Abstract
Study Design: This document details the planning phase of a systematic mapping review. Objective: The objective of this mapping review is to identify, describe, and organize evidence currently available from systematic reviews and primary studies regarding different co-interventions and surgical modalities used in orthognathic surgery (OS) and their outcomes. Methods: Systematic reviews (SRs), randomized controlled trials (RCTs), and observational studies that evaluate perioperative OS co-interventions and surgical modalities will be identified in an exhaustive search of MEDLINE, EMBASE, Epistemonikos, Lilacs, Web of Science, and CENTRAL. Grey literature will also be screened. Results: Expected results include identification of all PICO questions available in the evidence regarding OS and generation of evidence bubble maps, involving a matrix of all identified co-interventions, surgical modalities, and outcomes presented in the studies. This will achieve identification of research gaps and prioritization of new research questions. Conclusions: The significance of this review will result in a systematic identification and characterization of the available evidence, leading to a reduction in research waste and a guidance of future efforts in developing studies for unsolved questions.
Introduction
Orthognathic surgery (OS), a widely used procedure to correct dentofacial deformities that affects 20% of the population [1], involves surgical manipulation of facial skeletal elements [1,2,3,4,5,6]. The repositioning of maxillar and mandibular bones improves function, facial architecture [7], facial harmony, and esthetics [6,8]. According to the American Association of Oral and Maxillofacial Surgeons (AAOMS) [5], conditions that indicate the need for OS include anteroposterior, vertical, or transverse discrepancies, asymmetries, dysfunctions, temporomandibular joint disorders, speech impairments, airway dysfunctions, and psychosocial disorders [5]. Although data is scant, the records of the American Dental Association (ADA) and the AAOMS [9] would indicate that the number of OS procedures performed each year is increasing; in 2007, for instance, 8755 OS procedures were performed in the USA [9].
OS complications and outcomes are many and include relapse, bleeding, infection, lack of fixation stability, neurosensory disturbances, temporomandibular joint dysfunction, etc. [1,6,10]. Great variability exists regarding not only perioperative co-interventions (e.g., antibiotics, antifibrinolytics, and anesthetic techniques) but also surgical modalities used in OS (e.g., materials and types of screws and plates, and surgical instruments for osteotomies) aimed at optimizing surgical results [6].
Determining recommendations for OS requires analyzing all available evidence concerning this subject. The emerging method of mapping reviews constitutes a valuable mechanism for this particular area, given the broad field covered and the vast body of literature [11,12].
To our knowledge, ours is the first evidence-mapping synthesis that scopes all existing evidence regarding alternatives for OS co-interventions and surgical modalities.
Evidence mapping is an innovative and visual approach that illustrates existing evidence on the effects of specific interventions in a specific area [11,13]. As a user-friendly and didactic approach to displaying available evidence in extensive research areas, it systematically characterizes existing evidence, identifies knowledge gaps, and highlights research gaps [11,12,13,14]. It is therefore a possible first step to developing systematic reviews (SRs) or frameworks for informing policy development [11,12,13,14]. Furthermore, in identifying research gaps that have already been resolved, it avoids wasted research effort in developing primary studies or SRs.
This mapping review aims to identify, describe, and organize evidence currently available from SRs and primary studies regarding different OS co-interventions and surgical modalities, and their intraoperative and postoperative outcomes. It also aims to assess the quality of the existing evidence and highlight clinical questions regarding this topic. In facilitating the identification of research gaps, this review is expected to point the way to new studies.
This review will consist in two phases. First, a general mapping of all the co-interventions and surgical modalities developed in orthognathic surgery will be performed. Moreover, for the second phase, a more in-depth analysis will be performed of the quality and direction of results for three selected main outcomes: (1) postoperative infection, (2) intraoperative bleeding, and (3) relapse of the condition.
Methods
This review was drafted using the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-P) [14]. It adheres to the Global Evidence Mapping Initiative methods (GEM) [12], incorporating the quality of supporting evidence.
Mapping Boundaries and Context
To frame this mapping review, a preliminary search to establish eligibility criteria for study inclusion was performed.
Eligibility Criteria
The population, intervention, comparison, and outcomes (PICO) framework will be used to guide the eligibility criteria.
Studies. To include a complete mapping of co-interventions and surgical modalities regarding efficacy and safety, the following study designs will be considered for inclusion: SRs of randomized controlled trials (RCTs) and observational studies, as well as primary studies that corresponded to RCTs, and prospective and retrospective observational studies (case control and cohort studies). Furthermore, only studies published as full texts and publications ahead of print will be included. Narrative reviews, case series, case reports, and qualitative and cross-sectional studies will be excluded.
Population. Adult and adolescent participants (aged over 10 years) undergoing OS will be included. Syndromic patients will be excluded.
Interventions. Co-interventions and surgical modalities used during the OS perioperative period (mono- or bimaxillary surgery, including all types of osteotomies) will be considered. Studies regarding distraction osteogenesis and those whose focus relied on orthodontic procedures or surgical planning will be excluded.
Comparators. Different approaches to performing OS, different co-interventions, and use of placebo or no treatment (control group) associated with co-interventions will be included. Studies with no comparison group or studies in which the comparison group did not undergo OS will be excluded.
Outcomes. All outcomes arising from the intra- or postoperative OS period will be considered and mapped. Studies of co-interventions with preoperative or economic outcomes will be excluded. Since this mapping review will only include clinical outcomes, quality of life studies will be excluded.
Literature Search
A literature search will be performed in the following databases: MEDLINE (via PubMed), EMBASE (via OVID), Epistemonikos, Lilacs, Web of Science, and CENTRAL. The search strategy will be adapted for each database, considering the differences in controlled vocabulary and syntax rules. No date or language restrictions will be applied. The search strategy developed for MEDLINE is displayed in Appendix A.
The PROSPERO and clinicaltrials.gov registration platforms will also be searched for unpublished studies. A snowball approach will be used for screening reference lists to identify potentially eligible studies.
Data Collection and Extraction
Two independent reviewers will screen titles and abstract for results obtained from the search. The full texts of selected studies will be assessed independently by two authors. Studies that do not meet the inclusion criteria will be recorded with reasons for their exclusion. Any disagreements, either in the title/abstract or full-text screening phases, will be resolved by consensus or, if necessary, by a third author. The Covidence platform will be used for this process, and, as recommended in the Cochrane Handbook for Systematic Reviews of Intervention [15], the selection process will be documented in sufficient detail to generate a PRISMA flowchart.
Data will be extracted and tabulated by one author, using a previously piloted data extraction form, and will be double-checked by a content expert in the subject. The following information will be extracted from each SR: search date, year of publication, country, objective, number of primary studies included, number of participants, population, co-interventions, comparisons, and outcomes measured. SR research gaps will also be identified based on the aim, eligibility criteria, and conclusions (Table 1). The PICO format will be used throughout. For primary studies not included in any identified SRs, the following data will be extracted: year of publication, study design, objective, number of included patients, population, interventions, comparisons, and outcomes. The authors of the studies will be contacted if additional information was required.

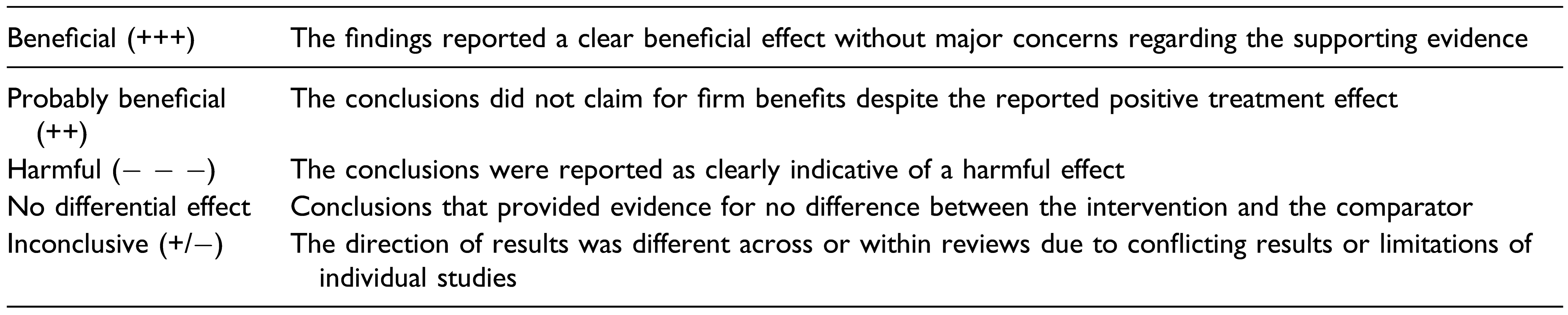
Table 1.
Classification of conclusions according to results reported by authors.
Methodological Quality Evaluation
Methodological quality will be independently evaluated for all the included SRs by two authors. Any disagreements will be resolved by consensus, or if necessary, by a third author. To evaluate the included SRs, the AMSTAR-2 instrument [16] will be used, which critically appraises SRs in 16 domains. The overall rating will be based on weaknesses in critical domains, namely, items 2, 3, 7, 9, 11, 13, and 15. Overall confidence in SR results—used for the mapping diagrams—will be rated in the following four quality categories: critically low (more than one critical flaw), low (one critical flaw), moderate (more than one non-critical flaw), and high (no flaw or just one non-critical flaw). The included primary studies will not be critically appraised since they are included to identify knowledge gaps rather than to inform clinical or policy decisions.
Data Synthesis and Analysis
Results will be summarized in tabular formats, describing the included study characteristics and all the identified PICO questions. A flow diagram will be created to classify the included studies, by design at the first level and by intervention at the second level (Figure 1). An evidence matrix to link primary studies with their SRs will be created using the Epistemonikos platform to identify primary studies not included in the SRs.
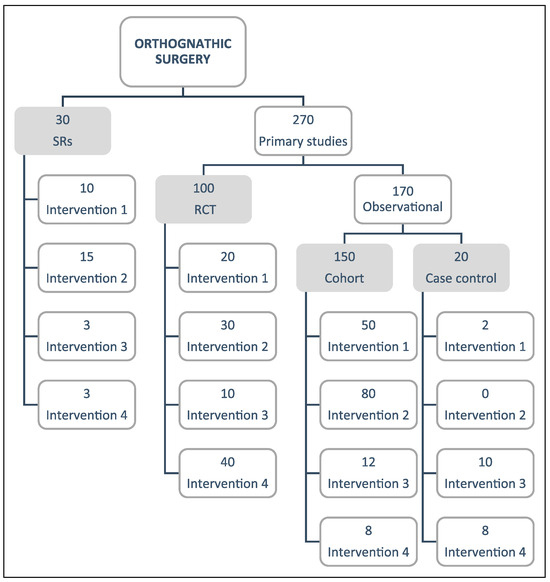
Figure 1.
Example of flow diagram.
Evidence bubble maps will be created to graphically depict the available evidence (Figure 2). All interventions reviewed in the included studies will be listed in rows, and reported outcomes listed in columns, and symbols indicate the study design (circles for SRs, squares for RCTs, and triangles for observational studies). Bubble size will represent the number of primary studies included in the SRs or the number of participants included in the primary studies. A number positioned over each bubble will indicate the number of studies. Colors will indicate confidence in SR findings (based on the AMSTAR-2 evaluation). The primary studies included in the maps will be those not included in any of the identified SRs.
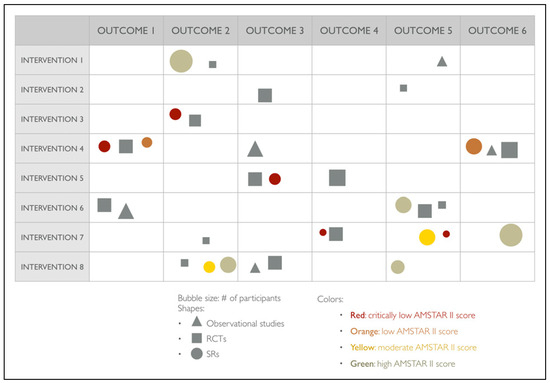
Figure 2.
Proposed evidence map for OS.
In the second phase, specific evidence maps will be developed for each of the selected outcomes of interest (blood loss, infection and relapse). The rows will list interventions included in the map, whereas the columns will consider if the treatment is reported as “beneficial,” “probably beneficial,” “no differential effect,” “harmful,” or “inconclusive.” The size of the bubble will indicate the relative number of studies included in the case of SRs or the number of participants in the case of RCTs. The squares will correspond to RCTs, the triangles, for observational studies, whereas the bubbles, for SRs. The colors in the SRs indicate the confidence in findings from the review, based on the AMSTAR II evaluation. The only primary studies included in this matrix will be the ones that are not reported by any of the identified SRs (Figure 3).
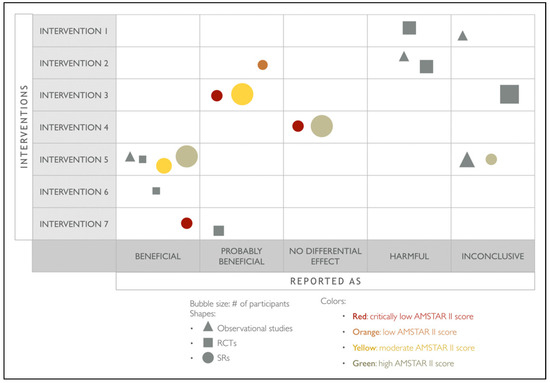
Figure 3.
Proposed evidence map for the outcomes blood loss, infection, and relapse.
Conclusion
The significance of this study into the research field of OS will result in a systematic identification and characterization of the available evidence, leading to a reduction in research waste, allowing a canalization of future efforts in developing studies for unsolved questions.
Author Contributions
J.B. conceived the protocol. J.B. drafted the manuscript and all the other authors contributed to it. The corresponding author is the guarantor and declares that all authors meet authorship criteria and that no other authors meeting the criteria have been omitted.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval
As researchers will not access information that could lead to the identification of an individual participant, obtaining ethical approval was waived.
Acknowledgments
We thank Ivan Sola for his help in the review of the search strategy.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Appendix A. Search Strategy for MEDLINE
- SR:
("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular distraction"[Title] OR "bone lengthening"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) AND (systematicreview[Filter])
- RCT:
(("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) NOT (("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) AND "Systematic"[Filter])) AND (("randomized controlled trial"[Publication Type] OR "controlled clinical trial"[Publication Type] OR "randomized"[Title/Abstract] OR "placebo"[Title/Abstract] OR "drug therapy"[MeSH Subheading] OR "randomly"[Title/Abstract] OR "trial"[Title/Abstract] OR "groups"[Title/Abstract]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]))
- Cohort:
("Case-Control Studies"[MeSH Terms] OR ("case"[Title] AND "control"[Title]) OR "case control"[Title/Abstract] OR "nested"[Title/Abstract] OR "match*"[Title/Abstract]) AND (("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) NOT ((("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) AND "Systematic"[Filter]) OR ((("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) NOT (("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[-Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) AND "Systematic"[Filter])) AND (("randomized controlled trial"[Publication Type] OR "controlled clinical trial"[Publication Type] OR "randomized"[Title/Abstract] OR "placebo"[Title/Abstract] OR "drug therapy"[MeSH Subheading] OR "randomly"[Title/Abstract] OR "trial"[Title/Abstract] OR "groups"[Title/Abstract]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]))) OR ((("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) NOT ((("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR"sagittal split"[Title]OR "bsso"[Title]OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) AND "Systematic"[Filter]) OR ((("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) NOT (("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) AND "Systematic"[Filter])) AND (("randomized controlled trial"[Publication Type] OR "controlled clinical trial"[Publication Type] OR "randomized"[Title/Abstract] OR "placebo"[Title/Abstract] OR "drug therapy"[MeSH Subheading] OR "randomly"[Title/Abstract] OR "trial"[Title/Abstract] OR "groups"[Title/Abstract]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]))))) AND ("Cohort Studies"[MeSH Terms] OR "cohort*"[Title/Abstract] OR "prospective*"[Title/ Abstract] OR "propensity"[Title/Abstract]))))
- Case control:
(("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[-Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) NOT ((("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) AND "Systematic"[Filter]) OR ((("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) NOT (("orthognathic surgery"[Title] OR "orthognathic surgical procedures"[Title] OR "sagittal split"[Title] OR "bsso"[Title] OR "ivro"[Title] OR "genioplasty"[Title] OR "mandibular advancement"[Title] OR "orthognathic surgical procedures"[MeSH Terms] OR "orthognathic surgery"[MeSH Terms]) AND "Systematic"[Filter])) AND (("randomized controlled trial"[ Publication Type] OR "controlled clinical trial"[Publication Type] OR "randomized"[Title/Abstract] OR "placebo"[Title/Abstract] OR "drug therapy"[MeSH Subheading] OR "randomly"[Title/Abstract] OR "trial"[Title/Abstract] OR "groups"[Title/Abstract]) NOT ("animals"[MeSH Terms] NOT "humans"[MeSH Terms]))))) AND ("Cohort Studies"[MeSH Terms] OR "cohort*"[Title/Abstract] OR "prospective*"[Title/Abstract] OR "propensity"[Title/Abstract])
References
- Chow, L.K.; Singh, B.; Chiu, W.K.; Samman, N. Prevalence of postoperative complications after orthognathic surgery: A 15-year review. J. Oral Maxillofac. Surg. 2007, 65, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.K.; Yang, E.J.; Oh, K.S.; Lo, L.J. Assessment of blood loss and need for transfusion during bimaxillary surgery with or without maxillary setback. J. Oral Maxillofac. Surg. 2013, 71, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Panula, K.; Finne, K.; Oikarinen, K. Incidence of complications and problems related to orthognathic surgery: A review of 655 patients. J. Oral Maxillofac. Surg. 2001, 59, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Pineiro-Aguilar, A.; Somoza-Martin, M.; Gandara-Rey, J.M.; Garcia-Garcia, A. Blood loss in orthognathic surgery: A systematic review. J. Oral Maxillofac. Surg. 2011, 69, 885–892. [Google Scholar] [CrossRef] [PubMed]
- American Association of Oral and Maxillofacial Surgeons. Criteria for Orthognathic Surgery; Published online; 2008. [Google Scholar]
- Naran, S.; Steinbacher, D.M.; Taylor, J.A. Current concepts in orthognathic surgery. Plast. Reconstr. Surg. 2018, 141, 925e–936e. [Google Scholar] [CrossRef] [PubMed]
- Delaire, J.; Schendel, S.A.; Tulasne, J.F. An architectural and structural craniofacial analysis: A new lateral cephalometric analysis. Oral Surg. Oral Med. Oral Pathol. 1981, 52, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Wallach, M.; Cuellar, J.; Verdugo-Paiva, F.; Alarcon, A. Long-term antibiotic prophylaxis regimen compared to short-term antibiotic prophylaxis regimen in patients undergoing orthognathic surgery. Medwave 2020, 20, e8072. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.M. Orthognathic surgery dilemma: Increasing access. J. Oral Maxillofac. Surg. 2011, 69, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Olate, S.; Sigua, E.; Aspirino, L.; De Moraes, M. Complications in orthognathic surgery. J. Craniofac. Surg. 2018, 29, e158–e161. [Google Scholar] [CrossRef] [PubMed]
- Miake-Lye, I.M.; Hempel, S.; Shamman, R.; Shekelle, P.G. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst. Rev. 2016, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Braege, P.; Clavisi, O.; Turner, T.; Tavender, E.; Collie, A.; Gruen, R.L. The global evidence mapping initiative: Scoping research in broad topic areas. BMC Med. Res. Methodol. 2011, 11, 92. [Google Scholar] [CrossRef]
- Snilsivelt, B.; Vojtkova, M.; Bhavsar, A.; Stevenson, J.; Gaarder, M. Evidence & gap maps: A tool for promoting evidence informed policy and strategic research agendas. J. Clin. Epidemiol. 2016, 79, 120–129. [Google Scholar] [CrossRef]
- Clavisi, O.; Braege, P.; Tavender, E.; Turner, T.; Gruen, R.L. Effective stakeholder participation in setting research priorities using a Global Evidence Mapping approach. J. Clin. Epidemiol. 2013, 66, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpson, M.; Li, T.; Page, M.J. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; Cochrane Training, 2021; Available online: www.training.cochrane.org/handbook.
- Shea, B.J.; Reeves, B.C.; Wells, G.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 21, j4008. [Google Scholar] [CrossRef]
Author Biography
Josefina Bendersky is a PhD candidate at the Methodology of Biomedical Research and Public Health programme, Universitat Autònoma de Barcelona, Spain.
© 2022 by the author. Multimed Inc.