Abstract
Objective: The purpose of this study was to determine the usefulness of active decompression and distraction sugosteogenesis (ADDS) for the management of non-syndromic odontogenic keratocysts (OKC). Materials and Methods: A retrospective case series study was designed and implemented. The study observed the Declaration of Helsinki on medical protocol and ethics and it was approved by the university’s Institutional Review Board (IRB). The medical files of all patients who underwent ADDS for OKCs of the jaws at the Department of Oral and Maxillofacial Surgery of a tertiary university-affiliated medical center were reviewed. Data were collected on patient’s age, gender, presenting signs and symptoms, lesion location, locularity, pre-ADDS, size of the lesion, post-ADDS, pain, days with bloody discharge and/or proteinaceous fluid inside the system’s external unit, days to achieve hermetic seal, size of the lesion 2 weeks after ADDS, percentage of reduction, patient’s complaints/complications, and follow-up period. Preand post-ADDS panoramic radiographs were reviewed for reduction parameters. Results: Six patients, 5 males and 1 female, with an average age of 45.16 years (range 16-74 years) were studied. ADDS was performed during 4 weeks in all patients. During the therapy, the extraoral unit collected blood during 2.83 days in average. In average, after the third day, the cystic cavity started to drain a proteinaceous fluid for about 9.33 days (range 6-15 days). The average pre-ADDS Standard Lesional Area Index (SLAI) was 18.17 cm2 (range 4.40 cm2-34.58 m2) and, after 2 weeks of ADDS, the average SLAI was 5.47 cm2 (range 0.49 cm2-15.39 cm2). The average percentage of reduction, after 2 weeks, was 73.93% (range 55.49%-97.51%), which yielded an overall good reaction of OKCs to ADDS. No significant reduction of the lesions was observed from week 2 to week 4, when ADDS ceased. All lesions were enucleated after 3 months. After an average of 14 months of follow-up (12 to 17 months), no signs of recurrence have been observed.
Introduction
The odontogenic keratocyst (OKC) is an intraosseous, benign odontogenic cyst having a characteristic lining of parakeratinized stratified squamous epithelium originating from the dental lamina or from primordial odontogenic epithelium.[1,2] Before it became best known for its locally aggressive behavior and high tendency to recur, surgeons treated it conservatively, i.e. marsupialization, decompression, enucleation, curettage. During the late 1900s, in an attempt to combat such high recurrence rates, aggressive approaches (marginal or segmental resection) were advocated.[3] Currently, it appears that surgeons are gradually returning to conservative therapies as primary or definitive approach, as suggested by Pogrel.[4,5,6]
Most of the commonly occurring odontogenic cysts enlarge principally due to the osmotic pressure of the cyst’s fluid, which causes bone resorption while the entity expands.[7] Relieving this intracystic pressure by the use of a plethora of drainage systems has been found to be a predictable method to reduce the entity’s size and change its environment.[8] Active Decompression and Distraction Sugosteogenesis (ADDS) is a novel, dual technique recently described for the management of odontogenic pathologies.[9,10,11,12,13] The aforementioned technique employs a vacuum-like device known as Evacuator for Odontogenic Cysts (Evocyst) to exert active intracystic negative pressure in order to induce bone formation (sugosteogenesis) while removing important cystic components.[11,12]
The purpose of this study is to further determine the usefulness of ADDS in the context of the more common OKC and to discuss its potential role in the management of other odontogenic cystic lesions.
Materials and Methods
Type of Study and Data Collection
We designed and implemented a retrospective case series research. The study observed the Declaration of Helsinki on medical protocol and ethics and it was approved by the university’s Institutional Review Board (IRB protocol #B0880121). In order to fulfill the aim of the study, we reviewed the medical files of all patients who underwent ADDS for OKC of the jaws at the Department of Oral and Maxillofacial Surgery of a tertiary university-affiliated medical center from 2019 to 2020. Data were collected regarding patient’s age, gender, presenting signs and symptoms, lesion location, locularity, pre-ADDS size of the lesion, post-ADDS pain, days with bleeding and proteinaceous fluid inside the system’s external unit, days to achieve hermetic seal, size of the lesion 2 weeks after ADDS, percentage of reduction, patient’s complaints/complications, and follow-up period. Patient’s panoramic X-rays (Sirona, Dentsply, Charlotte, NC) pre-ADDS and post-ADDS were reviewed. Images were taken employing the same technique. If further assessment was required, a computed tomography (CT) was ordered.
All measurements on panoramic X-rays (Sirona, Dentsply, Charlotte, NC) were taken by the same investigator (AW) according to equations A, B, and C, as proposed by Anavi et al.[14] In order to simplify the area measurement of the irregular forms of the OKCs, the Standard Lesion Area Index (SLAI) was utilized, as follows:

The percentage of reduction of the lesional area (POR) was recorded as a fraction of the final SLAI (SLAIf) from the initial SLAI (SLAIi), as follows:

As established by Nakamura et al,[15] the entity’s response to treatment was graded according to percentages, as follows:
- 80-100% reduction: good reaction to treatment.
- 50-80% reduction: moderate reaction.
- 0-50% reduction: poor reaction.
In order to eliminate possible distortions/magnifications due to the panoramic imaging technique, the rate of reduction (RR) in 1 month was determined,[14] as follows:

Exclusion criteria for this study included missing clinical or radiographic data, cystic histologic diagnosis different from OKC, failure to follow ADDS home instructions, failure to present to follow-up appointments at 2 weeks, 4 weeks, 3 months, 6 months, and 12 months. Other exclusion criteria included diabetes, drug abuse, Gorlin-Goltz syndrome, and other systemic conditions affecting the gnathic system. Data were recorded and analyzed using Microsoft Excel (Redmond, WA, USA).
Treatment Protocol
All patients were operated by the same surgeon (JPP) using a standardized technique and materials. All devices employed for ADDS were fabricated by the same investigator (JCN), as such appliance is not commercially available. Both ADDS technique and the Evocyst have been described in detail elsewhere.[9,10,11,12,13] After incisional biopsy and installment of the system, patients were instructed to return to clinic at 2 weeks postoperatively with a panoramic X-ray (Sirona, Dentsply, Charlotte, NC) to evaluate cystic and osseous response to ADDS and to verify histopathologic diagnosis of the lesion.
After the initial 2-week evaluation, patients were then followed at 4 weeks, 3 months, 6 months, and 12 months. For each of the aforementioned visits, symptoms, clinical features, radiographic changes, size of the lesion, and other pertinent variables were recorded. In agreement with the parameters outlined by Gao et al,[16] in 2014, final enucleation of all lesions was performed once the lesion measured less than 3 cm2. When deemed necessary, definitive surgery consisted of enucleation or enucleation and peripheral ostectomy.
Results
The studied population consisted of 6 patients, 5 (83.33%) males and 1 (16.66%) female with an average age of 45.16 years (range 16-74 years). Demographics, presenting signs, symptoms, and OKCs pre-ADDS radiographic characteristics are shown in Table 1. Table 2 presents variables observed during the ADDS phase. As per technique’s protocol,[12] ADDS was performed during 4 weeks in all patients.

Table 1.
Demographics and Presenting Signs and Symptoms.

Table 2.
Variables Observed During the ADDS Phase.
The method employed a custom-made vacuum-like system composed of an intraoral unit and an extraoral unit, which are interconnected. The extraoral unit is an active negative pressure, closed drainage system that connects to the intraoral unit. The equipment provided a negative pressure of 45 mmHg. The intraoral unit is a 2-way, double tubing system (irrigation and decompression tubes). The irrigation (upper) tube contains a needle port used to introduce a lavage solution (NSS 0.9%). A suction or decompression conduit attaches to the irrigation tube. The distal ends of both tubes are placed inside the cavity. The anterior end of the decompression tube connects to the vacuum. The intraoral unit secures to teeth with orthodontic wire. This ligature was verified at every visit to prevent dislodgement of the intraoral unit.
During the therapy, the extraoral unit collected blood during 2.83 days in average. After the third day, the cystic cavity drained a proteinaceous fluid (Figure 1) for about 9.33 days (range 6-15 days). Hermetic seal (achieved at the site where the intraoral tubes enter the cyst) was never obtained immediately; mediate hermetic seal, i.e. obtained between days 1-7, was obtained in 5 patients (83.30%); and late hermetic seal, i.e. between days 8-30 (end of therapy), was obtained in 1 patient (16.66%).

Figure 1.
Proteinaceous fluid collected by the extraoral unit.
Regarding OKC response to ADDS, Table 3 shows that the average pre-ADDS SLAI was 18.17 cm2 (range 4.40 cm2-34.58 m2) and, after 2 weeks of ADDS, the average SLAI was 5.47 cm2 (range 0.49 cm2-15.39 cm2). The average percentage of size reduction, after 2 weeks, was 73.93% (range 55.49%-97.51%), which yielded an overall good reaction of OKCs to ADDS (Figure 2 and Figure 3). RR was 0.01 in all cases. All lesions were enucleated following 3 months of the original intervention. After an average of 14 months of follow-up (12 to 17 months), no signs of recurrence have been observed. All patients were placed at a 10-year follow-up period.

Table 3.
OKC Response to ADDS.
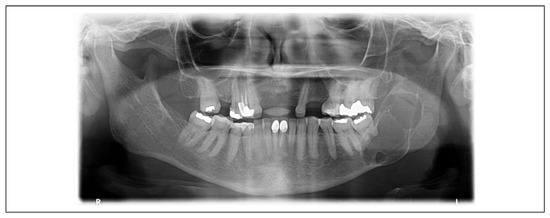
Figure 2.
Fifty-nine-year-old female patient presenting with a multiloculated lesion involving the left mandibular body, angle, and ramus. ADDS was initiated at the time of incisional biopsy.
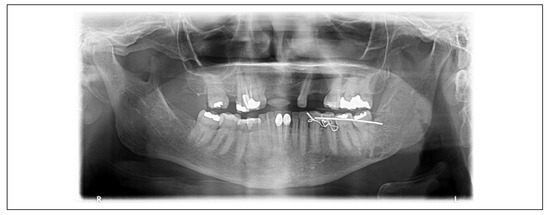
Figure 3.
Same 59-year-old female patient depicted before, after 2 weeks of ADDS. Almost the entire lesion has filled with bone. Only a small, well-corticallized radiolucent area remains below the lower left second molar.
Discussion
The aim of the present study was to determine the usefulness of ADDS for the management of OKCs. To achieve such goal, the authors designed and implemented a retrospective case series investigation. The studied population consisted of 6 patients with an average age of 45.16 years. The great majority of lesions (83.30%) occurred in the mandible. The mean SLAIi was 18.17 cm2 (range 4.40 cm2-34.58 m2) and, after 2 weeks of ADDS, the average SLAI was 5.47 cm2 (range 0.49 cm2-15.39 cm2). The mean POR, after 2 weeks, was 73.93% (range 55.49%-97.51), which shows that, in the studied population, all OKCs had a moderate to good reaction to ADDS, according to the parameters proposed by Nakamura et al.[15]
Marsupialization (cystostomy, Partsch I), as described by Partsch,[17] is a valid method for primary treatment of odontogenic cysts[2,4,5,6,8,15] and unicystic ameloblastomas.[18] Other conservative techniques, such as primary enucleation (cystectomy, Partsch II)[19]; decompression (drainierungs--Methode), as described by Neuschmidt[20] and popularized by Thomas[21] and Thoma,[22] have been found to be invaluable for the management of OKC.[4,5,8,14,16,23] Recently, by introducing the ADDS technique, Castro-Núñez[12] further subdivided decompression into passive and active. All 3 techniques (marsupialization, passive decompression, and active decompression) are based on the rationale that relieving the intraluminal cystic pressure causes the entity to decrease in size by gradual bone deposition from the periphery. From the results of this investigation, it appears that the later method achieves the same results, but at a much faster pace, presumably due to the osteoprogenitor cells response to the forces exerted by the Evocyst, as it will be discussed later in this article.
The results we obtained with ADDS are consonant with the ones reported by Anavi et al,[14] Nakamura et al,[15] and others[16,23,24,25,26] who have documented positive cystic response to marsupialization and passive decompression over a relative long period of time. Less treatment time, therefore, is the main and most remarkable difference between the employment of active decompression and passive drainage systems. Anavi et al,[14] for example, documented the passive decompression of 73 odontogenic cysts with a mean SLAIi of 14.5 cm2 (range 3.74 cm2 to 44 cm2). After a mean passive decompression time of 9.2 months (range 2-33 months), lesions reached a SLAIf mean area of 3.0 cm2 (range 0 cm2 to 22.3 cm2). In such study, the mean POR of SLAIf was 79.3% and the reaction was graded as good in 60% of lesions, moderate in 29%, and poor in 11%. In our study, reaction was graded as good in 33.32% of lesions and moderate in 66.64% of cases and, again, in only 2 weeks. While Anavi et al[14] achieved a RR of 0.11 for the mandible and 0.10 for the maxilla, ours was 0.01 irrespective of the location, which means that by using active decompression reduction occurred significantly faster.
Currently, this negative pressure-induced osteogenesis (sugosteogenesis) is done at the expense of patient’s discomfort, as 33.32% of them found the intraoral unit voluminous. The fact that the intraoral unit was considered somehow bulky by such percentage of patients should not be underestimated and they must be followed closely, as discomfort can limit oral hygiene. Although none of the subjects of this series developed either dental or periodontal conditions attributable to the intraoral unit, this research group is working on ways to reduce this drawback in order to make the intraoral unit comply with Tolstunov’s principles.[27]
The advantages of marsupialization and passive decompression are the gradual reduction of the cystic cavity, maintenance of pulp vitality, preservation of oral tissues and tooth buds in growing patients, prevention of dental extractions, avoidance of surgical trauma, and avoidance of mandibular fracture, among others.[4,5] Drawbacks include the need for patients’ cooperation, as they have to irrigate the cavity for several weeks and visit frequently the treating surgeon for several weeks/months.[4,5,6,8] Based on panoramic X-rays or CT scans, many methods and equations have been developed in order to evaluate the cyst’s response to such conservative approaches.[14,15,16,24,25,26]
Our results are in agreement with the aforementioned reports in terms of the positive response of the lesion to the therapy. ADDS, however, seems to be a more time-effective philosophy, as marsupialization/decompression requires somewhere between 2-80 months.[6,14,24] Kubota et al,[28] for instance, reported that a minimum decompression time of 12 months was required for a dentigerous cyst to decrease 50% of their original size, while Consolo et al[26] reported a volume reduction of 48.28% to 57.95% for various types of cysts after about 6 months and a reduction of 70.5% after 8 months, for a monthly reduction rate of 8.81%.
Although we used panoramic X-rays in this study, we agree with Chiapasco et al[29] that CT is a more exact tool to evaluate cystic lesions and their response to marsupialization/passive decompression. CT scans, however, are costly to use them routinely.[29] Moreover, current methods to evaluate osseous response to treatment are time-consuming, require special training and additional software, which challenge their use on daily basis, especially for practitioners outside academics/teaching institutions. Another limitation of the present study is the small sample employed and the relatively short follow up time.
As far as the accelerated bone response seen in patients subjected to ADDS, Castro-Núñez[12] has theorized about the possible underlying biological mechanisms. The fact that osseous tissue responds to physical forces is out of question, as it was demonstrated in 1892 by Julius Wolff in his seminal work Das Gesetz der Transformation der Knochen (The law of bone remodeling). Currently, concepts such as mechanotransduction, shear stress, oscillatory fluid flow, and many other provide the basis for our understanding of the bone remodeling process.
Regarding the effects of negative pressure on osteogenesis, Swain et al[30] have demonstrated how this type of force stimulates bone healing in animal models. ADDS might well be the first clinical demonstration of such findings. Taking the clinical and experimental data together, we believe that the accelerated osseous response seen in our patients is probably best explained by the tension that the Evocyst exerts on the osseous defect. This mechanical stimulation may promote angiogenesis, osteogenesis, and wound healing.
In conclusion, this paper has shown the favorable response of OKC to ADDS in a small group of patients. These results, therefore, must be evaluated cautiously. As a technique under development, no definitive protocols have been established. Furthermore, the long-term results remain to be seen. Currently, this research group is working on several projects aiming at developing smaller intraoral units to eliminate patient’s discomfort; drafting clinical studies involving a much larger sample; and determining the 3D volumetric response of other cystic lesions to ADDS.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their gratitude to professors Augusto Elias-Boneta and Sona Rivas-Tumanyan for their enthusiastic support to this project.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Soluk-Tekkes¸in, M.; Wright, J.M. The World Health Organization classification of odontogenic lesions: a summary of the changes of the 2017 (4th) edition. Turk Patoloji Derg. 2018, 34, 1–18. [Google Scholar]
- Pogrel, M.A. The keratocystic odontogenic tumor. Oral Maxillofacial Surg Clin N Am. 2012, 25, 21–30. [Google Scholar]
- Williams, T.P.; Connor, F.A. Surgical management of the odontogenic keratocyst: aggressive approach. J Oral Maxillofac Surg. 1994, 52, 964. [Google Scholar] [CrossRef]
- Pogrel, M.A. Treatment of keratocysts: the case for decompression and marsupialization. J Oral Maxillofac Surg. 2005, 63, 1667. [Google Scholar]
- Pogrel, M.A. Decompression and marsupialization as a treatment for the odontogenic keratocyst. Oral Maxillofac Surg Clin N Am. 2003, 15, 415. [Google Scholar]
- Pogrel, M.A. Marsupialization as a definitive treatment for the odontogenic keratocyst. J Oral Maxillofac Surg. 2004, 62, 651. [Google Scholar]
- Toller, P.A. The osmolality of fluids from cysts of the jaws. Br Dent J. 1970, 129, 275. [Google Scholar]
- Blanas, N.; Freund, B.; Schwartz, M.; Furst, I.M. Systematic review of the treatment and prognosis of odontogenic keratocyst. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000, 90, 553. [Google Scholar]
- Castro-Núñez, J. An innovative decompression device to treat odontogenic cysts. J Craniofac Surg. 2016, 27, 1316. [Google Scholar]
- Castro-Núñez, J.; Rey, D.; Amaya, L. An innovative intracystic negative pressure system to treat odontogenic cysts. J Craniofac Surg. 2017, 28, 1883–1884. [Google Scholar]
- Castro-Núñez, J. Active intracystic negative pressure could induce osteogenesis. J Craniofac Surg. 2018, 29, e370–e371. [Google Scholar] [PubMed]
- Castro-Núñez, J. Distraction sugosteogenesis: its biologic bases and therapeutic principles. J Craniofac Surg. 2018, 29, 2088–2095. [Google Scholar] [PubMed]
- Moreno-Rodríguez, P.; Guerrero, L.M.; Gómez-Delgado, A.; Castro-Núñez, J. Active decompression and distraction sugosteogenesis for the treatment of calcifying odontogenic cyst. Oral Maxillofac Surg. 2020, 25, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Anavi, Y.; Gal, G.; Miron, H.; Calderon, S.; Allon, D.M. Decompression of odontogenic cystic lesions: clinical long-term study of 73 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011, 112, 164–169. [Google Scholar]
- Nakamura, N.; Mitsuyasu, T.; Mitsuyasu, Y.; Taketomi, T.; Higuchi, Y.; Ohishi, M. Marsupialization for odontogenic keratocysts: long-term follow-up analysis of the effects and changes in growth characteristics. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002, 94, 543–553. [Google Scholar]
- Gao, L.; Wang, X.L.; Li, S.M.; et al. Decompression as a treatment for odontogenic cystic lesions of the jaws. J Oral Maxillofac Surg. 2014, 72, 327–333. [Google Scholar]
- Partsch, C. Uber kiefercysten. Dtsch Mschr Zahnheilkd. 1892, 10, 271–304. [Google Scholar]
- Nakamura, N.; Higuchi, Y.; Tashiro, H.; Ohishi, M. Marsupialization of cystic ameloblastoma: a clinical and histopathologic study of the growth characteristics before and after marsupialization. J Oral Maxillofac Surg. 1995, 53, 748–754. [Google Scholar]
- Partsch, C. Zur behandlung der kieferzysten. Dtsch Mschr Zahnheilkd. 1910, 28, 252–260. [Google Scholar]
- Neuschmidt, V. Drainierungs-methode der kieferzysten. Dtsch Zahnärztl Wschr. 1942, 21, 287. [Google Scholar]
- Thomas, E.H. Cysts of the jaws; saving involved vital teeth by tube drainage. J Oral Surg. 1947, 5, 1–9. [Google Scholar] [PubMed]
- Oral Surgery, 3rd ed.Thoma, K.H. (Ed.) Mosby, 1958; pp. 1033–1036. [Google Scholar]
- Hu, Y.-J.; Li, S.-Y.; Xu, L.-Q.; et al. Clinical study of large mandibular odontogenic keratocyst treated by decompression. China J Oral Maxillofac Surg. 2005, 3, 229–232. [Google Scholar]
- Lizio, G.; Sterrantino, A.F.; Ragazzini, S.; Marchetti, C. Volume reduction of cystic lesions after surgical decompression: a computerized three-dimensional computed tomography evaluation. Clin Oral Invest. 2013, 17, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G.; Hwang, J.J.; Lee, S.H.; Nam, W. Effect of decompression for patients with various jaw cysts based on a three-dimensional computed tomography analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2017, 123, 445–452. [Google Scholar] [CrossRef]
- Consolo, U.; Bellini, P.; Melini, G.M.; Ferri, A.; Lizio, G. Analysis of marsupialization of mandibular cysts in improving the healing of related bone defects. J Oral Maxillofac Surg. 2020, 78, 1355.e1–1355.e11. [Google Scholar] [CrossRef]
- Tolstunov, L. Marsupialization catheter. J Oral Maxillofac Surg. 2008, 66, 1077–1079. [Google Scholar] [CrossRef]
- Kubota Y, Yamashiro T, Oka S, et al. Relation between size of odontogenic jaw cysts and the pressure of fluid within. Br J Oral Maxillofac Surg. 2004, 42, 391–395. [Google Scholar] [CrossRef]
- Chiapasco, M.; Rossi, A.; Motta, J.J.; Crescentini, M. Spontaneous bone remodeling after enucleation of large mandibular cysts: a radiographic computed analysis of 27 consecutive cases. J Oral Maxillofac Surg. 2000, 58, 942–948. [Google Scholar] [CrossRef]
- Swain LD, Cornet DA, Manwaring ME, et al. Negative pressure therapy stimulates healing of critical-size calvarial defects in rabbits. Bonekey Rep. 2013, 2, 1–8. [Google Scholar]
© 2021 by the author. The Author(s) 2021.