In 2008, a report of monozygotic (MZ) twins born with Crouzon syndrome was published to demonstrate the differences that can be seen in this condition as it relates to genetic and nongenetic factors. Studying MZ twins with syndromic craniosynostosis is an interesting endeavor because it allows for the evaluation of these effects during the patient’s clinical course.[
1] The previous publication demonstrated phenotypic variation of twins with Crouzon syndrome, even though they are genetically identical. Despite identical
FGFR2 mutations (D321V), they had discordant craniosynostosis presentations, possibly influenced by either nongenetic factors, intrauterine forces, or maternal variables.[
1] The effects of these factors on development, cognitive function, and need for surgical intervention have rarely been reported due to the rarity of syndromic craniosynostosis combined with twinning. Given the incidence of identical twins at 4 per 1,000 and the incidence of Crouzon syndrome at 16 per million, the probability of identical twins with Crouzon syndrome can be calculated as 0.0000064%. There have been 14 case reports of twinning in syndromic craniosynostosis, with only two receiving follow-up.[
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15] Of these two, one reported the outcomes of patients with Crouzon syndrome at 5 years of age and the other reported the outcomes of Pfeiffer syndrome at 14 years of age.[
4,
14] The present report provides a clinical update for the previously reported identical twins with Crouzon syndrome, including surgical planning and management.
Case Report
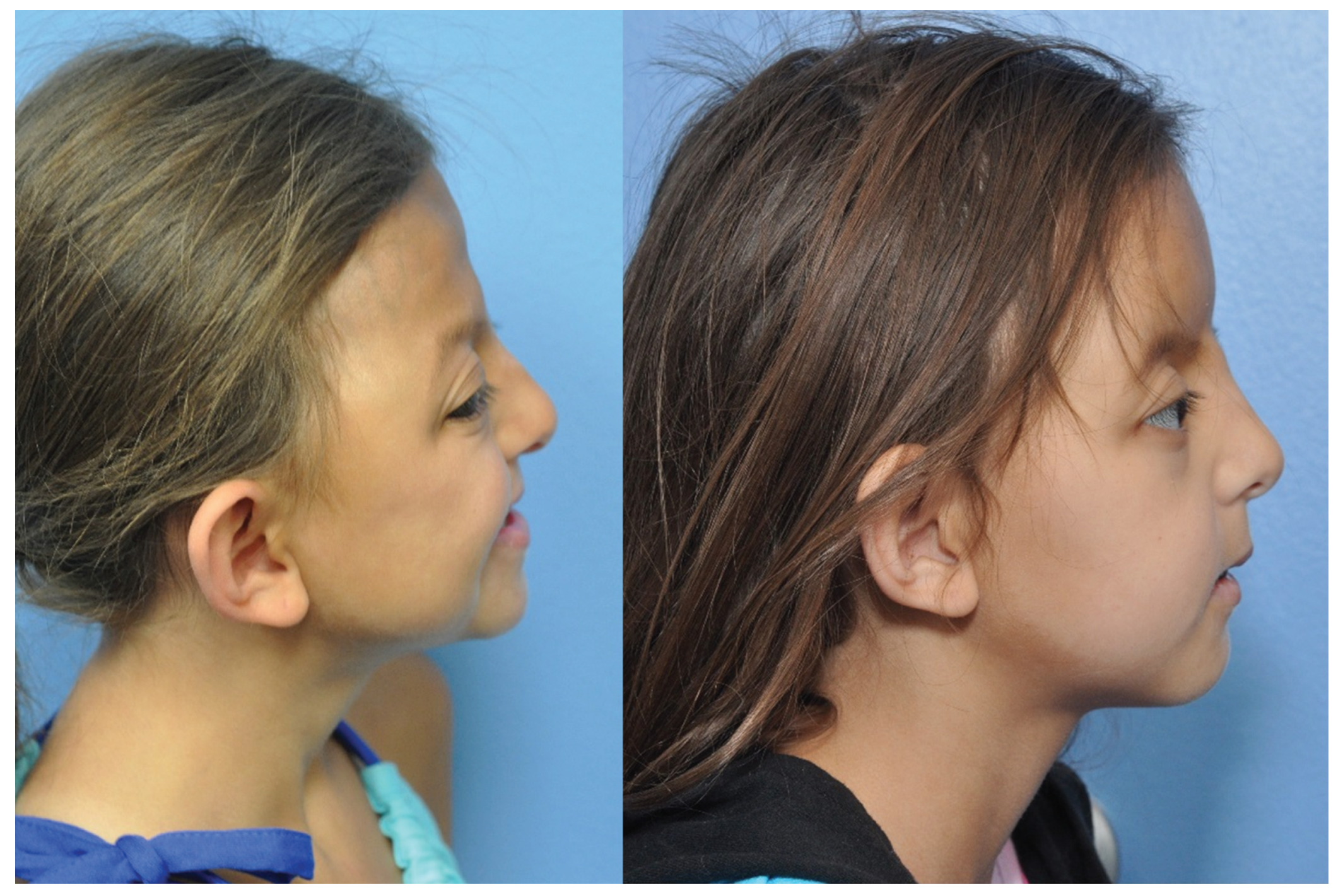
As previously reported, conception was achieved via in vitro fertilization, and the twins (A and B) were born at term to a 33-year-old primigravida mother via cesarean delivery. Family history was negative for craniosynostosis. Both were born in good health, demonstrated normal behavior, and met their developmental milestones. Craniofacial abnormalities were apparent by the first year of life and they were given a clinical diagnosis of Crouzon syndrome. Genetic testing identified a mutation in exon 10 of FGFR2 (c.962A > T [D321V]), which was present in both twins. Nearly, all patients with a clinical diagnosis of Crouzon syndrome have an identifiable mutation in FGFR2, with approximately 80% being located in exons 8–10. Crouzon syndrome is inherited in an autosomal dominant pattern, and mutations may be inherited from an affected parent or may be de novo. Despite identical FGFR2 mutations, the twins showed phenotypically discordant craniosynostosis.
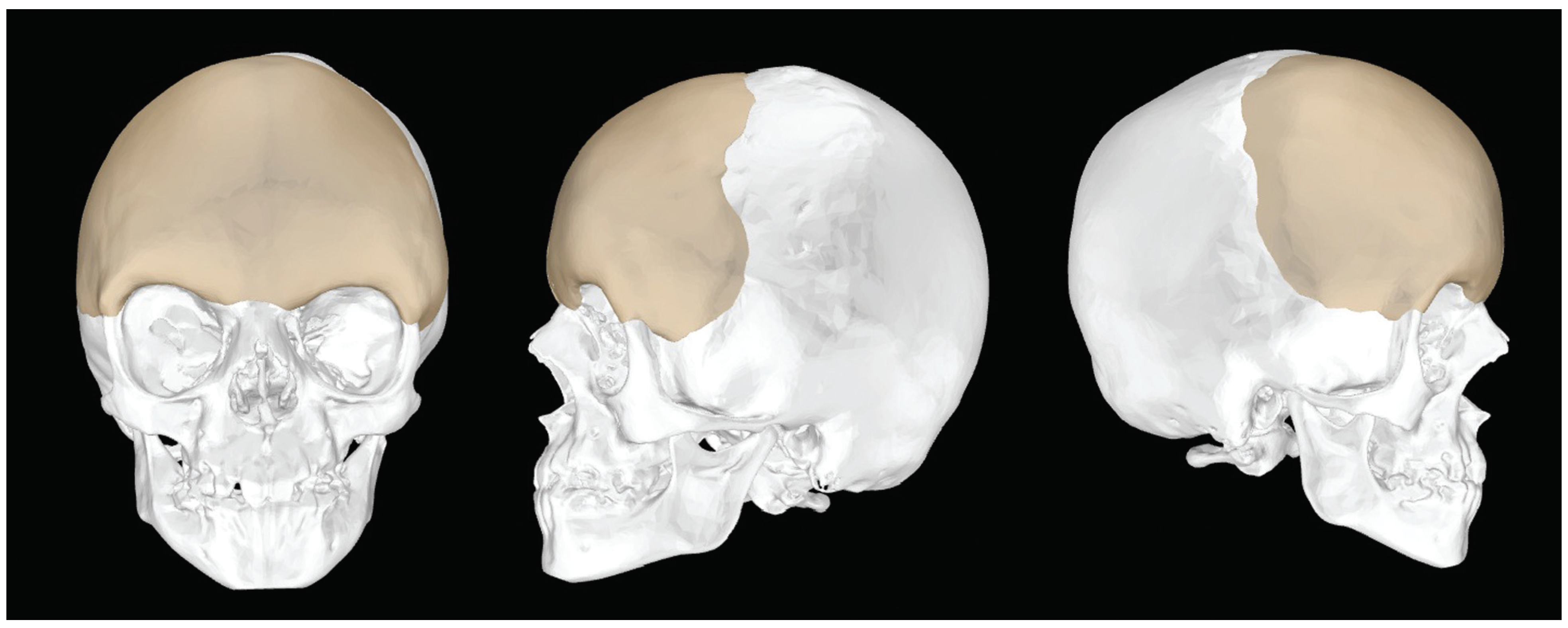
Figure 1.
CT-generated three-dimensional construction of skull with projected positioning of PEEK implant. PEEK, polyetheretherketone.
Figure 1.
CT-generated three-dimensional construction of skull with projected positioning of PEEK implant. PEEK, polyetheretherketone.
Twin A presented with a right unicoronal craniosynostosis, while twin B had a bicoronal craniosynostosis. Both twins underwent fronto-orbital advancement and remodeling in 2006. This temporarily treated their exorbitism and forehead retrusion with a clear improvement in the supraorbital and forehead regions. Twin A’s surgery was complicated by infection resulting in resorption of the frontal bone. A titanium cranioplasty was performed to reconstruct the defect in 2009.
By 8 years of age, both girls had significant forehead and mid-face retrusion with exorbitism. Their forehead abnormalities were different due to their presenting pathologies and postoperative courses (►
Figure 1 and
Figure 2). Their mid-face retrusion was similar with both displaying obstructive sleep apnea (snoring), exorbitism, and Angle class III malocclusion. There were no clinical, ophthalmologic, or radiological findings consistent with elevated intracranial pressure (ICP).
For improvement of the twins’ significant proptosis, traditional Le Fort III advancement and placement of an on-lay forehead cranioplasty were planned.
The implants were manufactured from polyetheretherketone (PEEK) and were tailored specifically to fit the contour of each patient’s forehead using both Medical Modeling (Golden, CO) and the input of the operative surgeon during a webinar (►
Figure 1). Interaction between engineer and surgeon accomplished an understanding of the soft tissue envelope stresses and limitations that would be encountered in registering the construct accurately. Given the differences in the shape of the forehead between the two girls, these implants had to be significantly different to achieve similar postoperative results. Implant projection was based on the normal distance between brow and anterior corneal distance as demonstrated by Pai et al.[
16] Both patients underwent the operation during the same week to facilitate family needs and postoperative recovery (►
Figure 2 and
Figure 3).
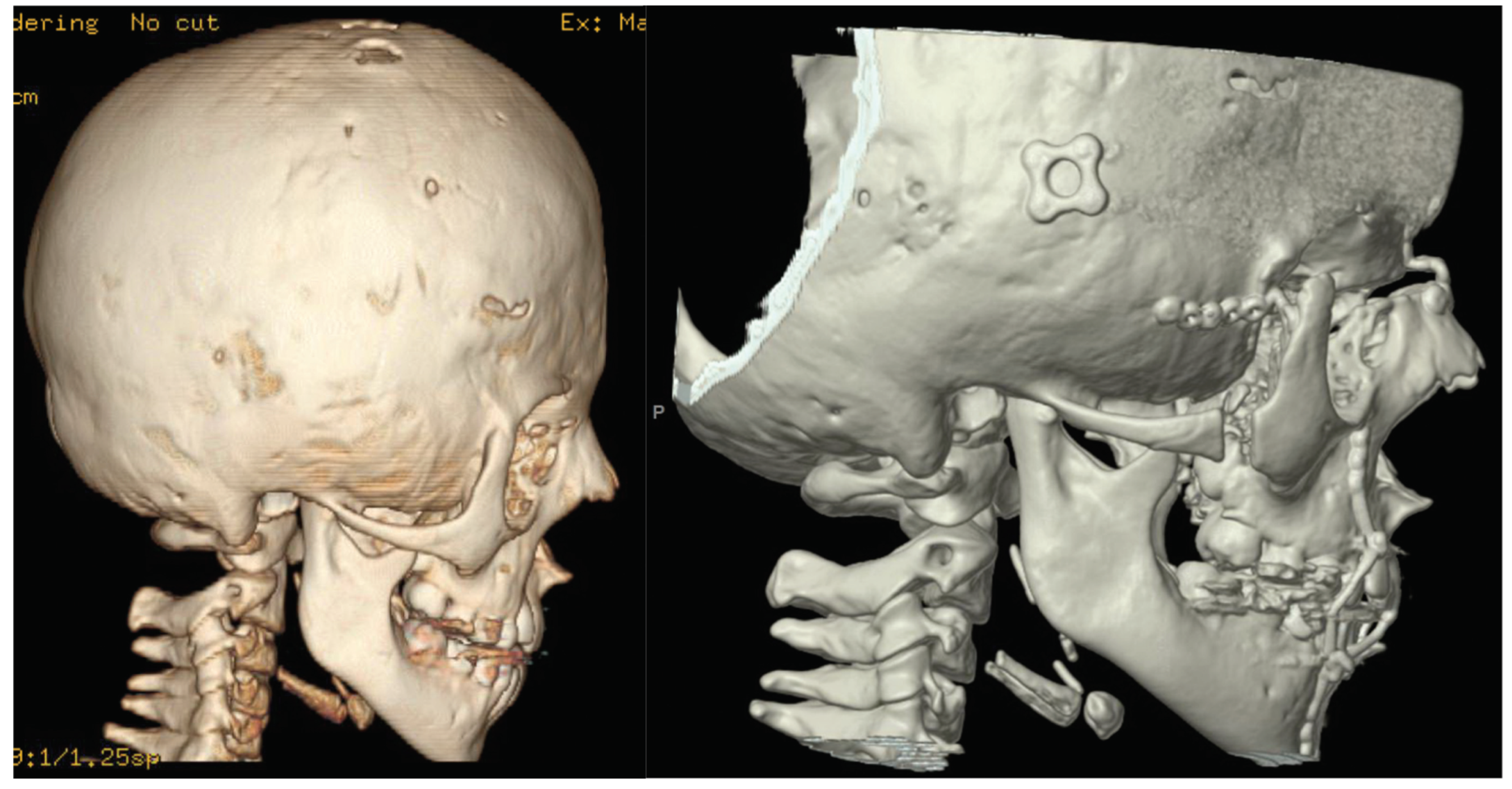
Figure 2.
Preoperative CT of twin A (left) and postoperative CT (right).
Figure 2.
Preoperative CT of twin A (left) and postoperative CT (right).
Figure 3.
Preoperative CT of twin B (left) and a close-up of postoperative CT (right).
Figure 3.
Preoperative CT of twin B (left) and a close-up of postoperative CT (right).
The twins tolerated the procedure without intraoperative complications. Postoperatively, twin A demonstrated neuropraxia of the left frontal branch of the facial nerve that manifested as mild brow weakness. Postoperative evaluation at 6 months revealed an improvement in forehead retrusion and asymmetry as well as resolution of their obstructive sleep apnea and class III malocclusion (►
Figure 4,
Figure 5,
Figure 6 and
Figure 7). Twin A’s neuropraxia was resolved by her 6-month postoperative visit. Both girls were in good spirits and happy with their appearance.
Discussion
The current report highlights phenotypic variability between identical twins with Crouzon syndrome and the operative management of this variability. A recent meta-analysis demonstrates phenotypic variability in identical twins with craniosynostosis as defined by severity, variability of suture fusion, and diagnosis.[
2] This review included both syndromic and nonsyndromic patients. Further investigation is needed to fully understand the phenotypic variability between identical twins with Crouzon syndrome.
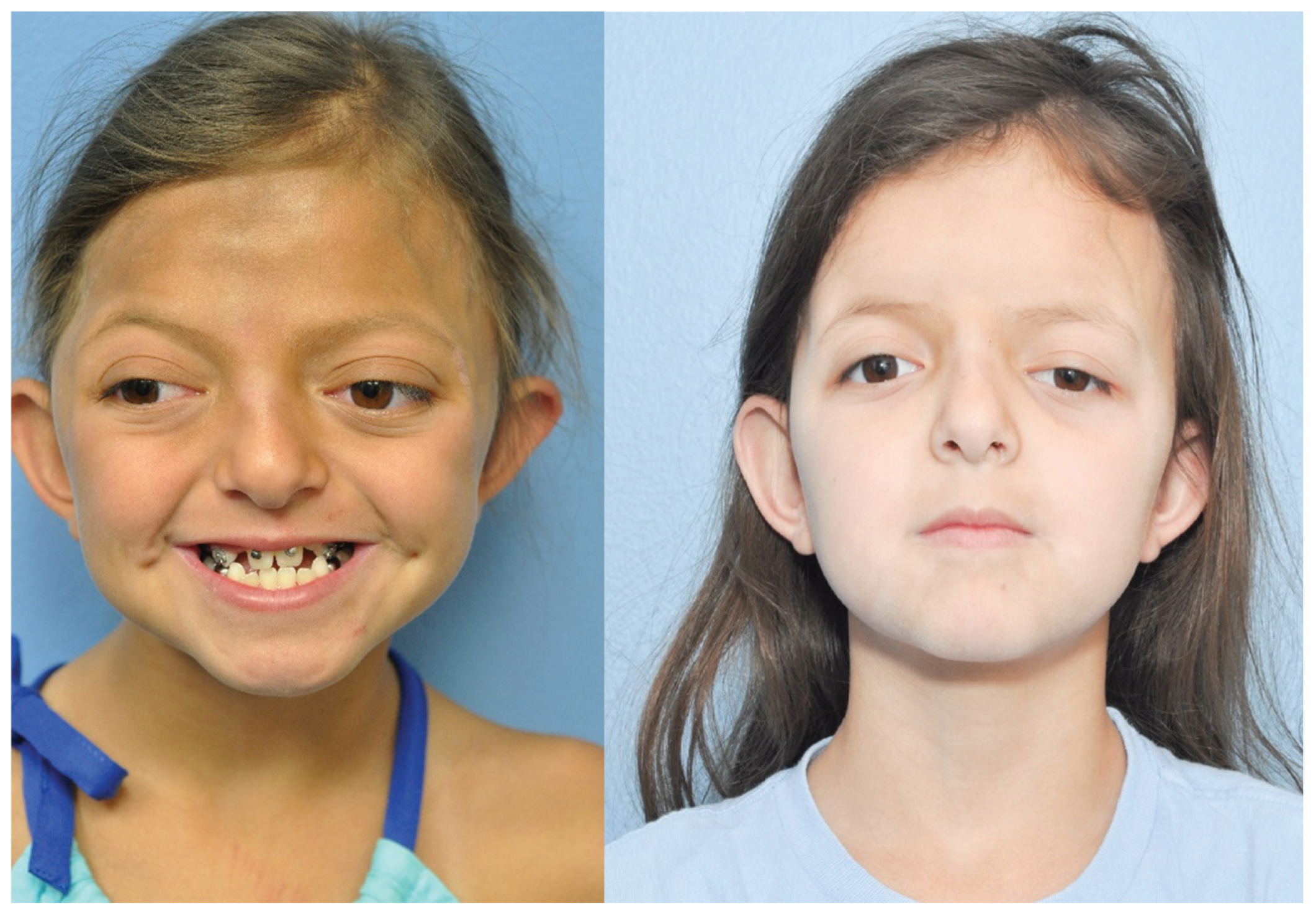
Figure 4.
Preoperative photo twin A (left) and postoperative (right)—anterior–posterior.
Figure 4.
Preoperative photo twin A (left) and postoperative (right)—anterior–posterior.
Figure 5.
Preoperative photo twin B (left) and postoperative (right)—anterior–posterior.
Figure 5.
Preoperative photo twin B (left) and postoperative (right)—anterior–posterior.
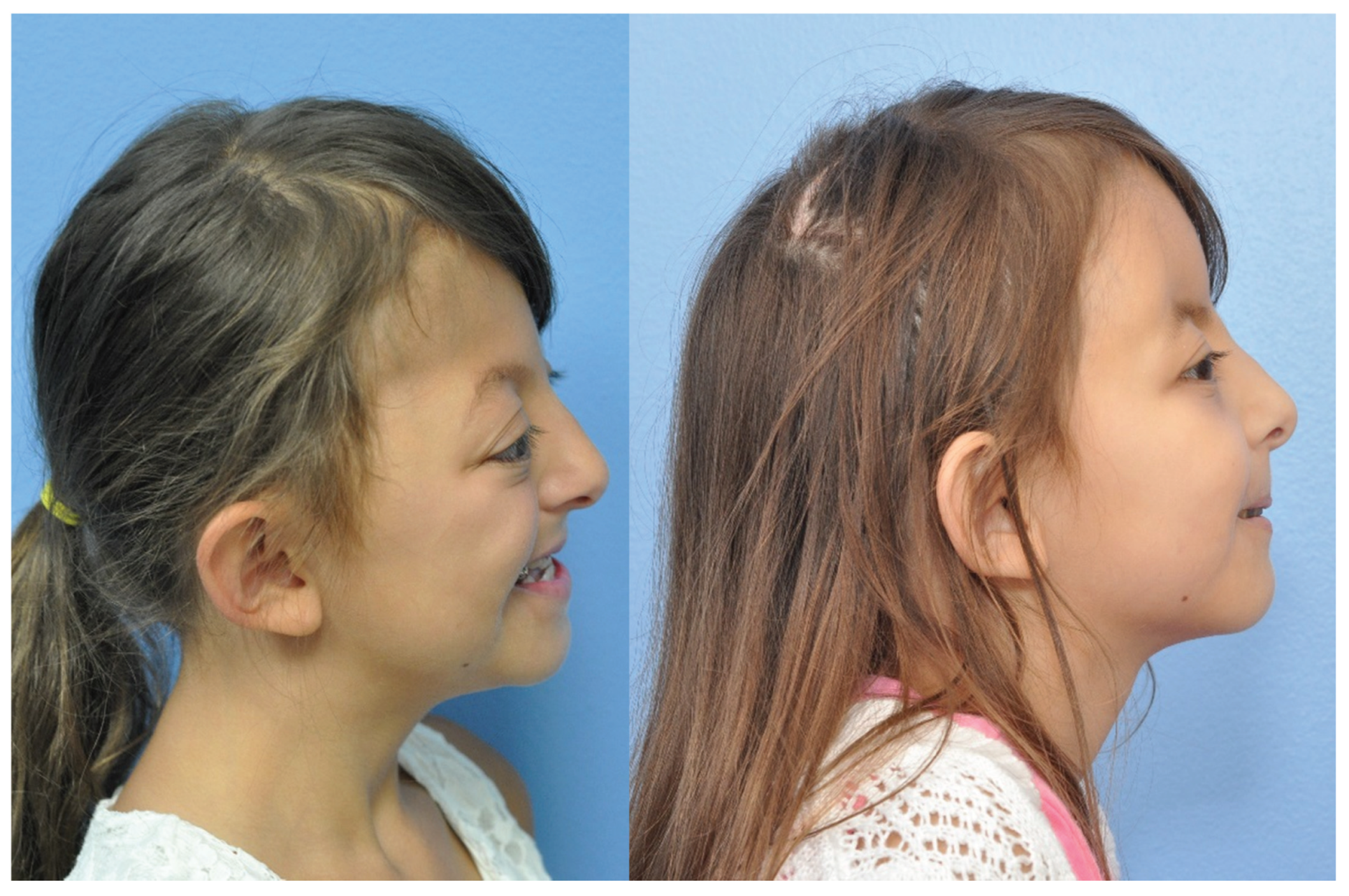
Figure 6.
Preoperative photo twin A (left) and postoperative (right)—lateral.
Figure 6.
Preoperative photo twin A (left) and postoperative (right)—lateral.
As demonstrated in this and our previous report, MZ discordance suggests that other factors influence phenotype. These likely include epigenetic modifications, postzygotic genetic variation, and the prenatal environment. Epigenetic modifications regulate gene expression and most commonly include DNA methylation and histone modification. Genes that lose their epigenetic modifications will have abnormal expression leading to too much or too little protein product. The resulting phenotype depends on the affected gene(s). In addition, discordance may result from genetic or chromosomal anomalies occurring after the zygotes split. These errors occur during mitosis and result in genetic mosaicism, a phenomenon in which the genetic anomaly is present in a percentage of the affected patient’s cells. The overall phenotype is dependent on the percentage of cells carrying the mutation. These could include sequence mutations within the DNA or chromosomal anomalies such as a deletion or duplication.
The prenatal environment is also thought to influence the craniosynostotic phenotype. Breech presentation has been reported twice in the literature as a possible cause of craniosynostosis, with one report being in nonsyndromic twins and the other in syndromic twins.[
3] In our case, twin B was in breech position.
Given the phenotypic variability as well as the differences in overall clinical course, our patients presented with different craniofacial morphologies. The goals of surgical care were to correct facial asymmetries and improve functional issues. These children presented with differing forehead morphology due to the differences in their initial presentation as well as their postoperative courses during their initial operation. They both suffered from midface retrusion as well as exorbitism. As a result, they had class III malocclusion and obstructive sleep apnea (snoring). Although the operation they would receive would be the same, the exact details would differ given the differences in their presentation. For example, twin A had the additional consideration of her frontal bone titanium cranioplasty. Her PEEK implant was constructed so that it would contour over the titanium. This was done to preserve the titanium implant, as removal of titanium after a prolonged period inside the body presents a significant risk of dural leak and cranial injury.
Figure 7.
Preoperative photo twin B (left) and postoperative (right)—lateral.
Figure 7.
Preoperative photo twin B (left) and postoperative (right)—lateral.
The timing of surgery was also important and important consideration. The goal is to correct their proptosis as soon as possible while avoiding the need for future revisionary surgery. Surgery at 8 years of age was deemed appropriate, as orbital growth is typically complete by this age. Previous studies have shown a significant difference between the need for revisionary surgery when Le Fort III is performed before and after the age of 8 years.[
17] Le Fort III surgery performed at or above the age of 8 years has not been shown to require later revisionary surgery. Although malocclusion was still a concern, correction of this would need to be achieved only after the jaw has finished growing at the time of skeletal maturity.
With the help of preoperative imaging as well as surgical planning software provided by Medical Modeling, we were able to simulate their surgery as well as design implants to fit and correct their specific deformities. This step, during the preoperative planning stages, helps decrease operative time by eliminating the need intraoperatively to fashion a forehead implant or determine the exact distance the Le Fort III segment should be stretched and plated. These stages are done in the preoperative setting on the digital skull as well as on a three-dimensional (3D) model so that plates are prebent and ready for implantation. The surgical time for each twin was between 5 and 6 hours.
Conclusion
Seven years after the initial report of the importance of genetic and epigenetic factors on MZ twins with syndromic craniosynostosis, who present with variable phenotypes, we present the clinical course and operative management for these patients. With the help of preoperative imaging, Medical Modeling, and preoperative model surgery, we were able to better understand the surgical anatomy and expedite the surgical procedure. By utilizing preoperative planning and 3D modeling complex, craniofacial deformities can be understood and corrected in a safe and expeditious manner.