnia, P.; Sorolla, J.P.; Acosta, S.; Andrades, P. Liposuction and lipoinjection treatment for congenital and acquired lipodystrophies in children. Plast Reconstr Surg 2009, 124, 134–143. [Google Scholar] [CrossRef]
- Coleman, S.R.; Saboeiro, A.; Sengelmann, R. A comparison of lipoatrophy and aging: volume deficits in the face. Aesthetic Plast Surg 2009, 33, 14–21. [Google Scholar] [CrossRef]
- Foyatier, J.L.; Mojallal, A.; Voulliaume, D.; Comparin, J.-P. [Clinical evaluation of structural fat tissue graft (Lipostructure) in volumetric facial restoration with face-lift About 100 cases]. Ann Chir Plast Esthet 2004, 49, 437–455. [Google Scholar] [CrossRef]
- Bucky, L.P.; Kanchwala, S.K. The role of autologous fat and alternative fillers in the aging face. Plast Reconstr Surg 2007, 120 (Suppl. 6), 89S–97S. [Google Scholar] [CrossRef] [PubMed]
- Mojallal, A.; Foyatier, J.L. [The effect of different factors on the survival of transplanted adipocytes]. Ann Chir Plast Esthet 2004, 49, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.L.; Coleman, S.R.; Cui, X.; Ferguson, R.E., Jr.; Vasconez, H.C. Autologous fat grafts harvested and refined by the Coleman technique: a comparative study. Plast Reconstr Surg 2008, 122, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Mojallal, A.; Auxenfans, C.; Lequeux, C.; Braye, F.; Damour, O. Influence of negative pressure when harvesting adipose tissue on cell yield of the stromal-vascular fraction. Biomed Mater Eng 2008, 18, 193–197. [Google Scholar] [CrossRef]
- Mojallal, A.; Shipkov, C.; Braye, F.; Breton, P.; Foyatier, J.L. Influence of the recipient site on the outcomes of fat grafting in facial reconstructive surgery. Plast Reconstr Surg 2009, 124, 471–483. [Google Scholar] [CrossRef]
- Kawamoto, H.K. Fat injection and craniofacial surgery. In Fat Injection From Filling to Regeneration; Coleman, S.R., Mazzola, R.F., Eds.; Quality Medical Publishing Inc: Saint Louis, MO, 2009; pp. 447–474. [Google Scholar]
- Coleman, S.R. Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 1995, 19, 421–425. [Google Scholar] [CrossRef]
- Fagrell, D.; Eneström, S.; Berggren, A.; Kniola, B. Fat cylinder transplantation: an experimental comparative study of three different kinds of fat transplants. Plast Reconstr Surg 1996, 98, 90–96, discussion 97–98. [Google Scholar] [CrossRef]
- Gutowski, K.A. ASPS Fat Graft Task Force. Current applications and safety of autologous fat grafts: a report of the ASPS fat graft task force. Plast Reconstr Surg 2009, 124, 272–280. [Google Scholar]
- Glasgold, R.A.; Glasgold, M.J.; Lam, S.M. Complications following fat transfer. Oral Maxillofac Surg Clin North Am 2009, 21, 53–58, vi. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Yang, H.N.; Kim, J.C.; Shyn, K.H. Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol 1996, 80, 1026–1027. [Google Scholar] [CrossRef] [PubMed]
- Caviggioli, F.; Klinger, F.; Villani, F.; Fossati, C.; Vinci, V.; Klinger, M. Correction of cicatricial ectropion by autologous fat graft. Aesthetic Plast Surg 2008, 32, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A. The adipose-derived stem cell: looking back and looking ahead. Mol Biol Cell 2010, 21, 1783–1787. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Galiè, M.; et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg 2007, 119, 1409–1422, discussion 1423–1424. [Google Scholar] [CrossRef]
- Kokai, L.E.; Rubin, J.P.; Marra, K.G. The potential of adipose-derived adult stem cells as a source of neuronal progenitor cells. Plast Reconstr Surg 2005, 116, 1453–1460. [Google Scholar] [CrossRef]

© 2013 by the author. The Author(s) 2013.
Share and Cite
Clauser, L.C.; Consorti, G.; Elia, G.; Galié, M.; Tieghi, R. Three-Dimensional Volumetric Restoration by Structural Fat Grafting. Craniomaxillofac. Trauma Reconstr. 2014, 7, 63-69. https://doi.org/10.1055/s-0033-1356757
Clauser LC, Consorti G, Elia G, Galié M, Tieghi R. Three-Dimensional Volumetric Restoration by Structural Fat Grafting. Craniomaxillofacial Trauma & Reconstruction. 2014; 7(1):63-69. https://doi.org/10.1055/s-0033-1356757
Chicago/Turabian StyleClauser, Luigi C., Giuseppe Consorti, Giovanni Elia, Manlio Galié, and Riccardo Tieghi. 2014. "Three-Dimensional Volumetric Restoration by Structural Fat Grafting" Craniomaxillofacial Trauma & Reconstruction 7, no. 1: 63-69. https://doi.org/10.1055/s-0033-1356757
APA StyleClauser, L. C., Consorti, G., Elia, G., Galié, M., & Tieghi, R. (2014). Three-Dimensional Volumetric Restoration by Structural Fat Grafting. Craniomaxillofacial Trauma & Reconstruction, 7(1), 63-69. https://doi.org/10.1055/s-0033-1356757


