Impact of Therapeutic Alcohol Administration on Perioperative Quality of Life (QoL) and Fracture Healing in Patients with Alcohol Use Disorder Undergoing Surgery for Maxillofacial Trauma—A Randomized Pilot Trial
Abstract
1. Introduction
2. Materials and Methods
- Adults aged 25–50 years, taking into consideration the official age in India for alcohol consumption (21 years) and the age range with peak bone mass in the adult skeleton (20–50 years) [9]
- Diagnosed with AUD—mild and moderate grade (DSM-5 criteria) [10]
- AUDIT scale—risk zone 1, 2, and 3 (Alcohol Use Disorder Identification Test by WHO) [11]
- CIWA-Ar score < 14 (Clinical Institute Withdrawal Assessment of Alcohol Scale—Revised) [12]
- Daily intake of at least 60 g of Ethanol (150 mL of distilled spirit) for the last 1 year
- ASA Grade III (due to alcoholism)
- GCS score of 15 and ambulatory and
- Diagnosed with isolated mandibular fractures requiring surgical intervention.
- Patients less than 21 years and greater than 50 years
- Patients with head injury and polytrauma
- Patients with systemic problems that compromise wound healing
- Patients with “drinking in moderation” (2 drinks or less in a day for men; 1 drink or less for women, according to the National Institute on Alcohol Abuse and Alcoholism)
- Patients with smoking
- Patients who are non-compliant with the perioperative protocol
- AUDIT zone 4, CIWA-Ar score > 14
- AST/ALT ratio more than 2 (AST—aspartate aminotransferase; ALT—alanine aminotransferase)
- Patients not consenting to enrolment into the study
2.1. Study Design
2.2. MTAA Protocol
2.3. Surgical Technique and Postoperative Management
2.4. Patient Safety Protocol
2.5. Outcome Measures
2.5.1. Perioperative Stress
2.5.2. Quality of Life
2.5.3. Soft Tissue Healing
2.5.4. Hard Tissue Healing
- Serum osteocalcin levels were measured via ELISA preoperatively and on postoperative day 30.
- Radiographic assessment was performed using orthopantomograms at the preoperative phase, 4th and 8th weeks postoperatively, and graded using Moed’s Scale [19]: Score 1: No callus formation, 2: Minimal callus, 3: Moderate callus, and 4: Complete callus bridging.
2.6. Statistical Analysis
3. Results
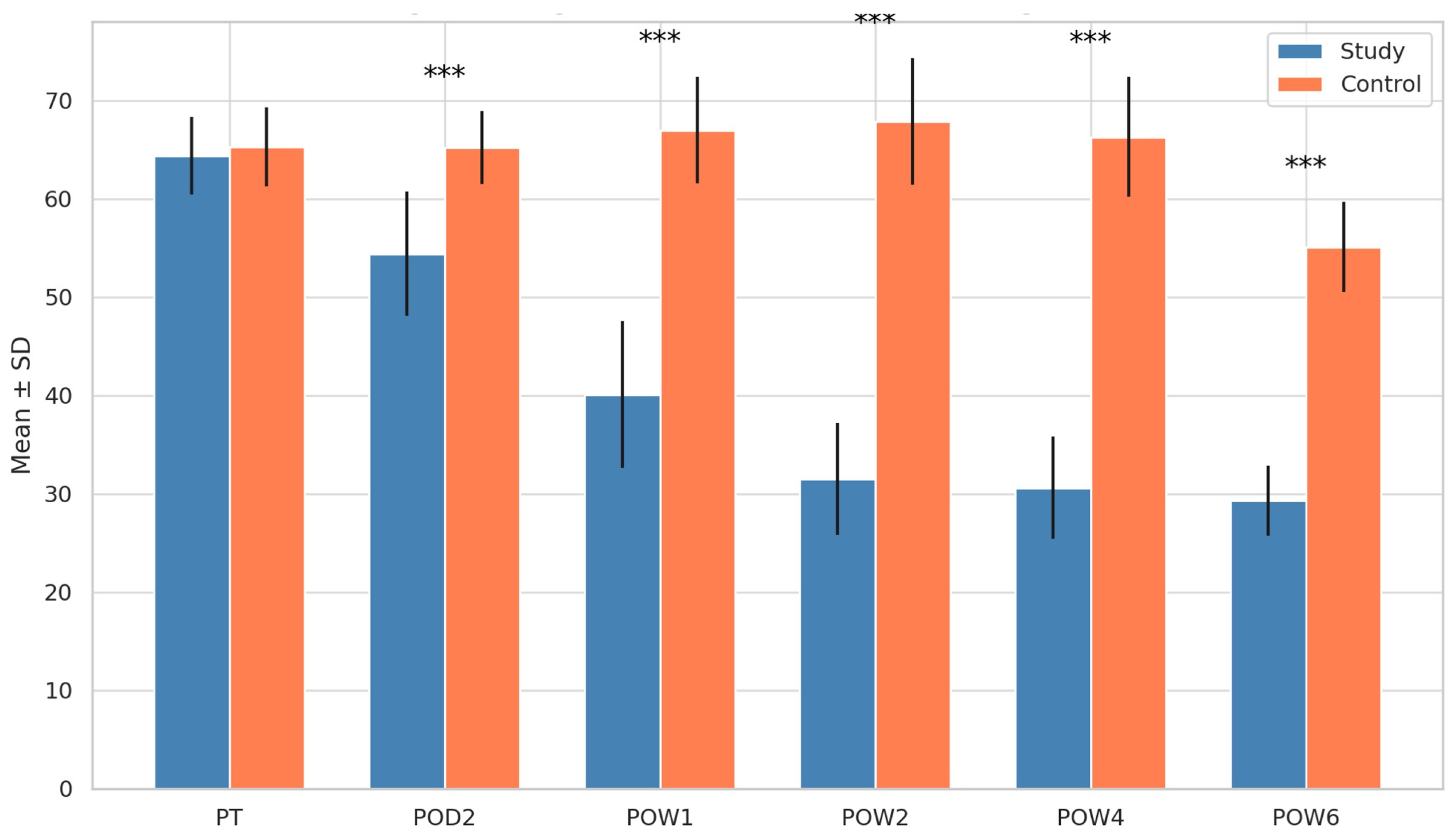
3.1. Stress Levels (Zung Score)
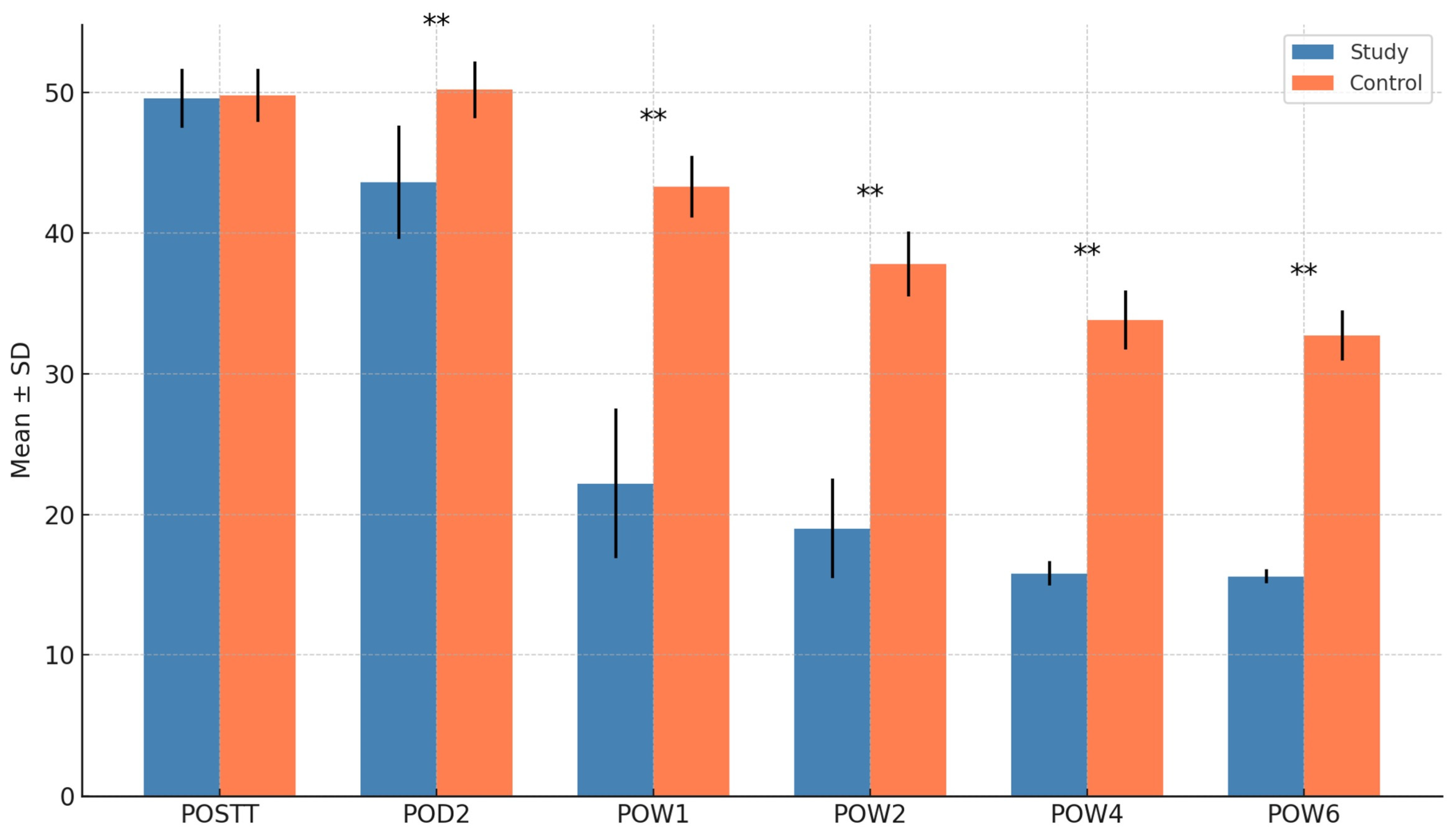
3.2. Quality of Life (OHIP Score)
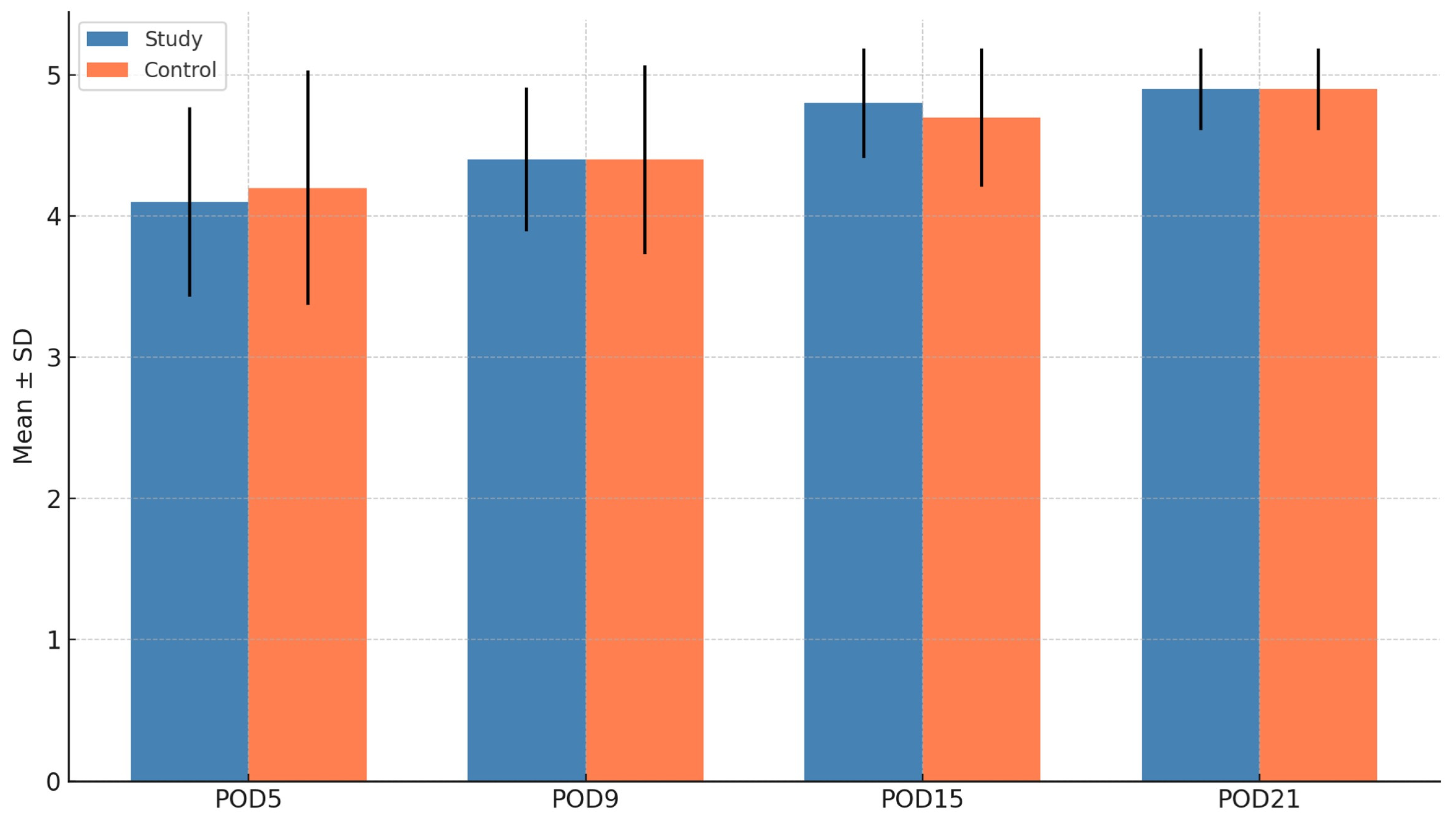
3.3. Soft Tissue Healing (Landry Score)
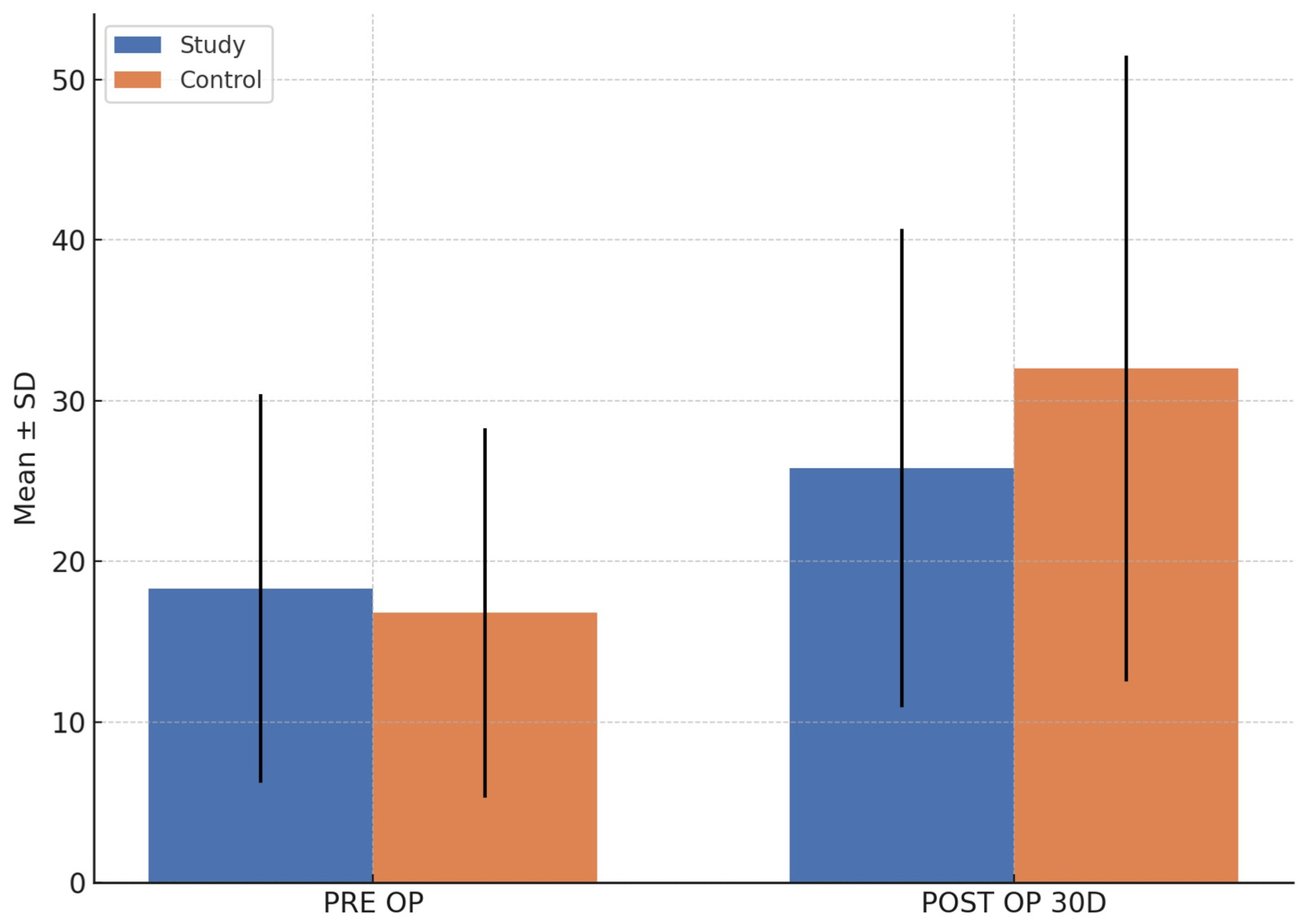
3.4. Biochemical Marker (Serum Osteocalcin)
3.5. Hard Tissue Healing (Moed Score)
3.6. Safety Outcomes
3.6.1. Adverse Events
3.6.2. Protocol Violations
3.6.3. Alcohol Withdrawal Syndrome (AWS) Episodes
- Prevalence: Most patients exhibited mild symptoms as discussed, with none presenting moderate or severe symptoms.
- Management: There were no patients requiring pharmacological help or admission. The patients were treated by the psychiatrist with examination and counselling. No cases of delirium tremens or seizures were reported.
4. Discussion
4.1. Perioperative Stress and Quality of Life
4.2. Tissue Healing Outcomes
4.3. Evolution of Alcohol Therapy
4.4. Clinical Implications and Ethical Considerations
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoonpongsimanont, W.; Ghanem, G.; Chen, Y.; Sahota, P.K.; Carroll, C.; Barrios, C.; Lotfipour, S. Underreporting of Alcohol Use in Trauma Patients: A Retrospective Analysis. Subst. Abuse 2021, 42, 192–196. [Google Scholar] [CrossRef]
- Laverick, S.; Patel, N.; Jones, D.C. Maxillofacial Trauma and the Role of Alcohol. Br. J. Oral Maxillofac. Surg. 2008, 46, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Ungur, A.L.; Neumann, T.; Borchers, F.; Spies, C. Perioperative Management of Alcohol Withdrawal Syndrome. Visc. Med. 2020, 36, 160–166. [Google Scholar] [CrossRef]
- Gottlieb, M.; Chien, N.; Long, B. Managing Alcohol Withdrawal Syndrome. Ann. Emerg. Med. 2024, 84, 29–39. [Google Scholar] [CrossRef]
- Moyer, A.; Finney, J.W.; Swearingen, C.E.; Vergun, P. Brief Interventions for Alcohol Problems: A Meta-Analytic Review of Controlled Investigations in Treatment-Seeking and Non-Treatment-Seeking Populations. Addict. Abingdon Engl. 2002, 97, 279–292. [Google Scholar] [CrossRef]
- Gentilello, L.M.; Rivara, F.P.; Donovan, D.M.; Jurkovich, G.J.; Daranciang, E.; Dunn, C.W.; Villaveces, A.; Copass, M.; Ries, R.R. Alcohol Interventions in a Trauma Center as a Means of Reducing the Risk of Injury Recurrence. Ann. Surg. 1999, 230, 473–480; discussion 480–483. [Google Scholar] [CrossRef]
- Stockwell, T.; Zhao, J.; Pauly, B.; Chow, C.; Vallance, K.; Wettlaufer, A.; Saunders, J.B.; Chick, J. Trajectories of Alcohol Use and Related Harms for Managed Alcohol Program Participants over 12 Months Compared with Local Controls: A Quasi-Experimental Study. Alcohol Alcohol. 2021, 56, 651–659. [Google Scholar] [CrossRef]
- Brothers, T.D.; Kaulbach, J.; Tran, A. Unhealthy Alcohol Use in a 65-Year-Old Man Awaiting Surgery. Can. Med. Assoc. J. 2021, 193, E1250–E1252. [Google Scholar] [CrossRef]
- Hereford, T.; Kellish, A.; Samora, J.B.; Reid Nichols, L. Understanding the Importance of Peak Bone Mass. J. Pediatr. Orthop. Soc. N. Am. 2024, 7, 100031. [Google Scholar] [CrossRef] [PubMed]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). Alcohol Use Disorder: A Comparison Between DSM–IV and DSM–5. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-use-disorder-comparison-between-dsm (accessed on 20 June 2025).
- AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. Available online: https://www.who.int/publications/i/item/WHO-MSD-MSB-01.6a (accessed on 10 August 2025).
- Puz, C.A.; Stokes, S.J. Alcohol Withdrawal Syndrome: Assessment and Treatment with the Use of the Clinical Institute Withdrawal Assessment for Alcohol-Revised. Crit. Care Nurs. Clin. N. Am. 2005, 17, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Spies, C.; Eggers, V.; Szabo, G.; Lau, A.; von Dossow, V.; Schoenfeld, H.; Althoff, H.; Hegenscheid, K.; Bohm, B.; Schroeder, T.; et al. Intervention at the Level of the Neuroendocrine-Immune Axis and Postoperative Pneumonia Rate in Long-Term Alcoholics. Am. J. Respir. Crit. Care Med. 2006, 174, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Kaisdotter Andersson, A.; Kron, J.; Castren, M.; Muntlin Athlin, A.; Hok, B.; Wiklund, L. Assessment of the Breath Alcohol Concentration in Emergency Care Patients with Different Level of Consciousness. Scand. J. Trauma Resusc. Emerg. Med. 2015, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Alvanzo, A.; Kleinschmidt, K.; Kmiec, J.A. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. J. Addict. Med. 2020, 14 (Suppl. S1), 1–72. [Google Scholar] [CrossRef]
- Ranganathan, V.; Panneerselvam, E.; Chellappazham, S.; Balasubramaniam, S.; Raja Vb, K. Evaluation of Depression Associated with Post-Traumatic Stress Disorder After Maxillofacial Injuries-A Prospective Study. J. Oral Maxillofac. Surg. 2018, 76, 1282.e1–1282.e9. [Google Scholar] [CrossRef]
- Conforte, J.J.; Alves, C.P.; Sánchez Mdel, P.R.; Ponzoni, D. Impact of Trauma and Surgical Treatment on the Quality of Life of Patients with Facial Fractures. Int. J. Oral Maxillofac. Surg. 2016, 45, 575–581. [Google Scholar] [CrossRef]
- Gopalan, A.; Panneerselvam, E.; Doss, G.T.; Ponvel, K.; Raja Vb, K. Evaluation of Efficacy of Low Intensity Pulsed Ultrasound in Facilitating Mandibular Fracture Healing-A Blinded Randomized Controlled Clinical Trial. J. Oral Maxillofac. Surg. 2020, 78, 997.e1–997.e7. [Google Scholar] [CrossRef]
- Roy, A.; Thulasiraman, S.; Panneerselvam, E.; Thulasi Doss, G.; Selvaraj, M.N.; Ganesh, S.K.; Raja Vb, K.; Kangusamy, B. Evaluation of the Efficacy of Salmon Calcitonin Nasal Spray on Bone Healing Following Open Reduction and Internal Fixation of Mandibular Fractures—A Randomized Controlled Trial. J. Cranio-Maxillofac. Surg. 2021, 49, 1151–1157. [Google Scholar] [CrossRef]
- Morris, P.R.; Mosby, E.L.; Ferguson, B.L. Alcohol Withdrawal Syndrome: Current Management Strategies for the Surgery Patient. J. Oral Maxillofac. Surg. 1997, 55, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Spies, C.D.; Rommelspacher, H. Alcohol Withdrawal in the Surgical Patient: Prevention and Treatment. Anesth. Analg. 1999, 88, 946–954. [Google Scholar] [CrossRef]
- Hodges, B.; Mazur, J.E. Intravenous Ethanol for the Treatment of Alcohol Withdrawal Syndrome in Critically Ill Patients. Pharmacotherapy 2004, 24, 1578–1585. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Benefits and Risks of Moderate Alcohol Consumption on Cardiovascular Disease: Current Findings and Controversies. Nutrients 2019, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- DiPaula, B.; Tommasello, A.; Solounias, B.; McDuff, D. An Evaluation of Intravenous Ethanol in Hospitalized Patients. J. Subst. Abuse Treat. 1998, 15, 437–442. [Google Scholar] [CrossRef]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef]
- Eggers, V.; Tio, J.; Neumann, T.; Pragst, F.; Müller, C.; Schmidt, L.G.; Kox, W.J.; Spies, C.D. Blood Alcohol Concentration for Monitoring Ethanol Treatment to Prevent Alcohol Withdrawal in the Intensive Care Unit. Intensive Care Med. 2002, 28, 1475–1482. [Google Scholar] [CrossRef]
- Weinberg, J.A.; Magnotti, L.J.; Fischer, P.E.; Edwards, N.M.; Schroeppel, T.; Fabian, T.C.; Croce, M.A. Comparison of Intravenous Ethanol versus Diazepam for Alcohol Withdrawal Prophylaxis in the Trauma ICU: Results of a Randomized Trial. J. Trauma 2008, 64, 99–104. [Google Scholar] [CrossRef]
- Dissanaike, S.; Halldorsson, A.; Frezza, E.E.; Griswold, J. An Ethanol Protocol to Prevent Alcohol Withdrawal Syndrome. J. Am. Coll. Surg. 2006, 203, 186–191. [Google Scholar] [CrossRef]
- Janda, S.M.; Fazio, A.; Henann, N.E.I.V. Alcohol in Prevention of Delirium Tremens. DICP Ann. Pharmacother. 1990, 24, 545. [Google Scholar] [CrossRef]
- Merry, J.; Marks, V. The Effects of Alcohol, Barbiturate, and Diazepam on Hypothalamic-Pituitary-Adrenal Function in Chronic Alcoholics. Lancet Lond. Engl. 1972, 2, 990–991. [Google Scholar] [CrossRef]
- Perrin, S.; Fillol, A.; Moriceau, S.; Le Tirant, L.; Allache, A.; Serre, F.; Stevens, N.; Auriacombe, M.; Cambon, L.; Martin-Fernandez, J. Exploring and Describing Alcohol Harm Reduction Interventions: A Scoping Review of Literature from the Past Decade in the Western World. Harm. Reduct. J. 2024, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Parkes, T.; Carver, H.; Matheson, C.; Browne, T.; Pauly, B. ‘It’s like a Safety Haven’: Considerations for the Implementation of Managed Alcohol Programs in Scotland. Drugs Educ. Prev. Policy 2022, 29, 477–489. [Google Scholar] [CrossRef]
- Smith-Bernardin, S.M.; Suen, L.W.; Barr-Walker, J.; Cuervo, I.A.; Handley, M.A. Scoping Review of Managed Alcohol Programs. Harm. Reduct. J. 2022, 19, 82. [Google Scholar] [CrossRef]
- Panneerselvam, E.; Krishnan, R. Rx. Alcohol for Facial Trauma: Treating Fractured Bones and Minds Together!! J. Oral Maxillofac. Surg. 2025, in press. [Google Scholar] [CrossRef]
- Parappilly, B.P.; Garrod, E.; Longoz, R.; Eligh, E.; van Heukelom, H.; Fairgrieve, C.K.; Pauly, B. Exploring the Experience of Inpatients with Severe Alcohol Use Disorder on a Managed Alcohol Program (MAP) at St. Paul’s Hospital. Harm. Reduct. J. 2020, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S.; Fairgrieve, C.; Dong, H.; Garrod, E.; van Heukelom, H.; Parappilly, B.P.; McLean, M.; Tsui, J.I.; Samet, J.H. A Hospital-Based Managed Alcohol Program in a Canadian Setting. J. Addict. Med. 2023, 17, 190–196. [Google Scholar] [CrossRef]
- Management of Substance Use in Acute Care Settings in Alberta: Guidance Document. CRISM Prairies. Available online: https://crismprairies.ca/management-of-substance-use-in-acute-care-settings-in-alberta-guidance-document/ (accessed on 8 June 2025).
- Sattar, S.P.; Qadri, S.F.; Warsi, M.K.; Okoye, C.; Din, A.U.; Padala, P.R.; Bhatia, S.C. Use of Alcoholic Beverages in VA Medical Centers. Subst. Abuse Treat. Prev. Policy 2006, 1, 30. [Google Scholar] [CrossRef]
- Quelch, D.; Pucci, M.; Thompson, T.; Copland, A.; Appleyard, C.; Arora, A.; Roderique-Davies, G.; John, B.; Bradberry, S. Peri-Operative Management of Alcohol Withdrawal with Ethanol Prescribing: A Case Study. Clin. Toxicol. 2024, 62, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Dubowski, K.M.; Essary, N.A. Measurement of Low Breath-Alcohol Concentrations: Laboratory Studies and Field Experience. J. Anal. Toxicol. 1999, 23, 386–395. [Google Scholar] [CrossRef]
- Brooks, H.L.; Kassam, S.; Salvalaggio, G.; Hyshka, E. Implementing Managed Alcohol Programs in Hospital Settings: A Review of Academic and Grey Literature. Drug Alcohol Rev. 2018, 37 (Suppl. S1), S145–S155. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. (n.d.). Mixing Alcohol with Medicines. Available online: https://www.niaaa.nih.gov/sites/default/files/publications/Harmful_Interactions.pdf (accessed on 21 April 2025).
- Kaner, E.F.; Beyer, F.R.; Muirhead, C.; Campbell, F.; Pienaar, E.D.; Bertholet, N.; Daeppen, J.B.; Saunders, J.B.; Burnand, B. Effectiveness of Brief Alcohol Interventions in Primary Care Populations. Cochrane Database Syst. Rev. 2018, 2, CD004148. [Google Scholar] [CrossRef]
- Golka, K.; Wiese, A. Carbohydrate-Deficient Transferrin (CDT)—A Biomarker for Long-Term Alcohol Consumption. J. Toxicol. Environ. Health B Crit. Rev. 2004, 7, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 statement: Updated guideline for reporting randomised trials. BMJ 2025, 389, e081123. [Google Scholar] [CrossRef] [PubMed]

| N = 12 | Groups | ||||||
| Study | Control | ||||||
| Gender | Frequency | ||||||
| Female | 0 | 0 | |||||
| Male | 12 | 12 | |||||
| Age (Years) | Mean (S.D) | Mean | p value | ||||
| 36 (6.2) | 34.8 (7.1) | 0.428 | |||||
| Zung Score (Stress) | Mean (S.D) | 95% CI | Mean | 95% CI | p value | ||
| LL | UL | LL | UL | ||||
| 64.4 (3.92) | 61.9 | 66.9 | 65.3 (4.01) | 62.8 | 67.9 | 0.577 | |
| 54.4 (6.33) | 50.4 | 58.4 | 65.2 (3.71) | 62.8 | 67.5 | <0.001 *** | |
| 40.1 (7.49) | 35.3 | 44.8 | 67 (5.44) | 63.5 | 70.5 | <0.001 *** | |
| 31.5 (5.68) | 27.9 | 35.1 | 67.8 (6.44) | 63.8 | 72.0 | <0.001 *** | |
| 30.6 (5.20) | 27.3 | 33.9 | 66.3 (6.10) | 62.5 | 70.2 | <0.001 *** | |
| 29.3 (3.55) | 27.1 | 31.6 | 55.1 (4.56) | 52.2 | 58.0 | <0.001 *** | |
| OHIP Score (QoL) | Mean (S.D) | LL | UL | Mean | LL | UL | p value |
| 49.6 (2.11) | 48.2 | 50.9 | 49.8 (1.90) | 48.6 | 51.0 | 0.763 | |
| 43.6 (4.03) | 41.0 | 46.1 | 50.2 (2.04) | 48.9 | 51.5 | <0.001 *** | |
| 22.2 (5.31) | 18.8 | 25.5 | 43.3 (2.18) | 41.9 | 44.6 | <0.001 *** | |
| 19 (3.54) | 16.7 | 21.3 | 37.8 (2.29) | 36.4 | 39.3 | <0.001 *** | |
| 15.8 (0.87) | 15.2 | 16.3 | 33.8 (2.09) | 32.4 | 35.1 | <0.001 *** | |
| 15.6 (0.51) | 15.3 | 15.9 | 32.7 (1.78) | 31.5 | 33.8 | <0.001 *** | |
| Landry Score (Soft tissue) | Mean (S.D) | LL | UL | Mean | LL | UL | p value |
| 4.1 (0.67) | 3.7 | 4.5 | 4.2 (0.83) | 3.6 | 4.7 | 0.790 | |
| 4.4 (0.51) | 4.1 | 4.7 | 4.4 (0.67) | 4.0 | 4.8 | 0.999 | |
| 4.8 (0.39) | 4.6 | 5.1 | 4.7 (0.49) | 4.4 | 5.0 | 0.368 | |
| 4.9 (0.29) | 4.7 | 5.1 | 4.9 (0.29) | 4.7 | 5.1 | 0.999 | |
| Moed Score (Hard tissue) | Mean (S.D) | LL | UL | Mean | LL | UL | p value |
| 1 (0) | 1.0 | 1.0 | 1 (0) | 1.0 | 1.0 | - | |
| 2.8 (0.39) | 2.6 | 3.1 | 3 (0) | 3.0 | 3.0 | 0.166 | |
| 3.8 (0.39) | 3.6 | 4.1 | 3.9 (0.29) | 3.7 | 4.1 | 0.557 | |
| S. Osteocalcin (Biochemical) | Median | LL | UL | Median | LL | UL | p value |
| 11.6 | 4.7 | 22.8 | 18.1 | 8.9 | 28.2 | 0.319 | |
| 22.6 | 12.5 | 30.0 | 33.1 | 14.0 | 48.7 | 0.478 | |
| Benzodiazepines (Pharmacological Management) | Harm Reduction Strategies | |
|---|---|---|
| Primary Role |
|
|
| Mechanism |
|
|
| Regimens |
|
|
| Main Advantages |
|
|
| Limitations/Risks |
|
|
| Ideal indications |
|
|
| Outcome Focus |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the AO Foundation. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panneerselvam, E.; Krishnan, R.; Velayudham, J. Impact of Therapeutic Alcohol Administration on Perioperative Quality of Life (QoL) and Fracture Healing in Patients with Alcohol Use Disorder Undergoing Surgery for Maxillofacial Trauma—A Randomized Pilot Trial. Craniomaxillofac. Trauma Reconstr. 2025, 18, 37. https://doi.org/10.3390/cmtr18030037
Panneerselvam E, Krishnan R, Velayudham J. Impact of Therapeutic Alcohol Administration on Perioperative Quality of Life (QoL) and Fracture Healing in Patients with Alcohol Use Disorder Undergoing Surgery for Maxillofacial Trauma—A Randomized Pilot Trial. Craniomaxillofacial Trauma & Reconstruction. 2025; 18(3):37. https://doi.org/10.3390/cmtr18030037
Chicago/Turabian StylePanneerselvam, Elavenil, Rajkumar Krishnan, and Jaikumar Velayudham. 2025. "Impact of Therapeutic Alcohol Administration on Perioperative Quality of Life (QoL) and Fracture Healing in Patients with Alcohol Use Disorder Undergoing Surgery for Maxillofacial Trauma—A Randomized Pilot Trial" Craniomaxillofacial Trauma & Reconstruction 18, no. 3: 37. https://doi.org/10.3390/cmtr18030037
APA StylePanneerselvam, E., Krishnan, R., & Velayudham, J. (2025). Impact of Therapeutic Alcohol Administration on Perioperative Quality of Life (QoL) and Fracture Healing in Patients with Alcohol Use Disorder Undergoing Surgery for Maxillofacial Trauma—A Randomized Pilot Trial. Craniomaxillofacial Trauma & Reconstruction, 18(3), 37. https://doi.org/10.3390/cmtr18030037






