Is Simple Reimplantation a Viable Option in Pediculated Auricular Avulsions? A Systematic Review of the Literature
Abstract
1. Introduction
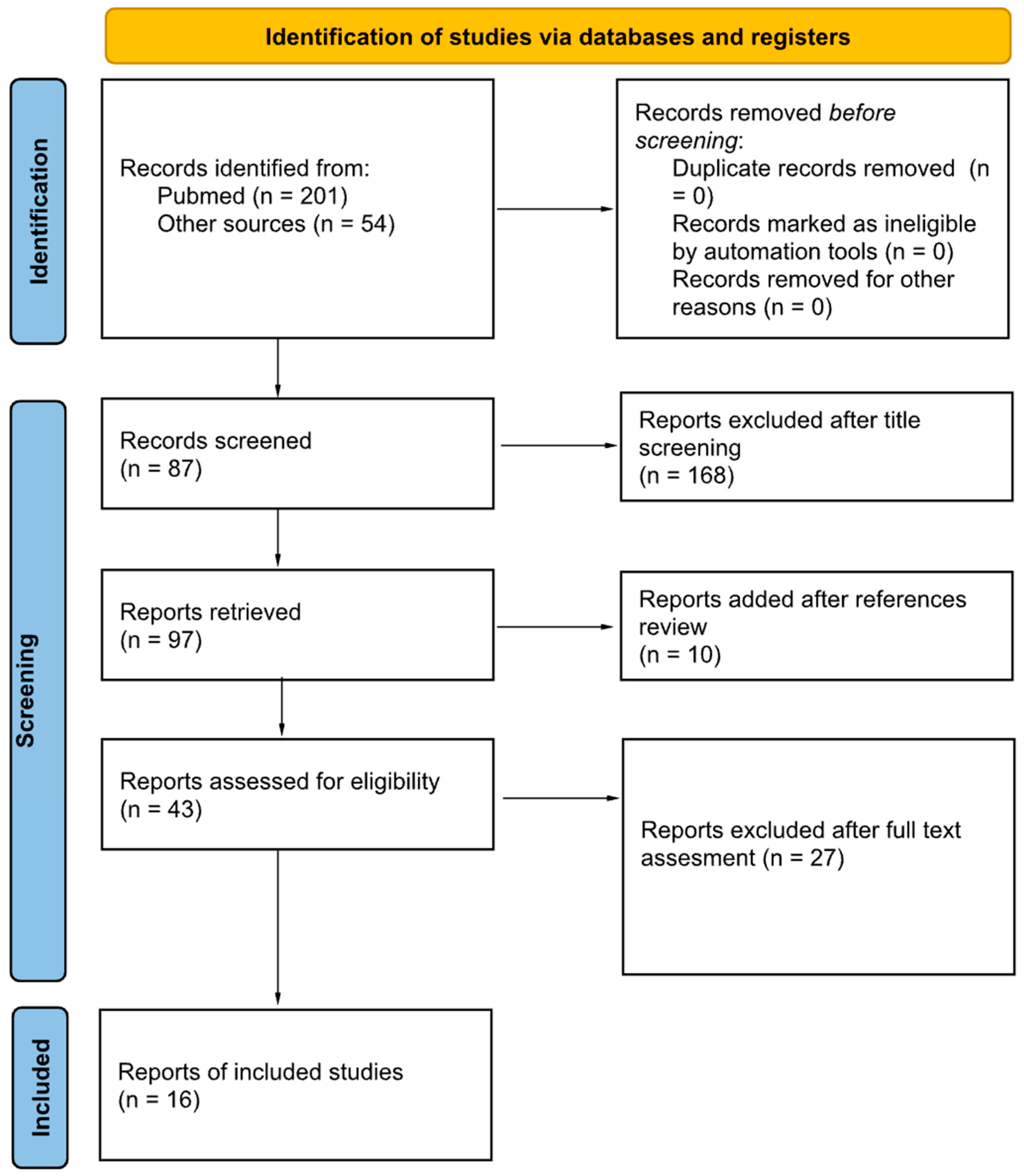
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Steffen, A.; Katzbach, R.; Klaiber, S. A comparison of ear reattachment methods: A review of 25 years since Pennington. Plast. Reconstr. Surg. 2006, 118, 1358–1364. [Google Scholar] [CrossRef]
- Gailey, A.D.; Farquhar, D.; Clark, J.M.; Shockley, W.W. Auricular avulsion injuries and reattachment techniques: A systematic review. Laryngoscope Investig. Otolaryngol. 2020, 5, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kind, G.M. Microvascular ear replantation. Clin. Plast. Surg. 2002, 29, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Lee, J.; Oh, S.J.; Koh, S.H.; Chung, C.H.; Lee, J.W. A review of microvascular ear replantation. J. Reconstr. Microsurg. 2013, 29, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Clodius, L. Local hypothermia for the avulsed external ear. Br. J. Plast. Surg. 1968, 21, 250–252. [Google Scholar] [CrossRef]
- Tomono, T.; Hirose, T. Treatment of the subtotally amputated auricle. J. Plast. Reconstr. Surg. 1980, 23, 41–46. [Google Scholar]
- Bernstein, L.; Nelson, R.H. Replanting the severed auricle: An update. Arch. Otolaryngol. 1982, 108, 587–590. [Google Scholar] [CrossRef]
- Safak, T.; Kayikcioglu, A. A traumatic ear amputation attached with a narrow pedicle. Ann. Plast. Surg. 1998, 40, 106–107. [Google Scholar] [CrossRef]
- Yotsuyanagi, T.; Yamashita, K.; Watanabe, Y.; Kobayashi, S.; Ikeda, K. Reconstruction of a subtotally amputated auricle: A case report. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2001, 35, 425–427. [Google Scholar] [CrossRef]
- Komorowska-Timek, E.; Hardesty, R.A. Successful reattachment of a nearly amputated ear without microsurgery. Plast. Reconstr. Surg. 2008, 121, 165e–169e. [Google Scholar] [CrossRef]
- Erdmann, D.; Bruno, A.D.; Follmar, K.E.; Stokes, T.H.; Gonyon, D.L.; Marcus, J.R. The helical arcade: Anatomic basis for survival in near-total ear avulsion. J. Craniofac. Surg. 2009, 20, 245–248. [Google Scholar] [CrossRef]
- Ozçelik, D.; Unveren, T.; Toplu, G. Subtotal ear amputation with a very narrow pedicle: A case report and review of the literature. Ulus. Travma Acil Cerrahi Derg. 2009, 15, 306–310. [Google Scholar]
- Bada, A.M.; Pope, G.H. Use of hyperbaric oxygen as adjunct in salvage of near-complete ear amputation. Plast. Reconstr. Surg. Glob. Open 2013, 1, e5. [Google Scholar] [CrossRef]
- Aremu, S.K. Nonmicroscopic reconstruction of subtotally amputated/torn auricles: Report of 3 cases. Ear Nose Throat J. 2014, 93, E1–E3. [Google Scholar]
- Kemaloglu, C.A.; Kilic, F.; Gunay, G.K. Reconstruction of a subtotally amputated auricle with a very narrow inferior pedicle. Case Rep. Plast. Surg. Hand Surg. 2015, 2, 77–79. [Google Scholar] [CrossRef]
- Zhang, C.; Teng, L.; Xu, J.J.; Lu, J.J.; Xie, F.; Yang, L.Y.; Li, S.-Y.; Wu, H.-H.; Sun, H.; Yang, B. Incomplete ear amputation. J. Craniofac. Surg. 2018, 29, 2231–2233. [Google Scholar] [CrossRef]
- D’Arcangelo, M.; Al-Ali, M.A.; Abu-Zidan, F.M. Primary reattachment of near-complete ear amputation: A successful outcome. Ear Nose Throat J. 2020, 99, NP156–NP159. [Google Scholar] [CrossRef]
- Albdour, M.; Ammar, H.M.; Alnaser, M.M.S.; Alzaben, F.S.; Malek, S. Non-microvascular successful management of near-total ear avulsion. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3386. [Google Scholar] [CrossRef]
- Allam, K.; Mohamed, M.; Haredy, M.; Abdelmegeed, A. Retrospective study of management of near total avulsion of auricles and review of the literature. Egypt. J. Plast. Reconstr. Surg. 2021, 45, 259–264. [Google Scholar] [CrossRef]
- Widiarni, W.D.; Putri, B.; Respati, W.R. Prognostic factors in partial auricular avulsion after one-stage reconstruction. Trauma Case Rep. 2023, 47, 100891. [Google Scholar] [CrossRef]
- Momeni, A.; Liu, X.; Januszyk, M.; Wan, D.C.; Buncke, G.M.; Buntic, R.F.; Parrett, B.M. Microsurgical ear replantation—Is venous repair necessary? A systematic review. Microsurgery 2016, 36, 345–350. [Google Scholar] [CrossRef]
- Park, C.; Shin, K.S.; Kang, H.S.; Lee, Y.H.; Lew, J.D. A new arterial flap from the postauricular surface: Its anatomic basis and clinical application. Plast. Reconstr. Surg. 1988, 82, 498–504. [Google Scholar] [CrossRef]
- O’Toole, G.; Bhatti, K.; Masood, S. Replantation of an avulsed ear using a single arterial anastomosis. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 326–329. [Google Scholar] [CrossRef]
- Hussey, A.J.; Kelly, J.I. Microsurgical replantation of an ear with no venous repair. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2010, 44, 64–65. [Google Scholar] [CrossRef]
- Facchin, F.; Lancerotto, L.; Arnez, Z.M.; Bassetto, F.; Vindigni, V. Leeching as salvage venous drainage in ear reconstruction: Clinical case and review of literature. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1820. [Google Scholar] [CrossRef]

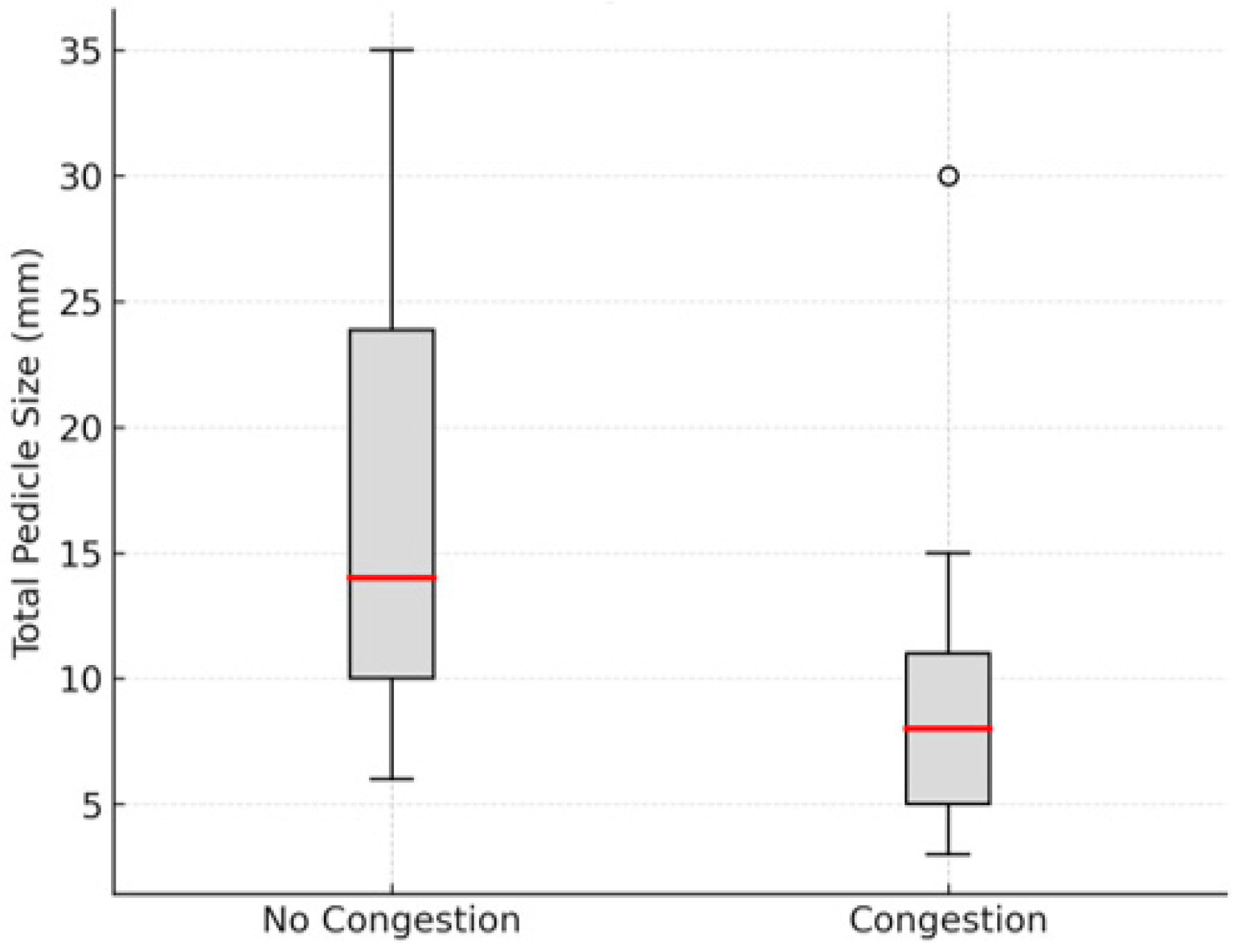
| Author and Year | Age (Years) | Sex | Cause | Location and Total Pedicle Size (mm) | Pedicle Congestion | Medical Treatment Timing (Pre/Post Congestion) | Adjuvant Therapy |
|---|---|---|---|---|---|---|---|
| L. Clodius et al. 1968 [5] | NR | M | MVA | Sup, 30 mm | No | Pre | LMWH, Cryotherapy |
| NR | M | MVA | Inf, 35 mm | No | Pre | LMWH, Cryotherapy | |
| Tomono T and Hirase, 1980 [6] | 6 | M | NR | Inf, 30 mm | No | Pre | LMWH, Niacin |
| 6 | M | NR | Inf, 10 mm | No | Pre | LMWH, Niacin | |
| Berstein et al., 1982 [7] | 28 | F | Bite | Sup, 10 mm | Yes | Post | UFH, Cryotherapy, Dextran, Incisions |
| Tunc Safak et al., 1998 [8] | 40 | M | MVA | Sup, 3 mm | Yes | None | None |
| Takatoshi Yotsuyanagi et al., 2001 [9] | 42 | F | WR | Sup, 10 mm | No | None | None |
| Komorowska-Timek et al., 2005 [10] | 35 | M | WR | Sup and Inf, 7 mm | Yes | Pre | Leech, Dextran, HBOT |
| Detlev Erdmann et al., 2008 [11] | 23 | F | MVA | Sup, 15 mm | Yes | Post | Leech |
| 3 | M | Fall | Sup, 8 mm | Yes | Post | Leech | |
| 52 | M | WR | Sup, 5 mm | Yes | Post | Leech | |
| Ozcelik D et al., 2009 [12] | 36 | M | MVA | Sup, 6 mm | No | Pre | Dextran |
| Bada and Pope, 2013 [13] | 4 | M | Bite | Inf, 30 mm | Yes | Post | HBOT |
| Aremu SK et al., 2014 [14] | 12 | M | MVA | Sup, 20 mm | No | None | None |
| 31 | M | Bite | Inf, - mm | No | None | None | |
| 45 | M | Assault | Sup, - mm | No | None | None | |
| Kemaloglu CA et al., 2015 [15] | 57 | M | WR | Inf, 5 mm | Yes | Pre | LMWH |
| Zhang C, et al., 2018 [16] | 16 | M | Assault | 2 Inf, 8 mm | No | Pre | LMWH |
| Mauro D’Arcangelo et al., 2020 [17] | 34 | M | MVA | Sup, 7 mm | No | Pre | LMWH |
| 31 | M | MVA | Inf, 25 mm | No | Pre | LMWH | |
| 50 | M | Assault | Sup and Inf, 20.5 mm | No | Pre | LMWH | |
| 34 | M | Assault | Inf, 10 mm | No | Pre | LMWH | |
| 32 | M | MVA | Inf, 6 mm | No | Pre | LMWH | |
| Mahammad Albdour et al., 2021 [18] | 55 | M | MVA | Sup, 5 mm | Yes | Post | LMWH, Incisions |
| Karam A. Allam et al., 2021 [19] | 19 | M | MVA | Inf, 8 mm | Yes | Post | Incisions |
| 25 | M | Assault | Inf, 9 mm | No | None | None | |
| 45 | M | MVA | Inf, 12 mm | Yes | Post | Pentoxifilin, Vit E | |
| 33 | M | Assault | Inf, 14 mm | No | None | None | |
| 27 | F | Assault | Inf, 10 mm | No | None | None | |
| 20 | M | MVA | Inf, 16 mm | No | None | None | |
| 12 | M | MVA | Inf, 19 mm | No | None | None | |
| W. Dini Widiarni et al., 2023 [20] | 28 | M | Assault | Inf, 30 mm | No | None | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the AO Foundation. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Román Padilla, J.C.; Ortiz Peces, L.; Alavedra Martínez, P.; Cebrián Carretero, J.L. Is Simple Reimplantation a Viable Option in Pediculated Auricular Avulsions? A Systematic Review of the Literature. Craniomaxillofac. Trauma Reconstr. 2025, 18, 36. https://doi.org/10.3390/cmtr18030036
Román Padilla JC, Ortiz Peces L, Alavedra Martínez P, Cebrián Carretero JL. Is Simple Reimplantation a Viable Option in Pediculated Auricular Avulsions? A Systematic Review of the Literature. Craniomaxillofacial Trauma & Reconstruction. 2025; 18(3):36. https://doi.org/10.3390/cmtr18030036
Chicago/Turabian StyleRomán Padilla, Jose Carlos, Luis Ortiz Peces, Pol Alavedra Martínez, and Jose Luis Cebrián Carretero. 2025. "Is Simple Reimplantation a Viable Option in Pediculated Auricular Avulsions? A Systematic Review of the Literature" Craniomaxillofacial Trauma & Reconstruction 18, no. 3: 36. https://doi.org/10.3390/cmtr18030036
APA StyleRomán Padilla, J. C., Ortiz Peces, L., Alavedra Martínez, P., & Cebrián Carretero, J. L. (2025). Is Simple Reimplantation a Viable Option in Pediculated Auricular Avulsions? A Systematic Review of the Literature. Craniomaxillofacial Trauma & Reconstruction, 18(3), 36. https://doi.org/10.3390/cmtr18030036







