Introduction
Prosthetic treatment of the temporomandibular joint (TMJ) is far from new, with the first alloplastic interpositioning dating back to the mid-19th century and total joint replacement (TJR) first reported in 1965.[
1] Since then, TJR has seen significant changes, using different designs and materials, as well as the development of both stock and computer-assisted design/computer-assisted manufacturing (CAD-CAM) systems. Most well-known current systems are the stock and patient-fitted Zimmer Biomet Microfixation TMJ Replacement System (Jacksonville, Florida) and the TMJ Concepts Patient-Fitted Total TMJ Replacement System (Ventura, California). Although several other PSIs are available on the market, these 2 systems are currently the only US Food and Drug Administration (FDA)-approved TJR systems available.[
1,
2]
The stock Biomet system makes use of 3 differently sized mandibular and fossa components, requiring the surgeon to select a size and intraoperatively alter the recipient’s bone to achieve a desirable fit.[
2,
3] In contrast, PSI joint replacements, such as the TMJ Concepts prosthesis, rely on CAD-CAM technology. A preoperative computed tomographic scan of the region of interest is digitally converted to a data set by which the TJR components are designed, considering any anatomical abnormalities and the need for occlusal correction. As such, the surgeon is not forced to adapt the anatomical structures to achieve a tight fit, and operating time is reduced. Fixation screw placement can be optimized, minimizing the risk of inferior alveolar nerve damage.[
2,
4,
5] As stated by Mercuri,[
5,
6] it is expected that PSIs, also known as custom(ized) systems, provide significantly better results compared to stock prostheses. Reimbursement stakeholders worry whether the results outweigh the higher production cost. Keeping in mind that the number of TMJ-TJRs is increasing over time and is projected to exceed 1000 procedures within 15 years in the United States alone,[
7] we set out to evaluate both systems by means of a meta-analysis, as to guide craniomaxillofacial (CMF) surgeons and reimbursement stakeholders.
Objective
To the best of our knowledge, 2 published meta-analyses compared the results of stock and PSI TMJ-TJR systems.[
8,
9] Whereas the meta-analysis by Zou et al[
9] evaluated both the short- (≤3 years) and long-term results (>3 years), Johnson et al[
8] did not make this distinction and evaluated the Biomet Lorenz, TMJ Concepts, and Nexus CMF systems over the entire follow-up period as a whole. As a result, both types of prosthetic systems are compared to one another without clearly defined end points in time. This resulted in the inclusion of articles with a 6-month follow-up being compared with those with a 60-month follow-up. This increases the risk of skewing the postoperative results.
This systematic review and meta-analysis aims to evaluate and compare postoperative results in patients who were treated with either a PSI or a stock prosthesis, at well-defined moments in time, to determine whether there are significant differences in postoperative results between these 2 approaches. We hypothesized that the use of a CAD-CAM approach with the development of a PSI would lead to better postoperative results.
Materials and Methods
Study Design
We performed a systematic review by conducting a computerized literature search. The search was performed up to August 15, 2018, following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The following databases were used: PubMed Central, Web of Science, Cochrane Library Plus, and EMBASE. The following heading was used to define the search string: (“Temporomandibular Joint” OR TMJ) AND (“Prosthesis” OR “Prostheses” OR “Implant” OR “Total Joint Replacement”). The search was conducted using both Medical Subject Headings (MeSH) and free-text words. The exact combination in which these search terms were used depends on the database and is listed in
Table 1. A manual search of reference lists of the included articles was also performed.
For an article to be included, the patient sample had to consist of humans who received either unilateral or bilateral stock or custom(ized) TMJ-TJR systems. Both preoperative maximal mouth opening (MMO) and pain scores should be available, as well as those of at least 1 year postoperatively. These data had to be available at well-defined end points in time (eg, 1, 2, and/or 3 years after surgery). If any information on diet was provided, these data were also included. There were no boundaries set for age or sex, and the minimal patient population was set to 5. Articles evaluating postoperative results of the Vitek-Kent prosthesis were not considered for inclusion due to the negative long-term results following the use of incompatible materials.[
2]
Randomized controlled trials (RCTs), non-RCTs, comparative and prospective studies, retrospective studies, and case series were included. Case reports and expert opinions were excluded to maintain scientific soundness. Systematic reviews and meta-analyses concerning the use of a stock or patient-specific TJR were reviewed to identify possible eligible studies. Only articles written in English, Dutch, French, or German were included, and the full text had to be accessible.
Study Bias
All included studies were assessed for risk of bias. For non-RCTs and other observational studies, both prospective and retrospective, bias was assessed using the Methodological Index for Non-Randomized Studies (MINORS) scale, first introduced in 2003 by Slim et al.[
10] The items were scored 0 if not reported; 1 when reported, but inadequately; and 2 when reported adequately. As an unbiased assessment of study end points was not possible in the noncomparative studies due to the nature of the subject, this criterion was left out of the analysis. While the item “adequate statistical analysis” is normally only used for comparative trials, it was also used for the included articles to evaluate the quality of analysis between pre- and postoperative results.
Study Variables and Data Collection
After assessing the eligibility of all studies retrieved, the following data were extracted when available: authors, year of publication, number of patients included, sex, mean age of patients (in years), type of TMJ-TJR, time of follow-up (in months), MMO (in mm), and pain and diet measurements using a Visual Analog Scale (VAS) measurement. All VAS scores were based on the patients’ subjective evaluation and ranged from 0 to 10. For pain, a score of 0 meant a total absence of pain, while a score of 10 was considered the worst imaginable pain someone could experience. A dietary VAS score of 0 indicated that the patient could only eat liquids, while a score of 10 reflected solid foods.
The use of a TMJ prosthesis was considered the predictor variable, and the MMO and VAS pain scores were the main outcome variables. The diet VAS score was considered the secondary outcome variable, which was further analyzed to determine the effect of physiotherapy.
Several authors were contacted to determine whether there was any duplication within their patient groups. As a result, not all data provided by Mercuri et al[
11,
12,
13] were included. Also the articles by Gruber et al[
14] and Sidebottom and Gruber[
15] used the same patient population. We decided to use the data provided by Sidebottom and Gruber[
15] at 1 year and at 3 years postoperatively by Gruber et al[
14] to obtain as many patients as possible. Gonzalez-Perez et al[
16] reported on the same patient group twice, albeit 1article discussed the stock TMJ-TJR, while the other evaluated both the stock and the custom(ized) TJR systems.[
17] Only the data obtained from the article discussing both patient groups were included.
Statistical Analysis
The outcomes between the stock and the PSI systems were based on the weighted mean gain of the MMO, the weighted mean gain or reduction in VAS scores for pain and diet, and their standard error of weighted mean difference (seWMD). Weighted mean difference and seWMD between pre- and postoperative MMO, pain, and diet scores were calculated using the following formulas:
Forest plots were constructed for both primary and secondary outcomes, showing the summary and 95% confidence interval (95% CI) estimated in the meta-analyses. Mean difference was pooled using the generic inverse variance method. A random-effect model (DerSimonian-Laird method) was used, and variation in effects due to differences in study populations and methods was expected. Heterogeneity between subgroups was evaluated using the χ
2 test and
I2 metrics, where
P < .1 or
I2 > 50% indicates significant heterogeneity.[
18] The meta-analysis was performed using Review Manager 5.3 (Cochrane IMS, Copenhagen, Denmark).
Ethics Approval
Internal ethical committee approval and confirmation of adherence to the Helsinki Declaration were not necessary for this literature review.
Results
Study Inclusions
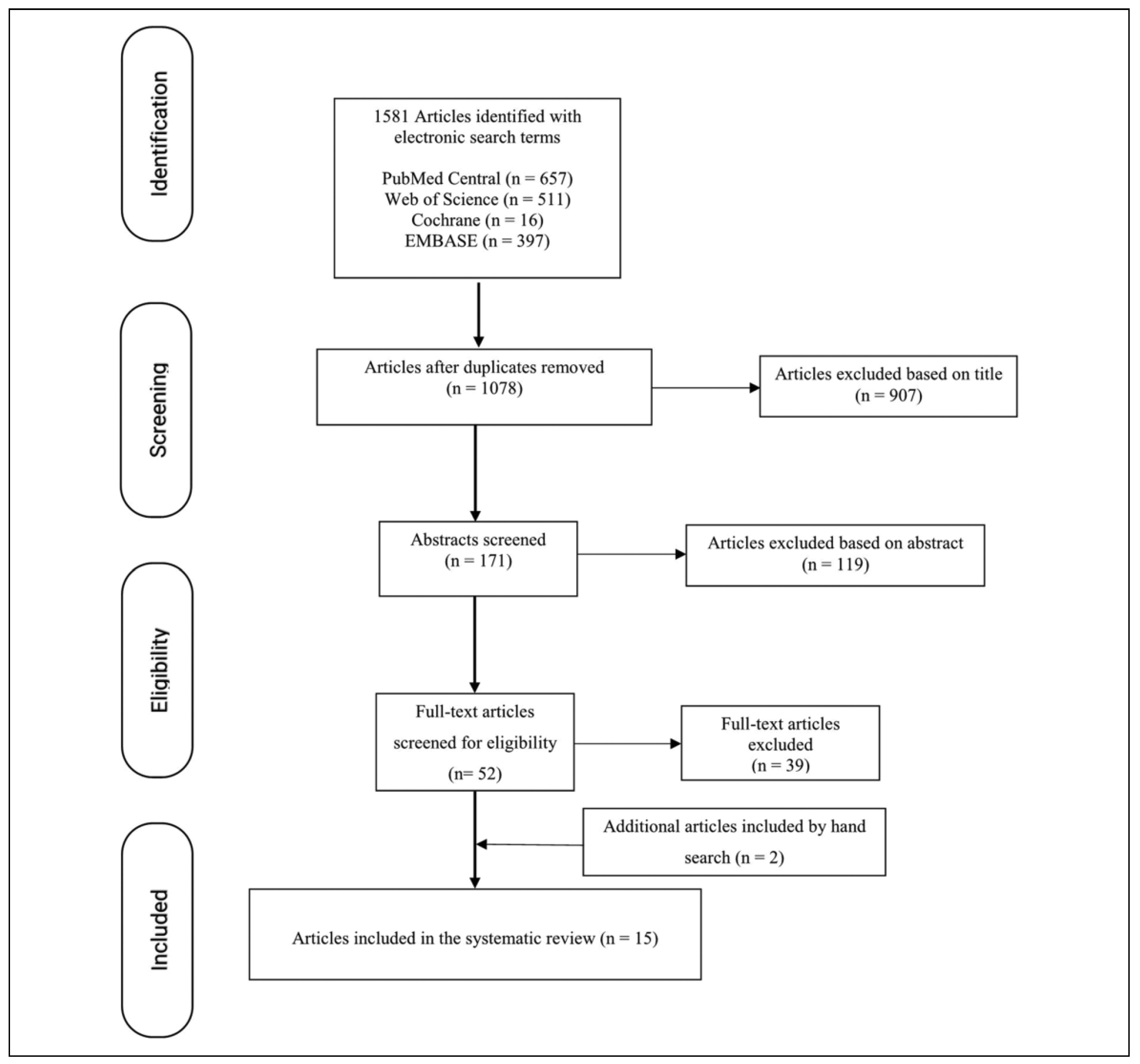
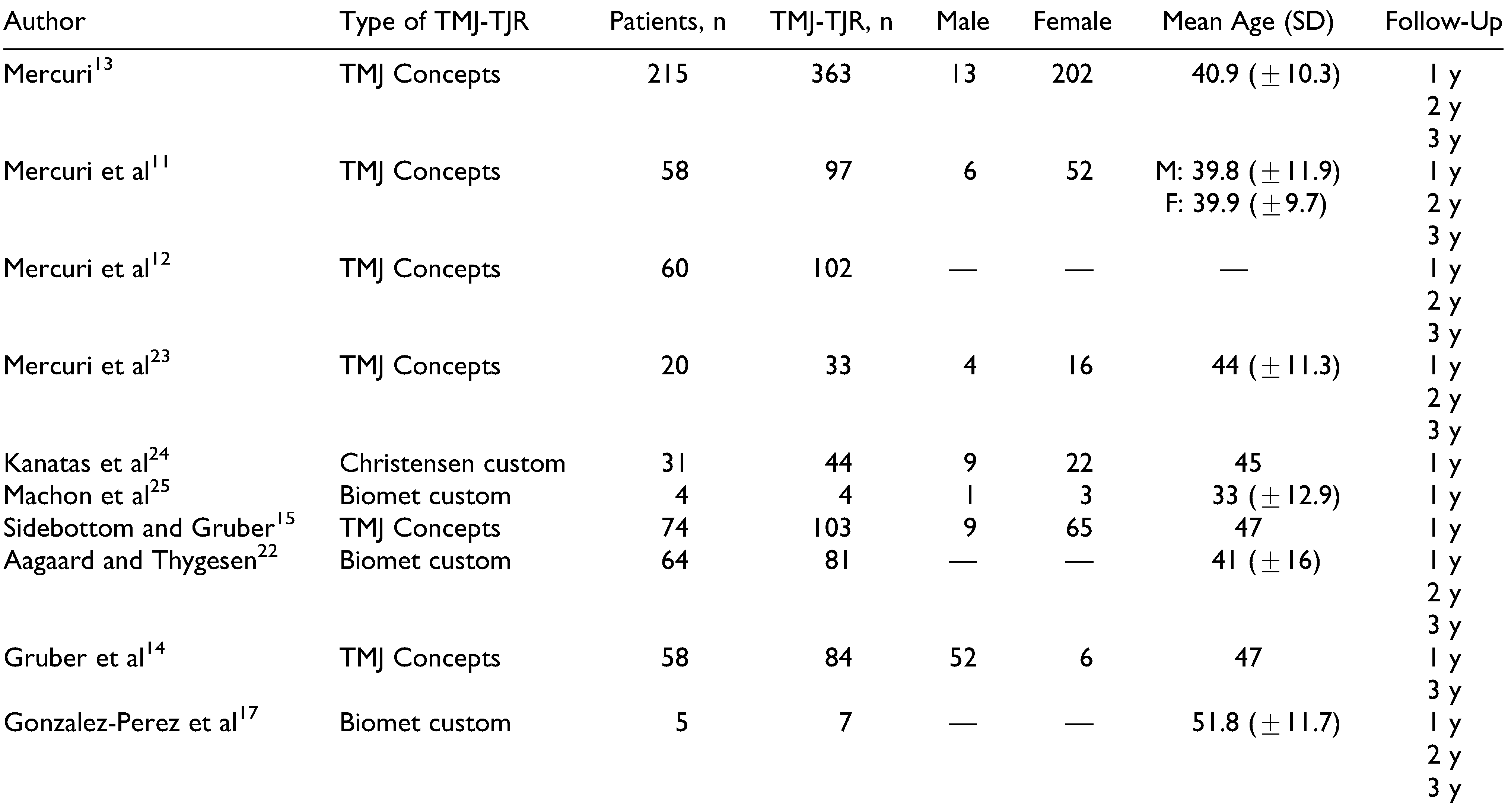
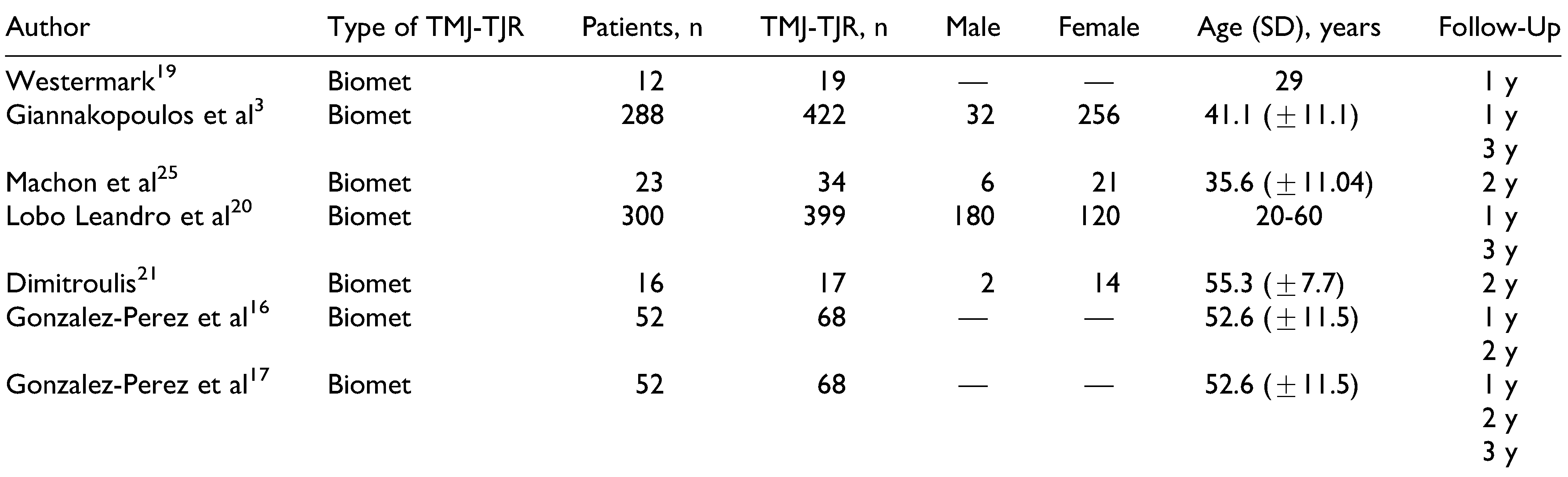
The initial search and selection was independently performed by 2 of the authors. Their results were then compared, and a third reviewer was asked to evaluate the reference in case of conflict. This search returned 1581 published articles. After removing the duplicates, 1078 articles were screened, and 1026 were excluded based on the contents of the title (n = 907) and abstract (n = 119). By reading the final 52 articles and applying the inclusion criteria, a total of 13 articles were analyzed. Two additional articles were identified by manually searching the reference list of one of the meta-analyses. The performed search is summarized in the PRISMA flow diagram (
Figure 1). Five articles met the inclusion criteria for stock prostheses,[
3,
16,
19,
20,
21] while 8 were included for patient-specific TMJ-TJR.[
11,
12,
13,
14,
15,
22,
23,
24] Machon et al[
25] and Gonzalez-Perez et al[
17] evaluated both the stock system and the PSI. The basic characteristics of the included articles are given in
Table 2,
Table 3,
Table 4 and
Table 5. As stated earlier, not all articles that were included in the systematic review were included in the meta-analysis, as to prevent duplication of patient population. In total, 12 of the 15 included articles provided data that were included in the meta-analysis.[
3,
13,
14,
15,
16,
19,
20,
21,
22,
23,
24,
25] A total of 413 patients were treated with either a unilateral or a bilateral patient-matched implant, while 691 patients were treated with a stock implant. Not all articles reported on sex, with a clear female dominance of 411 female patients versus 220 male patients for stock implants.[
3,
20,
21,
25] This was more so the case for the PSI, with 308 and 36 female and male patients, respectively.[
13,
15,
23,
24,
25] Both groups were also relatively similar in age. A more detailed overview of the study populations for stock and custom(ized) TMJ-TJR can be found in
Table 2 and
Table 3.
We chose not to divide the included prosthetic systems on basis of brand for this meta-analysis. As a result, a direct comparison was made between stock and patient-fitted systems. While not intentional, all stock TMJ-TJRs consisted of the Biomet system, with less heterogeneity in the PSI group.
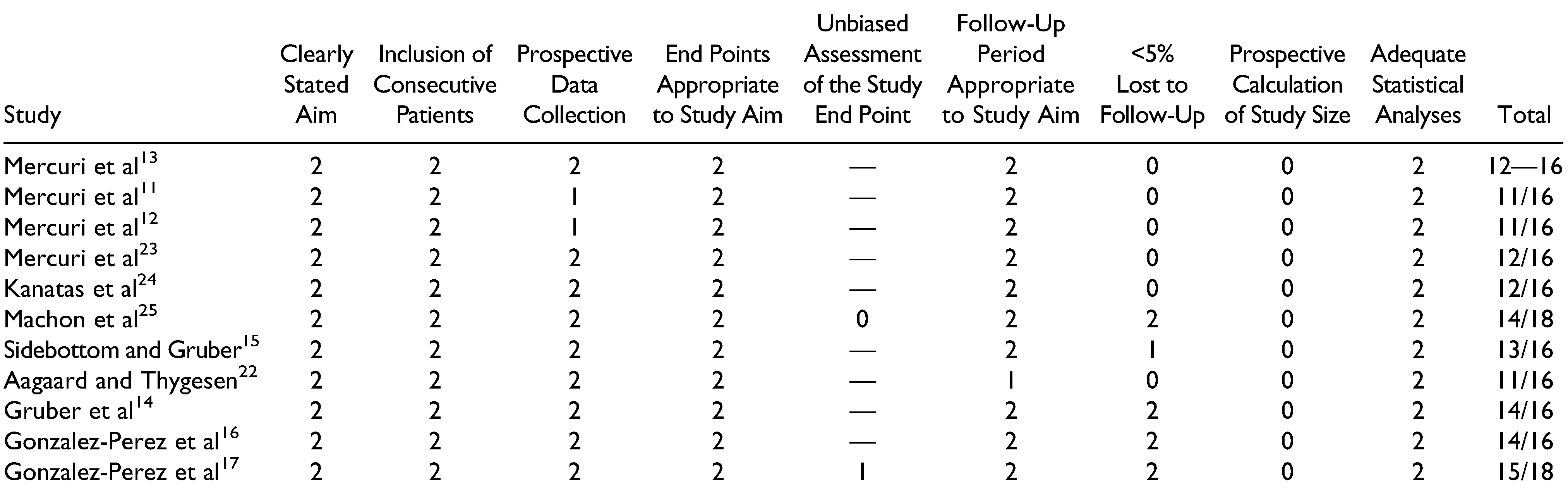
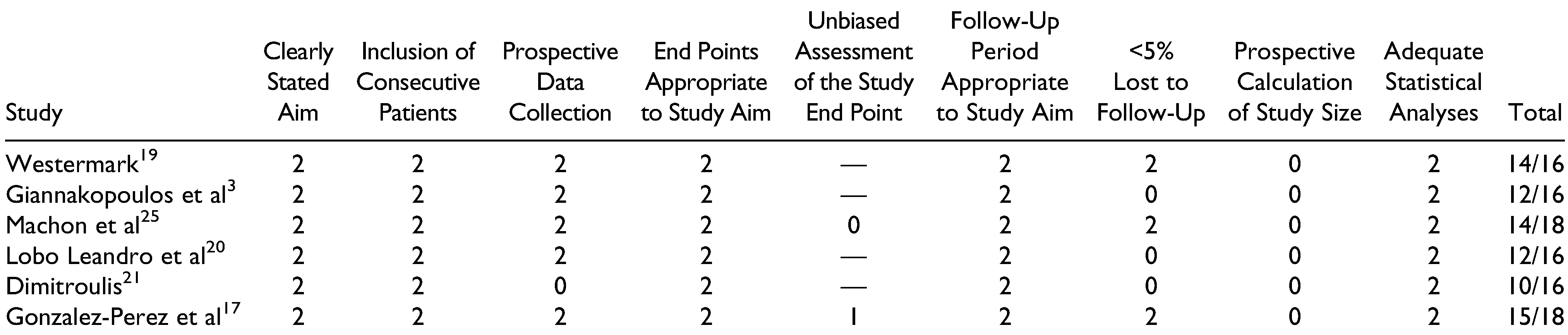
Risk of Bias
All 15 studies were assessed using the MINORS scale. Only those by Machon et al[
25] and Gonzalez-Perez et al[
17] were of comparative nature. Overall, the risk of bias was relatively low for articles dealing with stock and custom(ized) systems, with respective mean scores of 12/16 and 12.2/16 for the noncomparative articles. It should be noted that all included articles either did not report on or did not prospectively calculate the necessary study size. A second point on which many studies scored poorly was the loss in follow-up, frequently exceeding over 50% of the initially included patient population. Both comparative articles had a low risk of bias with scores of 15/18 and 14/18,[
17,
25] with points lost for not calculating the necessary patient population (
Table 6 and
Table 7).
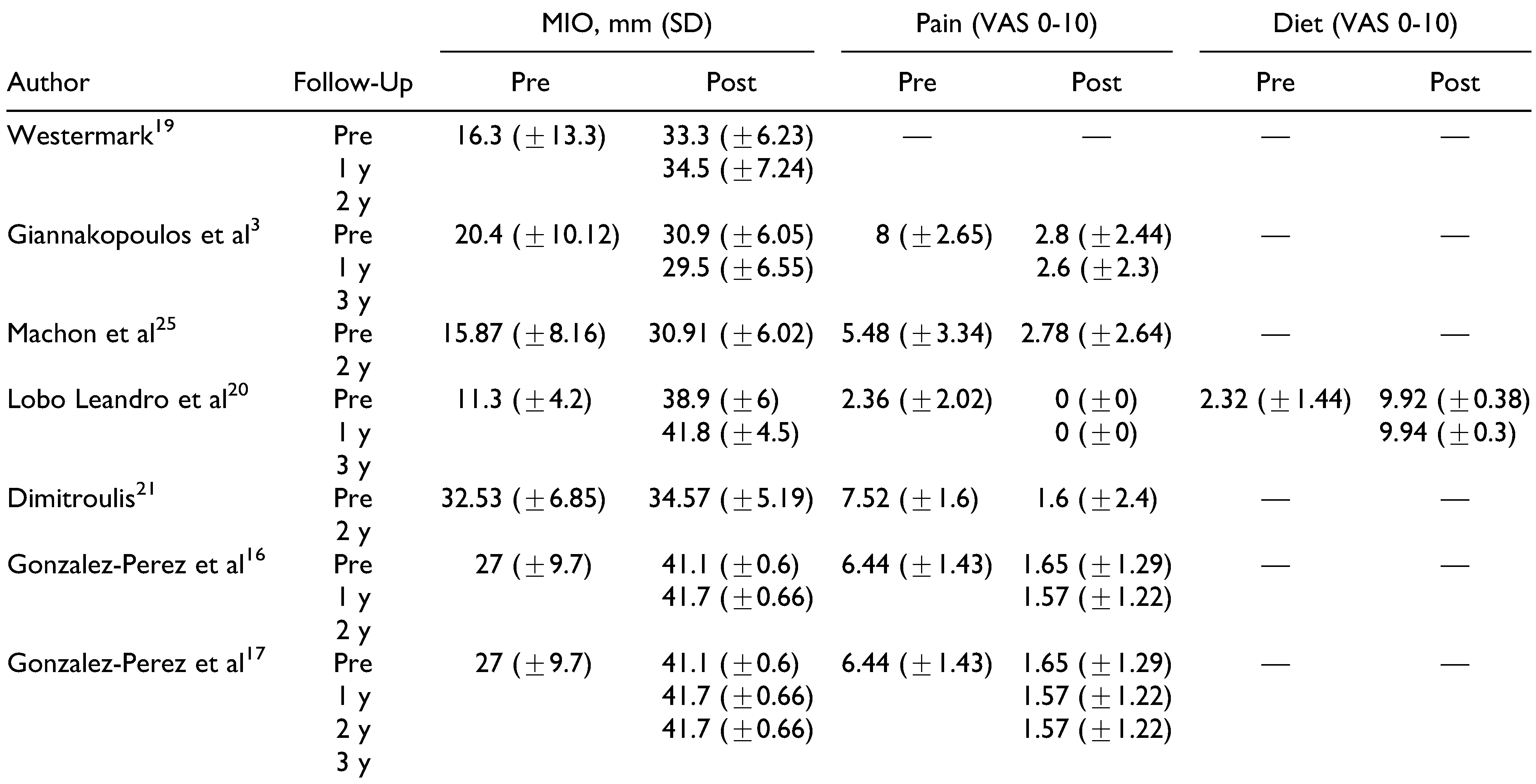
Study Results
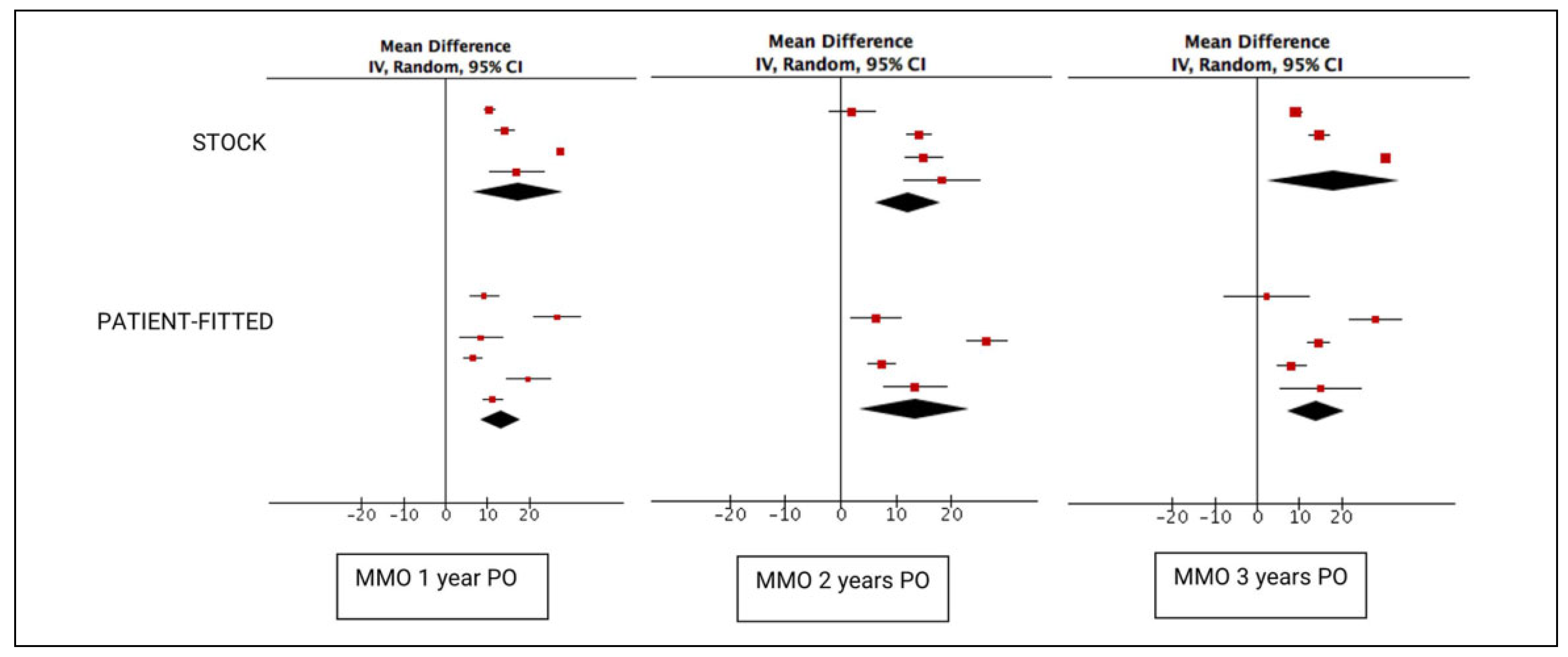
A total of 686 stock prosthesis were included in the 1-year follow-up results. This number significantly dropped to 122 for the 2-year follow-up and then increased to 468 for the 3-year follow-up. In comparison, 252 PSIs were available at the 1-year mark, 85 at 2 years, and 124 at 3 years. Both stock and patient-fitted systems achieved significant increases in postoperative MMO, with a mean increase of 17.32 mm (95% CI: 6.39-28.25) for stock implants and 13.27 mm (95% CI: 8.47-18.08) for custom(ized) implants (
Figure 2). However, the difference between the implant systems quickly decreased after 2 years. The difference between both groups was nonsignificant at 1 (
p = .51), 2 (
p = .84), and 3 (
p = .63) years.
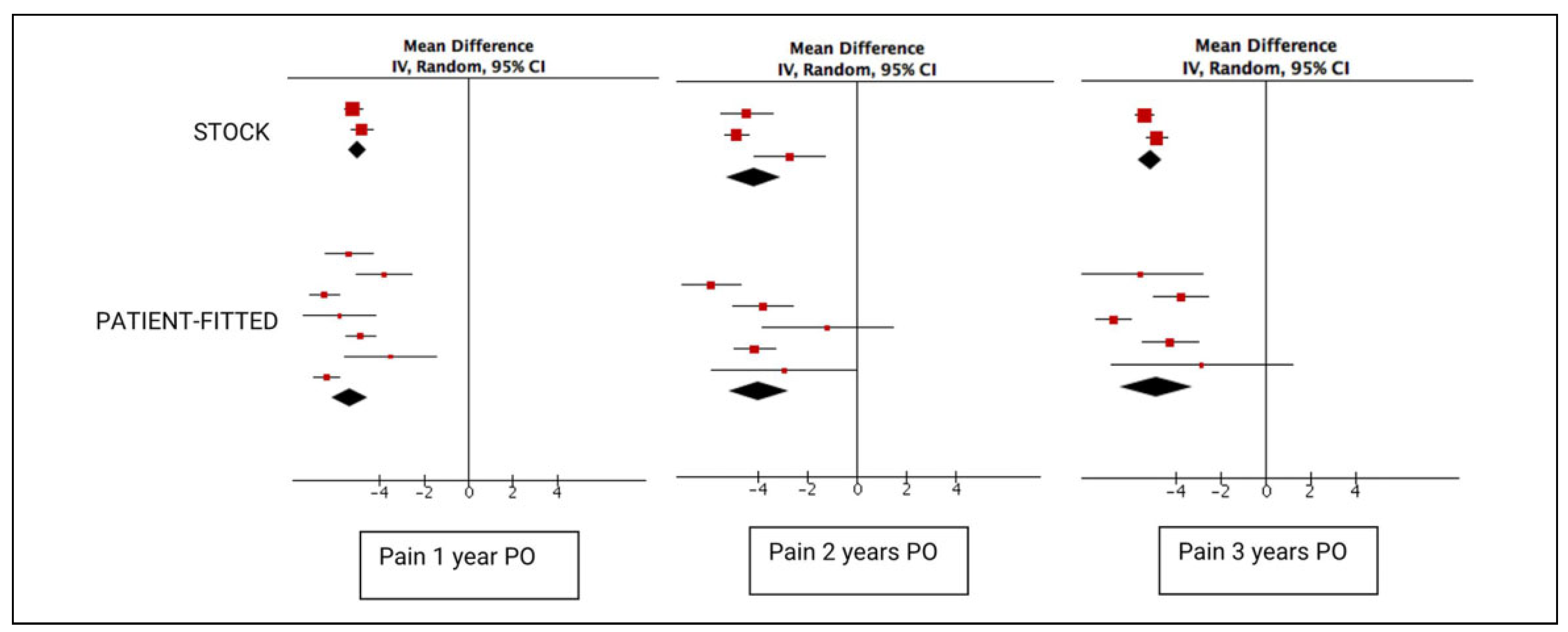
In total, 268 sides in patients treated with stock implants were evaluated for pain both pre- and postoperatively (
Figure 3). At 2 and 3 years, 103 and 256 sides were evaluated, respectively. A significant decrease in the VAS pain score was noted, with a 5.02 (95% CI: −5.42 to −4.62) decrease on a 0 to 10 scale. While this decrease was higher for the 252 sides treated with a patient-fitted implant at the 1-year postoperative assessment at 5.34 (95% CI: −6.15 to −4.53), this difference was nonsignificant (
p = .49). This lack of significance persisted at 2 (
p = .81) and 3 years (
p = .76).
Only Lobo Leandro et al[
20] provided information on patient dietary capabilities, with 300 included patients at the 1-year mark and 212 patients at the 3-year mark (
Figure 4). A significant increase for both time points was seen for the dietary VAS score: 7.60 (95% CI: 7.45-7.75) and 7.62 (95% CI: 7.47-7.77) at 2 and 3 years, respectively. Patients treated with a PSI showed a significant increase in their dietary VAS score (5.45 [95% CI: 4.95-5.96] and 4.82 [95% CI: 2.98-6.67]),[
13,
14,
15,
17,
22,
23,
24,
25] albeit less significantly compared to the 1-year (
p < .001) and 3-year results (
p < .01) results published by Lobo Leandro et al.[
20]
Discussion
While our approach was different from that of Zou et al[
9] and Johnson et al,[
8] the statistical findings were similar for the 3 meta-analyses. Despite the conviction of Mercuri[
5] and many other surgeons, the currently available data do not seem to indicate a clear advantage of patient-fitted implant systems over their stock counterparts. However, several significant remarks must be made before reaching this conclusion, and several confounders should be mentioned.
Bias of Pooled Data
While Lobo Leandro et al[
20] noted similar postoperative MMO results compared to the other included articles, both their mean preoperative mouth opening at 11.3 mm and mean postoperative dietary VAS scores of 9.92 and 9.94 at 1 and 3 years postoperatively are significantly lower or higher compared to the other included articles. Furthermore, only Lobo Leandro et al[
20] provided dietary VAS scores for the stock prosthesis, significantly weakening the conclusion of these findings. Due to the large population size,[
20] these data have a significant effect on the metaanalysis results, heavily “benefitting” the overall results for the stock prosthesis. This remark was also made by Johnson et al[
8] in their meta-analysis. Excluding the data provided by Lobo Leandro et al[
20] had a significant effect on the effect size for MMO, leading to a smaller increase in MMO from 17.32 to 13 mm (95% CI: 9.60-16.39) and from 18.11 to 11.82 mm (95% CI: 6.33-17.30), and a smaller increase in MMO compared to patients treated with a patient-fitted implant. While the data of Lobo Leandro et al[
20] cannot simply be discarded, this demonstrates the sensitivity of the pooled data to bias.
Lack of Pathology Grading
Pathology grading was lacking in the included studies. While it is well-known that TJR should be considered the last resort for patients with end-stage joint disease, the studies had great variability in the clinical severity of the pathologies and indications for surgery.[
26,
27] For example, one of the indications was joint ankylosis. Sawhney[
28] made clear distinctions among 4 different types, whereas Durr et al[
29] identified 3 types (
Table 8 and
Table 9). While all 4 types of Sawhney[
28] come into consideration for TJR surgery, it is evident that differences in severity and type of ankylosis (osseous, fibrous, mixed, or extended) can affect results. Postoperative results obtained in the ankylosis group of one study were negatively influenced by the presence of more severe cases, even if they are diagnosed as being of the same type.[
28]
The Wilkes’ staging classification for internal derangement of the TMJ and the Helkimo index are the 2 scales often used to evaluate temporomandibular disorder (TMD) severity and joint degeneration.[
30,
31] The Helkimo index has 3 subindexes (anamnestic, clinical, and occlusal dysfunction), while the Wilkes classification is based on both clinical and radiological properties. Both indexes have wide ranges, with the final stage coming into consideration for TMJ-TJR surgery.[
30,
31] As such, while 2 patients might have a similar score on the Helkimo index,[
31] the amount of bony destruction can be significantly different. However, surgeons are currently unable to report this distinction in severity due to the lack of diagnostic tools for end-stage TMD. Nevertheless, anatomical abnormality affects both the choice of implant system and 1- versus 2-stage surgery, as well as the postoperative results. When comparing MMO, pain, and diet, the relative numbers of patients with ankylosis and severe inflammatory/degenerative joint disease in the study group can affect the postoperative improvements.[
5,
27,
32]
Many surgeons prefer the use of a patient-fitted system in case of more severe anatomical abnormalities.[
5,
8,
33,
34] This was illustrated by Gonzalez-Perez et al[
17] who opted for a PSI system in patients with large and complex defects. The amount of subjective and objective improvement diminishes as the severity of anatomical abnormalities and the number of previous treatments increase, due to compromised (neuro)muscular anatomy and function.[
13,
35,
36] When a patient-fitted system is preferred in case of severe TMJ degeneration or in revision surgery, it is obvious that its potential for postoperative improvement is more limited compared to a stock implant system that is usually indicated in less severe or primary cases. This is a major contributor to bias in the meta-analysis.
Surgical Risks and Operation Time
The immediate advantage of a patient-fitted prosthesis is that it requires no alteration of the patient’s anatomy. The total contact surface between the mandibular component and the mandible is optimal for improved osseointegration and stability.[
2] In contrast, when using a stock implant, either the bony surface has to be fitted to the implant or the implant must be bent or grinded down. This increases the total operation time and puts the materials at risk for fatigue and micromotions, which can lead to implant failure.[
2,
11,
12,
24,
37]
Zhao et al[
37] set out to evaluate the amount of bone that needed to be removed or grafted to achieve a good fit for the stock Biomet system in 63 joints they had treated between 2010 and 2016. Computer simulation revealed that a medium amount of bone trimming was needed (150-300 mm
3 bone) in 46% of skull bases, and a large volume (>300 mm
3) of bone trimming was necessary in 33% of cases. The mandibular bone required medium and large amounts of trimming in 27% and 29% of all cases, respectively. Furthermore, in 44% of all cases, a medium bone graft was needed elsewhere on the fossa to achieve a good fit; while in 35%, a large amount was needed. They concluded that a patient-fitted implant required less adaptation, which decreases surgery time and the risk of injury to the skull base and alveolar nerve.[
37]
Similarly, Abramowicz et al[
38] set out to evaluate the necessity for the use of a patient-fitted implant in 22 cases by evaluating whether a stock Biomet implant could be fitted to the stereolithographic models of patients who were treated with a TMJ Concepts device. They found that in 23% of all sites, no fit could be achieved by means of a stock implant. In an additional 27% of all sites, significant alterations had to be performed to either the skull base or condylar bone with a minimum of 3 mm of bone that needed to be removed. They concluded that in more complex cases, such as patients who underwent multiple operations or who have more severe anatomical abnormalities, the use of a patient-fitted solution should be preferred over a stock implant. However, for more straightforward and simple cases, they found that a stock implant was a more cost-effective solution.[
38]
Conclusion
This meta-analysis evaluated the MMO and VAS scores for pain and diet to provide pooled estimates for both patient-fitted and stock TMJ-TJR systems. While no significant differences were found between the implant systems, the provided data do not consider pathology severity, which can heavily influence postoperative outcomes and is prone to bias of pooled data. By means of a prospective randomized trial, this bias could be overcome, yet this forces the use of a certain implant system even if not deemed suited by the performing surgeon, posing an important ethical dilemma.
There is need for a detailed diagnostic evaluation tool to better describe the degree of joint degeneration as well as preoperative testing for allergies to the implant components to prevent the need for explantation due to soft tissue inflammation. Also, postoperative follow-up should give more attention to functionality and quality of life, rather than only maximal incisal opening and pain.
Using a patient-fitted implant in more straightforward cases decreases risk of damage to the alveolar and facial nerves by optimization of screw positioning and using a smaller approach during placement. In more complex cases, the need for secondary surgery can be prevented (eg, by using an extended TJR), thus compensating for the initial higher cost of a patient-fitted implant.
Lastly, while FDA-approved stock implants contain Co-Cr-Mo, to which 10% of the population is allergic, PSI can be completely manufactured out of Ti, significantly diminishing the risk of an allergic reaction and implant failure.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Annika Herrtwich, MD, for her assistance performing the search and selecting papers for inclusion as well as her useful and insightful remarks during manuscript drafting.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- De Meurechy, N.; Mommaerts, M.Y. Alloplastic temporomandibular joint replacement systems: a systematic review of their history. Int J Oral Maxillofac Surg. 2018, 47, 743–754. [Google Scholar] [CrossRef]
- De Meurechy, N.; Braem, A.; Mommaerts, M.Y. Biomaterials in temporomandibular joint replacement: current status and future perspectives—a narrative review. Int J Oral Maxillofac Surg. 2017, 47, 518–533. [Google Scholar] [CrossRef]
- Giannakopoulos, H.E.; Sinn, D.P.; Quinn, P.D. Biomet microfixation temporomandibular joint replacement system: a 3-year follow-up study of patients treated during 1995 to 2005. J Oral Maxillofac Surg. 2012, 70, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Wolford, L.M.; Pitta, M.C.; Reiche-Fischel, O.; Franco, P.F. TMJ Concepts/Techmedica custom-made TMJ total joint prosthesis: 5-year follow-up study. Int J Oral Maxillofac Surg. 2003, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G. Alloplastic temporomandibular joint replacement: rationale for the use of custom devices. Int J Oral Maxillofac Surg. 2012, 41, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G. Patient-fitted (“Custom”) alloplastic temporomandibular joint replacement technique. Atlas Oral Maxillofac Surg Clin North Am. 2011, 19, 233–242. [Google Scholar] [CrossRef]
- Onoriobe, U.; Miloro, M.; Sukotjo, C.; Mercuri, L.G.; Lotesto, A.; Eke, R. How many temporomandibular joint total joint alloplastic implants will be placed in the United States in 2030? J Oral Maxillofac Surg. 2016, 74, 1531–1538. [Google Scholar] [CrossRef]
- Johnson, N.R.; Roberts, M.J.; Doi, S.A.; Batstone, M.D. Total temporomandibular joint replacement prostheses: a systematic review and bias-adjusted meta-analysis. Int J Oral Maxillofac Surg. 2016, 46, 1–7. [Google Scholar] [CrossRef]
- Zou, L.; He, D.; Ellis, E. A Comparison of clinical follow-up of different total temporomandibular joint replacement prostheses: a systematic review and meta-Analysis. J Oral Maxillofac Surg. 2018, 76, 294–303. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Mercuri, L.G.; Wolford, L.M.; Giobbie-hurder, A. Long-term follow-up of the CAD/CAM patient fitted total temporomandibular joint reconstruction system. J Oral Maxillofac Surg. 2002, 60, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G.; Edibam, N.R.; Giobbie-Hurder, A. Fourteen-year follow-up of a patient-fitted total temporomandibular joint reconstruction system. J Oral Maxillofac Surg. 2007, 65, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G.; Wolford, L.M.; Sanders, B.; White, R.D.; Hurder, A.; Henderson, W. Custom CAD/CAM total temporomandibular joint reconstruction system. Preliminary multicenter report. J Oral Maxillofac Surg. 1995, 53, 106–115. [Google Scholar] [CrossRef]
- Gruber, E.A.; McCullough, J.; Sidebottom, A.J. Medium-term outcomes and complications after total replacement of the temporomandibular joint. Prospective outcome analysis after 3 and 5 years. Br J Oral Maxillofac Surg. 2015, 53, 412–415. [Google Scholar] [CrossRef]
- Sidebottom, A.J.; Gruber, E. One-year prospective outcome analysis and complications following total replacement of the temporomandibular joint with the TMJ Concepts system. Br J Oral Maxillofac Surg. 2013, 51, 620–624. [Google Scholar] [CrossRef]
- Gonzalez-Perez, L.M.; Fakih-Gomez, N.; Gonzalez-Perez-Somarriba, B.; Centeno, G.; Montes-Carmona, J.F. Two-year prospective study of outcomes following total temporomandibular joint replacement. Int J Oral Maxillofac Surg. 2016, 45, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, L.M.; Gonzalez-Perez-Somarriba, B.; Centeno, G.; Vallellano, C.; Montes-Carmona, J.F. Evaluation of total alloplastic temporo-mandibular joint replacement with two different types of prostheses: a three-year prospective study. Med Oral Patol Oral Cir Bucal. 2016, 21, e766–e775. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions. Version 5; Chichester, West Sussex; John Wiley & Sons: Hoboken, NJ, USA, 2011; http://handbook.cochrane.org. [Google Scholar]
- Westermark, A. Total reconstruction of the temporomandibular joint. Up to 8 years of follow-up of patients treated with Biomet (R) total joint prostheses. Int J Oral Maxillofac Surg. 2010, 39, 951–955. [Google Scholar] [CrossRef]
- Lobo Leandro, L.F.; Ono, H.Y.; de Souza Loureiro, C.C.; Marinho, K.; Garcia Guevara, H.A. A ten-year experience and follow-up of three hundred patients fitted with the Biomet/Lorenz microfixation TMJ replacement system. Int J Oral Maxillofac Surg. 2013, 42, 1007–1013. [Google Scholar] [CrossRef]
- Dimitroulis, G. Comparison of the outcomes of three surgical treatments for end-stage temporomandibular joint disease. Int J Oral Maxillofac Surg. 2014, 43, 980–989. [Google Scholar] [CrossRef]
- Aagaard, E.; Thygesen, T. A prospective, single-center study on patient outcomes following temporomandibular joint replacement using a custom-made biomet TMJ prosthesis. J Oral Maxillofac Surg. 2014, 69, e46. [Google Scholar] [CrossRef]
- Mercuri, L.G.; Ali, F.A.; Woolson, R. Outcomes of total alloplastic replacement with periarticular autogenous fat grafting for management of reankylosis of the temporomandibular joint. J Oral Maxillofac Surg. 2008, 66, 1794–1803. [Google Scholar] [CrossRef]
- Kanatas, A.N.; Needs, C.; Smith, A.B.; Moran, A.; Jenkins, G.; Worrall, S.F. Short-term outcomes using the Christensen patient-specific temporomandibular joint implant system: a prospective study. Br J Oral Maxillofac Surg. 2011, 50, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Machon, V.; Hirjak, D.; Beno, M.; Foltan, R. Total alloplastic temporomandibular joint replacement: the Czech-Slovak initial experience. Int J Oral Maxillofac Surg. 2012, 41, 514–517. [Google Scholar] [CrossRef]
- Sidebottom, A.J. Guidelines for the replacement of temporomandibular joints in the United Kingdom. Br J Oral Maxillofac Surg. 2008, 46, 146–147. [Google Scholar] [CrossRef]
- NICE. National Institute for Health and Care Excellence Scope. Natl Inst Heal Care Excell 2014, 1–9. https://www.nice.org.uk/guidance/gid-cgwave0704/resources/multimor bidity-final-scope2.
- Sawhney, C.P. Bony ankylosis of the temporomandibular joint: follow-up of 70 patients treated with arthroplasty and acrylic spacer interposition. Plast Reconstr Surg. 1986, 77, 29–40. [Google Scholar] [PubMed]
- Durr, E.D.; Turlington, E.G.; Foote, R.L. Radiation treatment of heterotopic bone formation in the temporomandibular joint articulation. Int J Radiat Oncol Biol Phys. 1993, 27, 863–869. [Google Scholar]
- Wilkes, C. Internal derangements of the temporomandibular joint: pathological variations. Arch Otolaryngol Neck Surg. 1989, 115, 469–477. [Google Scholar] [CrossRef]
- Helkimo, M. Studies on function and dysfunction of the masticatory system. II. Index for anamnestic and clinical dysfunction and occlusal state. Sven Tandlak Tidskr. 1974, 67, 101–121. [Google Scholar]
- Guarda-Nardini, L.; Manfredini, D.; Ferronato, G. Total temporomandibular joint replacement: a clinical case with a proposal for post-surgical rehabilitation. J Craniomaxillofac Surg. 2008, 36, 403–409. [Google Scholar] [CrossRef]
- Saeed, N.R.; Hensher, R.; McLeod, N.M.H.; Kent, J.N. Reconstruction of the temporomandibular joint autogenous compared with alloplastic. Br J Oral Maxillofac Surg. 2002, 40, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Elledge, R.; Attard, A.; Green, J.; et al. UK temporomandibular joint replacement database: a report on one-year outcomes. Br J Oral Maxillofac Surg. 2017, 55, 927–931. [Google Scholar] [CrossRef]
- Garrett, W.R.; Abbey, P.A.; Christensen, R. Temporomandibular joint reconstruction with a custom total temporomandibular joint prosthesis: use in the multiply operated patient. Surg Technol Int 1997, 6, 347–354. [Google Scholar] [PubMed]
- Henry, C.H.; Wolford, L.M. Treatment outcomes for temporomandibular joint reconstruction after Proplast-Teflon implant failure. J Oral Maxillofac Surg. 1993, 51, 352–360. [Google Scholar] [CrossRef]
- Zhao, J.; Zou, L.; He, D.; Ellis, E. Comparison of bone adaptation after modification in biomet standard alloplastic temporomandibular joint prostheses. J Cranio-Maxillofacial Surg. 2018, 46, 1707–1711. [Google Scholar] [CrossRef]
- Abramowicz, S.; Barbick, M.; Rose, S.P.; Dolwick, M.F. Adaptability of stock TMJ prosthesis to joints that were previously treated with custom joint prosthesis. Int J Oral Maxillofac Surg. 2012, 41, 518–520. [Google Scholar] [CrossRef]
- Westermark, A.; Heden, P.; Aagaard, E.; Cornelius, C.P. The use of TMJ Concepts prostheses to reconstruct patients with major temporomandibular joint and mandibular defects. Int J Oral Maxillofac Surg. 2011, 40, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Elledge, R.; Mercuri, L.; Speculand, B. Extended total temporomandibular joint replacements: a classification system. Br J Oral Maxillofac Surg. 2018, 56, 578–581. [Google Scholar] [CrossRef]
- Biglioli, F.; Colletti, G. Mini-retromandibular approach to condylar fractures. J Craniomaxillofac Surg. 2008, 36, 378–383. [Google Scholar] [CrossRef]
- Hallab, N.; Merritt, K.; Jacobs, J.J. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001, 83, 428–436. [Google Scholar] [CrossRef]
- de Graaf, N.P.J.; Feilzer, A.J.; Kleverlaan, C.J.; Bontkes, H.; Gibbs, S.; Rustemeyer, T. A retrospective study on titanium sensitivity: Patch test materials and manifestations. Contact Dermatitis. 2018, 79, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, M.Y.; Foster, M.E.; Gundlach, K.K.H. How to do clinical research in cranio-maxillo-facial surgery. J Craniomaxillofac Surg. 2012, 40, 97–102. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. The Author(s) 2020.