The Role of Bone Grafts in Preventing Medication-Related Osteonecrosis of the Jaw: Histomorphometric, Immunohistochemical, and Clinical Evaluation in Animal Model
Abstract
:Introduction
Materials and Methods
Study Design and Ethics
MRONJ Rat Model
Surgical Procedure
Euthanasia
Histomorphometric and Histological Analysis
Immunohistochemical Analysis
Clinical Evaluation
Statistical Assessment
Results
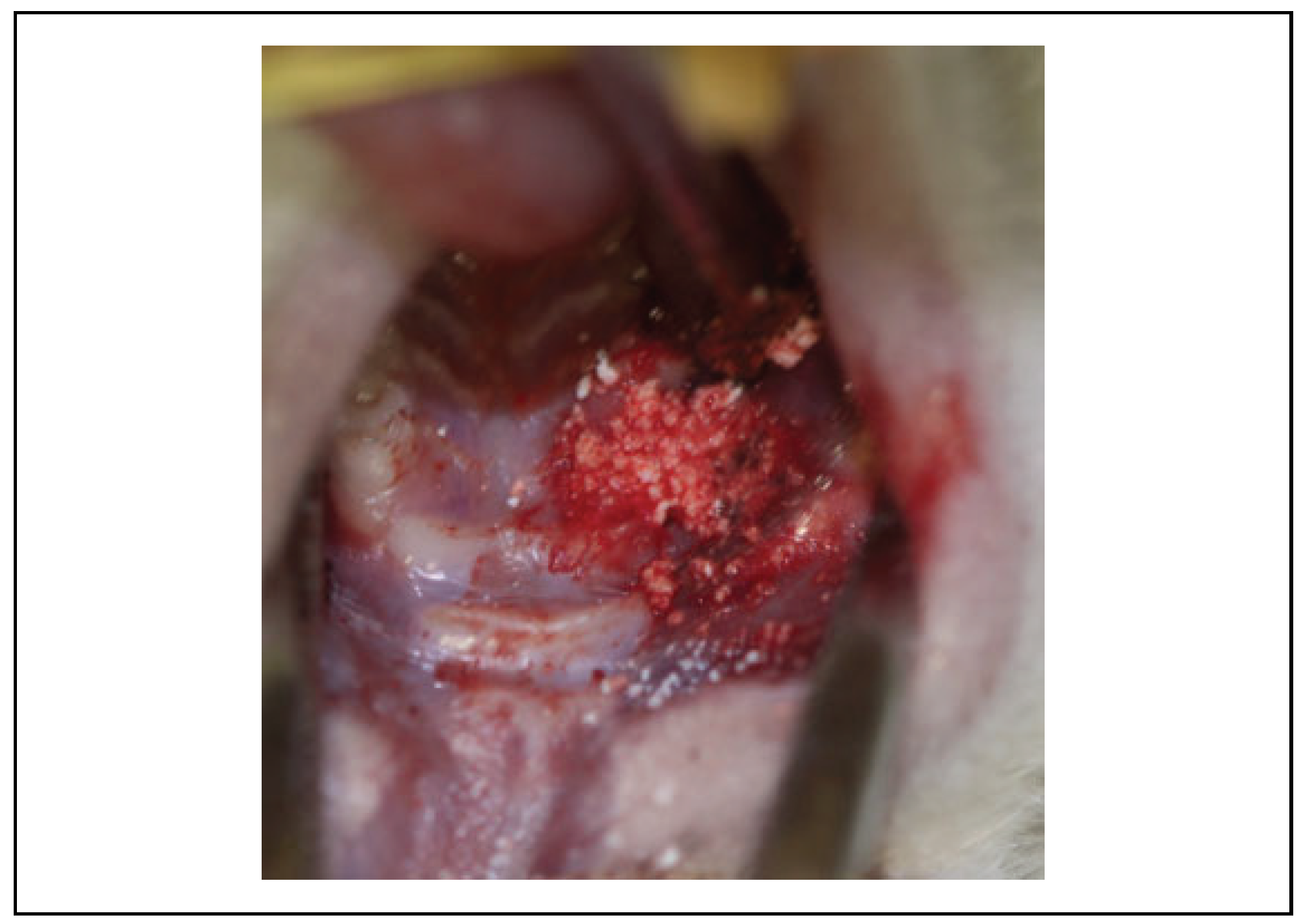
Clinical Evaluation
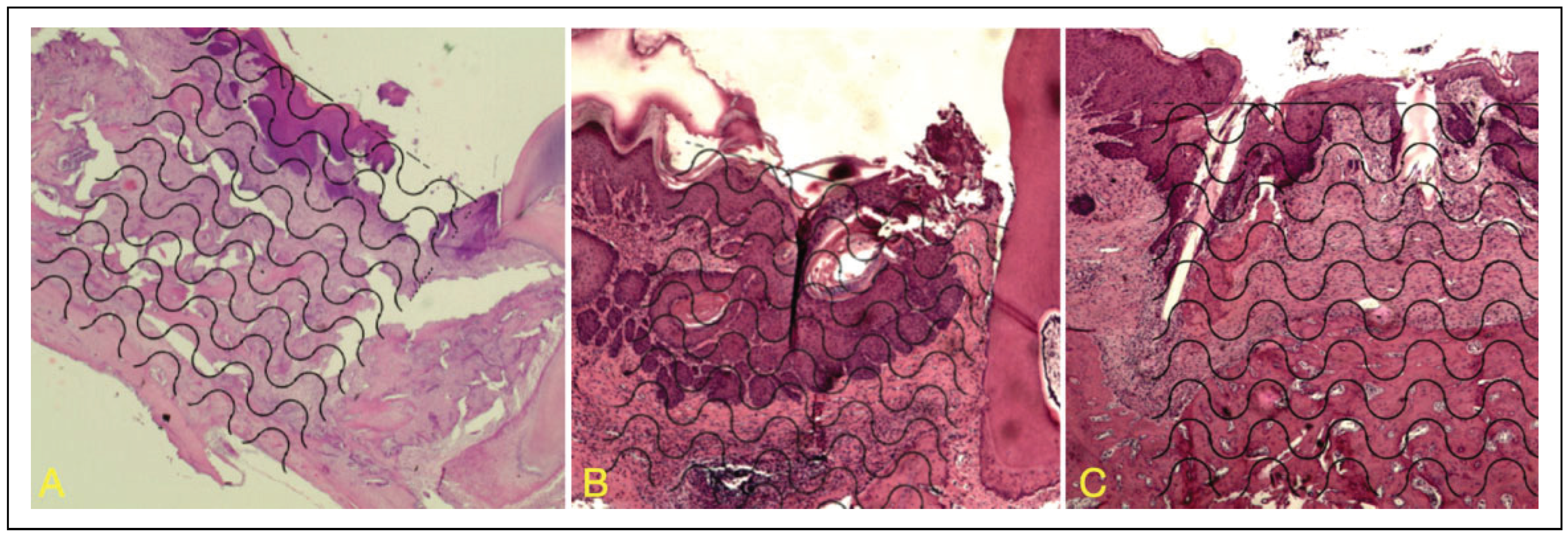
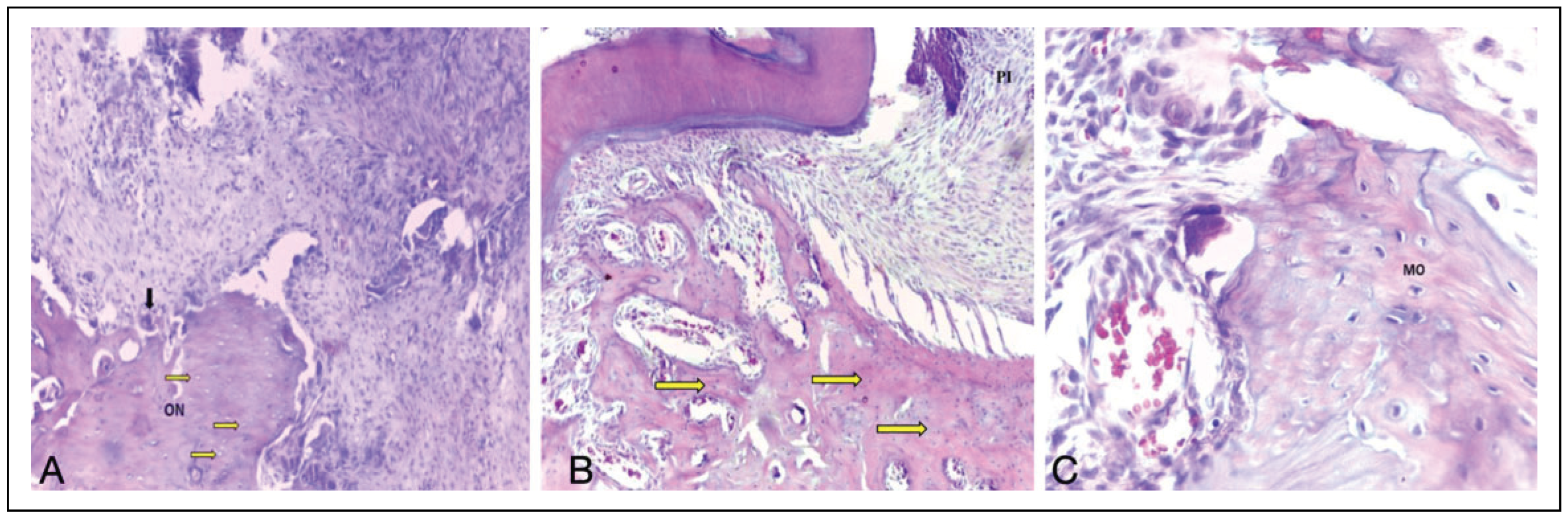
Histological Analysis
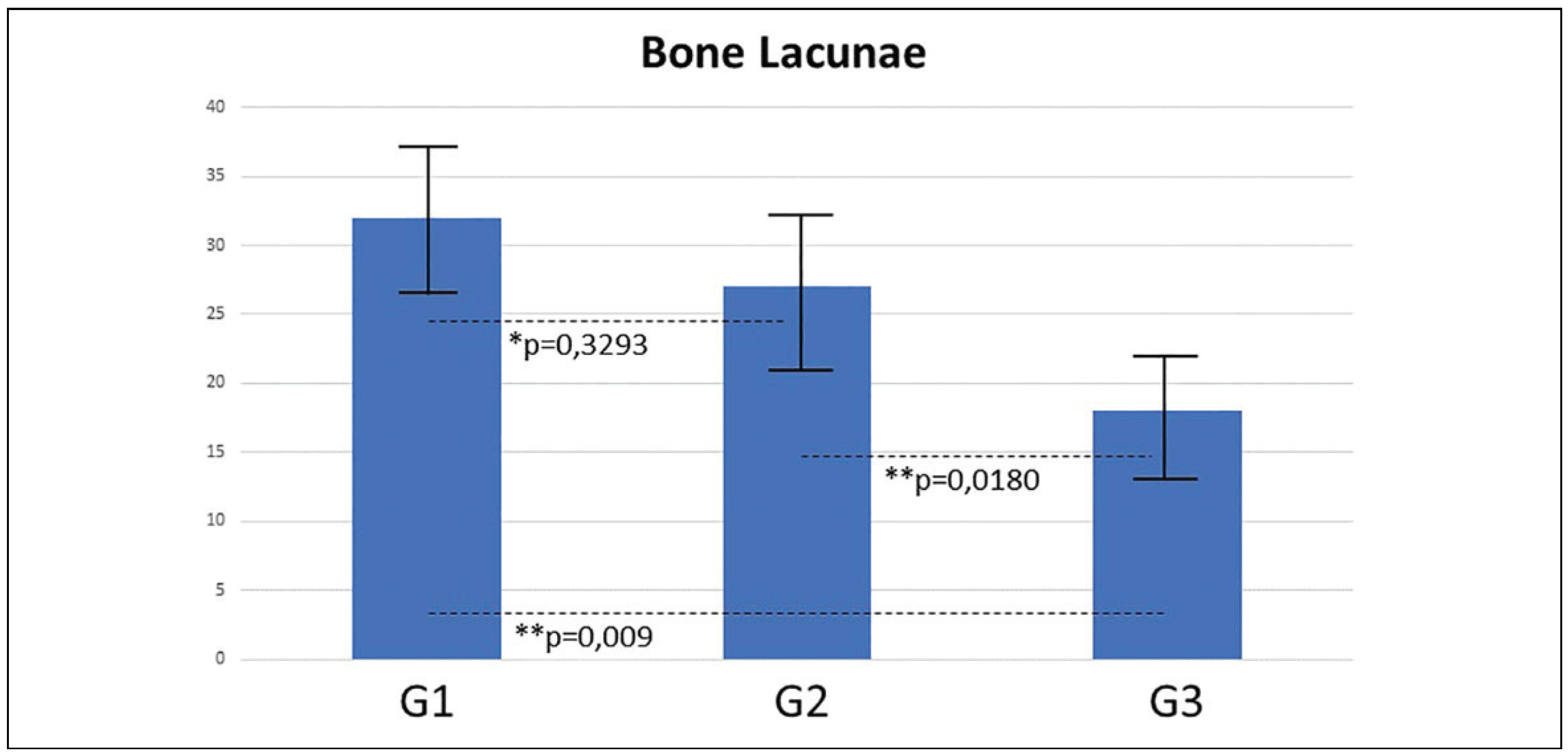
Histomorphometric Analysis
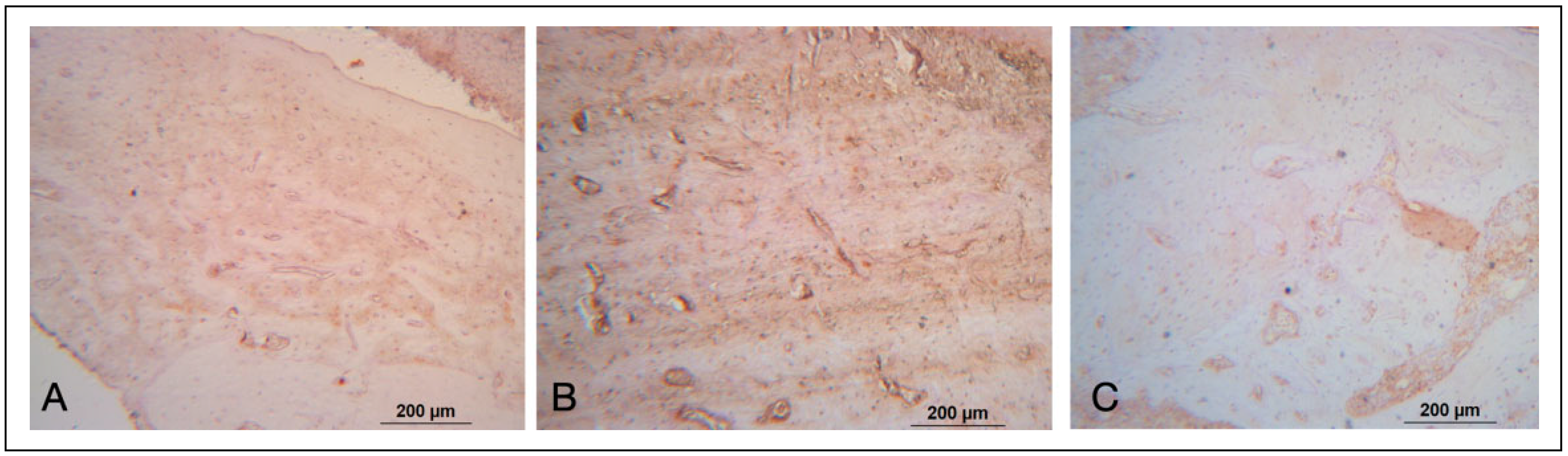
Immunohistochemical Analysis
Discussion
Conclusion
Funding
Acknowledgments
Conflicts of Interest
Author’s note
Ethical Approval
References
- Russell, R.G. Bisphosphonates: the first 40 years. Bone. 2011, 49, 2–19. [Google Scholar] [CrossRef]
- Sharma, P.; Chatterjee, P. Response monitoring to bisphosphonate therapy in monostotic Paget disease using (18)F-FDG PET/CT. Clin Nucl Med. 2015, 40, 499–500. [Google Scholar] [CrossRef]
- Marx, R.E. A decade of bisphosphonate bone complications: what it has taught us about bone physiology. Oral Craniofac Tissue Eng. 2012, 2, 309–320. [Google Scholar]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw—2009 update. J Aust Soc Endodontol Inc. 2009, 35, 119–130. [Google Scholar]
- Schwartz, H.C. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update and CTX. J Oral Maxillofac Surg. 2015, 73, 377. [Google Scholar] [PubMed]
- Marx, R.E.; Cillo, J.E.; Jr Ulloa, J.J. Oral bisphosphonate-induced osteonecrosis: risk factors, prediction of risk using serum CTX testing, prevention, and treatment. Official J Am Assoc Oral Maxillofac Surg. 2007, 65, 2397–2410. [Google Scholar]
- Otto, S.; Pautke, C.M.D.; Hafner, S.; et al. Pathologic fractures in bisphosphonate-related osteonecrosis of the jaw—review of the literature and review of our own cases. Craniomaxillofac Trauma Reconstr. 2013, 6, 147–154. [Google Scholar] [CrossRef]
- García-de Marcos, J.A.; Rey-Biel, J. Submental perforator flap for soft-tissue reconstruction in bisphosphonate-related osteonecrosis of the jaws. Craniomaxillofac Trauma Reconstr. 2017, 10, 299–305. [Google Scholar]
- Dal Pra, K.J.; Lemos, C.A.; Okamoto, R.; Soubhia, A.M.; Pellizzer, E.P. Efficacy of the C-terminal telopeptide test in predicting the development of bisphosphonate-related osteonecrosis of the jaw: a systematic review. Int J Oral Maxillofac Surg 46, 151–156.
- Shintani, T.; Hayashido, Y.; Mukasa, H.; et al. Comparison of the prognosis of bisphosphonate-related osteonecrosis of the jaw caused by oral and intravenous bisphosphonates. Int J Oral Maxillofac Surg. 2015, 44, 840–844. [Google Scholar]
- Hokugo, A.; Christensen, R.; Chung, E.M.; et al. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res. 2010, 25, 1337–1349. [Google Scholar] [PubMed]
- Biasotto, M.; Chiandussi, S.; Zacchigna, S.; et al. A novel animal model to study non-spontaneous bisphosphonates osteonecrosis of jaw. J Oral Pathol Med. 2010, 39, 390–396. [Google Scholar]
- Statkievicz, C.; Toro, L.F.; Mello-Neto, J.M.; et al. Photomodulation multiple sessions as a promising preventive therapy for medication-related osteonecrosis of the jaws after tooth extraction in rats. J Photochem Photobiol B 2018, 184, 7–17. [Google Scholar] [CrossRef]
- Sarkarat, F.; Motamedi, M.H.K.; Jahanbani, J.; Sepehri, D.; Kahali, R.; Nematollahi, Z. Platelet-rich plasma in treatment of zoledronic acid-induced bisphosphonate-related osteonecrosis of the jaws. Trauma Mon. 2014, 19, e17196. [Google Scholar]
- Barba-Recreo, P.; Del Castillo Pardo de Vera, J.L.; GeorgievHristov, T.; et al. Adipose-derived stem cells and platelet rich plasma for preventive treatment of bisphosphonate-related osteonecrosis of the jaw in a murine model. J Craniomaxillofac Surg. 2015, 43, 1161–1168. [Google Scholar] [PubMed]
- Milinkovic, I.; Cordaro, L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int J Oral Maxillofac Surg. 2014, 43, 606–625. [Google Scholar] [PubMed]
- Horowitz, R.; Holtzclaw, D.; Rosen, P.S. A review on alveolar ridge preservation following tooth extraction. J Evid Based Dent Pract 2012, 12, 149–160. [Google Scholar] [CrossRef]
- Traini, T.; Degidi, M.; Sammons, R.; Stanley, P.; Piattelli, A. Histologic and elemental microanalytical study of anorganic bovine bone substitution following sinus floor augmentation in humans. J Periodontol. 2008, 79, 1232–1240. [Google Scholar]
- Jo, S.H.; Kim, Y.-K.; Choi, Y.-H. Histological evaluation of the healing process of various bone graft materials after engraftment into the human body. Materials. 2018, 11, 714. [Google Scholar]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft in animals: a systematic review. Int J Oral Maxillofac Surg. 2012, 41, 114–120. [Google Scholar] [CrossRef]
- Gorla, L.d.O.; Spin-Neto, R.; Boos, F.; Pereira, R.d.S.; Garcia-Junior, I.; Hochuli-Vieira, E. Use of autogenous bone and betatricalcium phosphate in maxillary sinus lifting: a prospective, randomized, volumetric computed tomography study. Int J Oral Maxillofac Surg. 2015, 44, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, J.P.; Pereira, R.; Lima, F.B.; et al. Prospective and randomized evaluation of ChronOS and Bio-Oss in human maxillary sinuses: histomorphometric and immunohistochemical assignment for Runx 2, vascular endothelial growth factor, and Osteocalcin. J Oral Maxillofac Surg. 2018, 76, 325–335. [Google Scholar] [PubMed]
- Adachi, N.; Ayukawa, Y.; Yasunami, N.; et al. Preventive effect of fluvastatin on the development of medication-related osteonecrosis of the jaw. Sci Rep. 2020, 10, 1–8. [Google Scholar]
- Wasserzug, O.; Kaffe, I.; Lazarovici, T.S.; et al. Involvement of the maxillary sinus in bisphosphonate-related osteonecrosis of the jaw: radiologic aspects. Am J Rhinol Allergy. 2017, 31, 36–39. [Google Scholar] [CrossRef]
- Cardoso, C.L.; Curra, C.; Curi, M.M.; et al. Treatment of bisphosphonate-related osteonecrosis using platelet-rich plasma: microtomographic, microscopic, and immunohistochemical analyses. Braz Oral Res. 2019, 33, e050. [Google Scholar]
- Menezes, J.D.; Pereira, R.D.S.; Bonardi, J.P.; Griza, G.L.; Okamoto, R.; Hochuli-Vieira, E. Bioactive glass added to autogenous bone graft in maxillary sinus augmentation: a prospective histomorphometric, immunohistochemical, and bone graft resorption assessment. J Appl Oral Sci. 2018, 26. [Google Scholar]
- Howie, R.N.; Bhattacharyya, M.; Salama, M.E.; et al. Removal of pamidronate from bone in rats using systemic and local chelation. Arch Oral Biol. 2015, 60, 1699–1707. [Google Scholar]
- Zandi, M.; Dehghan, A.; Zandipoor, N.; Amini, P.; Doulati, S. Effect of different doses and durations of teriparatide therapy on resolution of medication-related osteonecrosis of the jaw: a randomized, controlled preclinical study in rats. J Craniomaxillofac Surg. 2018, 46, 466–472. [Google Scholar]
- Hassumi, J.S.; Mulinari-Santos, G.; Fabris, A.L.d.S.; et al. Alveolar bone healing in rats: micro-CT, immunohistochemical and molecular analysis. J Appl Oral Sci. 2018, 26, e20170326. [Google Scholar]


| Group | Grafting material | Number of animals |
|---|---|---|
| 1 | Control (Clot) | 6 |
| 2 | Inorganic bovine bone graft (Lumina Bone®) | 6 |
| 3 | β-TCP(chronOS®) | 6 |
| Total | 18 |
| Group | Necrotic Bone exposure | Normal soft tissue healing | Total of Animals |
|---|---|---|---|
| G1: Control Group | 06 | 0 | 6 |
| G2: Inorganic bovine bone graft (Lumina Bone®) | 0 | 6 | 6 |
| G3: b TCP(chronOS®) | 0 | 6 | 6 |
| TRAP | RANKL | OC | |
|---|---|---|---|
| Group 1 | + | + | ++ |
| Group 2 | ++ | ++ | ++ |
| Group 3 | ++ | ++ | ++ |
© 2021 by the author. The Author(s) 2021.
Share and Cite
da Silva, J.R.; Balbas, M.C.d.M.; Corrêa, C.Á.; Zanela, M.; Okamoto, R.; Pereira, R.d.S.; Homsi, N.; Hochuli-Vieira, E. The Role of Bone Grafts in Preventing Medication-Related Osteonecrosis of the Jaw: Histomorphometric, Immunohistochemical, and Clinical Evaluation in Animal Model. Craniomaxillofac. Trauma Reconstr. 2022, 15, 304-311. https://doi.org/10.1177/19433875211048367
da Silva JR, Balbas MCdM, Corrêa CÁ, Zanela M, Okamoto R, Pereira RdS, Homsi N, Hochuli-Vieira E. The Role of Bone Grafts in Preventing Medication-Related Osteonecrosis of the Jaw: Histomorphometric, Immunohistochemical, and Clinical Evaluation in Animal Model. Craniomaxillofacial Trauma & Reconstruction. 2022; 15(4):304-311. https://doi.org/10.1177/19433875211048367
Chicago/Turabian Styleda Silva, Jonathan Ribeiro, Maria Cristina de Moraes Balbas, Caroline Águeda Corrêa, Manuella Zanela, Roberta Okamoto, Rodrigo dos Santos Pereira, Nicolas Homsi, and Eduardo Hochuli-Vieira. 2022. "The Role of Bone Grafts in Preventing Medication-Related Osteonecrosis of the Jaw: Histomorphometric, Immunohistochemical, and Clinical Evaluation in Animal Model" Craniomaxillofacial Trauma & Reconstruction 15, no. 4: 304-311. https://doi.org/10.1177/19433875211048367
APA Styleda Silva, J. R., Balbas, M. C. d. M., Corrêa, C. Á., Zanela, M., Okamoto, R., Pereira, R. d. S., Homsi, N., & Hochuli-Vieira, E. (2022). The Role of Bone Grafts in Preventing Medication-Related Osteonecrosis of the Jaw: Histomorphometric, Immunohistochemical, and Clinical Evaluation in Animal Model. Craniomaxillofacial Trauma & Reconstruction, 15(4), 304-311. https://doi.org/10.1177/19433875211048367




