Orbital compartment syndrome in the context of trauma occurs due to an orbital hemorrhage. However, periorbital injury can present with various permutations of bone trauma, soft-tissue edema, and hematomas that might involve proptosis and restricted motility.
The orbital cavity approximates to a quadrilateral pyramid-shaped structure, made of bony walls, and a base made of soft tissue. As such, there is limited scope to accommodate an increased volume due to a mass effect, with the effect invariably being axial or nonaxial forward projection of the globe (proptosis) to a variable extent. The critical clinical signs of an orbital hemorrhage are increased orbital tension to palpation; ophthalmoplegia; increased intraocular pressure; possible optic nerve dysfunction such as reduced visual acuity (VA) and an afferent pupil defect; possible central retinal artery pulsation on funduscopy, indicating perfusion compromise due to increased intraorbital and intraocular pressure; or a central retinal artery occlusion with a cherry-red spot and pale disc if ocular perfusion compromise is established.
In contrast, traumatic orbital blow-out fractures that involve displacement of bony walls are likely to be orbital-volume enhancing and will allow accommodation of increased volume into the adjacent paranasal sinuses. This will be particularly true of type II and type III pure orbital-floor fractures [
1], where the floor is hinged on one side or totally punched out into the maxillary sinus, respectively. The critical clinical signs here will be normal or reduced orbital tension; motility restriction depending on fracture location; hypoesthesia in the infraorbital nerve territory; normal IOP; and possible bradycardia mediated through an oculocardiac reflex.
Orbital compartment syndrome may occur with or without associated bony injury. With the former case, the diagnosis is relatively straightforward; in the latter presentation, signs may overlap with signs of fractures and confuse the clinical picture, diagnosis, and management. In the context of periocular trauma, clinicians need to be aware of critical signs to distinguish potential sight-threatening injuries from less urgent cases. We report an unusual case of a traumatic orbital compartment syndrome despite the presence of a large, significantly displaced orbital-floor blow-out fracture. We discuss this case’s clinical features, diagnosis, clinical significance, and management. Related differential diagnoses are compared and contrasted.
Case Report
A 32-year-old man was involved in a motorcycle accident during a sports event in which he flipped and fell, with a branch going through his protective goggles and striking his right eye. He presented to the emergency room with periocular bruising and complaining of right midface, lip, and gum numbness. The patient was referred for an ophthalmology opinion for a suspected fracture. He did not have any previous ocular or medical history. On examination, his VA was 20/80 in the right eye and 20/20 in the left eye. Both upper and lower eyelids were tense with increased orbital tension on palpation. The conjunctiva was diffusely chemosed and cornea was clear on inspection; there was a hyphema on gross inspection in the anterior chamber. The pupil was reactive with no afferent pupil defect. The intraocular pressure was 50 mm Hg on handheld testing with a tonopen. The motility was restricted in up, down, and lateral gaze. Undilated funduscopy showed central retinal artery pulsation. There was hypoesthesia in the territory of the infraorbital nerve. Systemically, he was stable but noted to have a pulse of 45 beats per minute.
The clinical diagnosis, on the basis of a tense orbit, raised intraocular pressure, and restricted motility, was that of an intraorbital hemorrhage; however, the restriction of motility, infraorbital nerve hypoesthesia, and oculocardiac reflex also suggested an orbital floor fracture. Due to the raised intraocular pressure and central retinal artery pulsation, a decision was made to proceed with urgent surgical decompression by means of lateral canthotomy and cantholysis. Intraocular pressure reduction was further augmented with intravenous acetazolamide 500 mg stat and the same dose orally.
The postprocedure intraocular pressure was 23 to 29 mm Hg and there was a palpable reduction in orbital tension. Subsequent orbital imaging (
Figure 1,
Figure 2 and
Figure 3) confirmed a displaced/punched-out fracture of the orbital floor (unhinged, type III) [
1] with fracture of the medial wall, hematoma in the orbital compartment. The globe was entrapped between the orbital rim inferiorly, hematoma posterosuperiorly, and edematous preseptal tissue anteriorly. There was no orbital foreign body.
Further management included a stat dose of intravenous methylprednisolone 125 mg the following day and a tapering regimen of oral prednisolone starting at 20 mg.
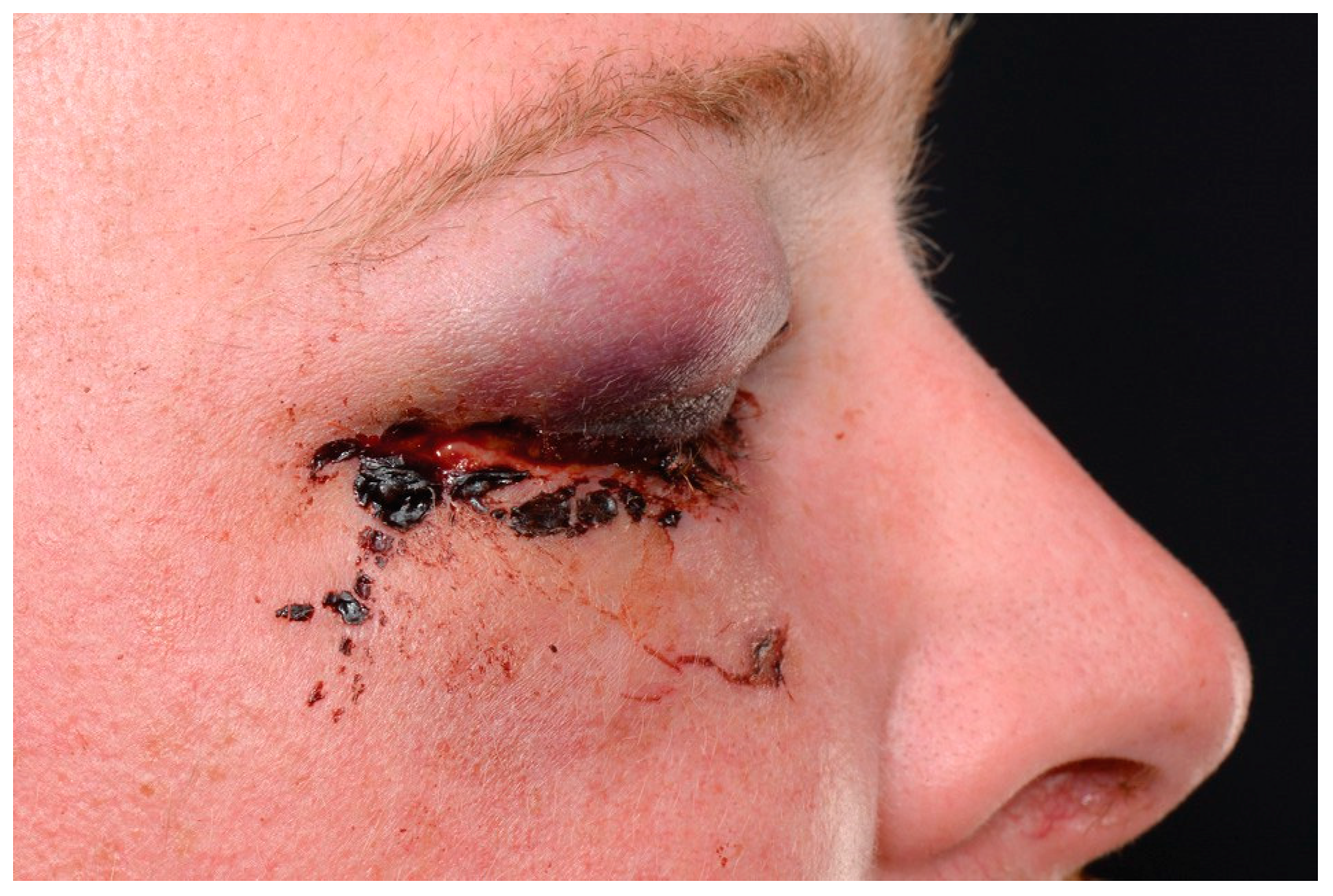
By day 2, the periocular swelling was diminishing (see
Figure 4) with a VA of 20/30 and intraocular pressure of 23 mm Hg. At day 10, the vision was 20/20, exophthalmometry indicated a 3-mm enophthalmos, consistent with resorption of orbital hematoma and edema and floor fracture. The motility was much improved. At 4 months, there was a 6-mm enophthalmos and 2-mm hypoglobus, minimal reduction of motility but persistent infraorbital nerve hypoesthesia.
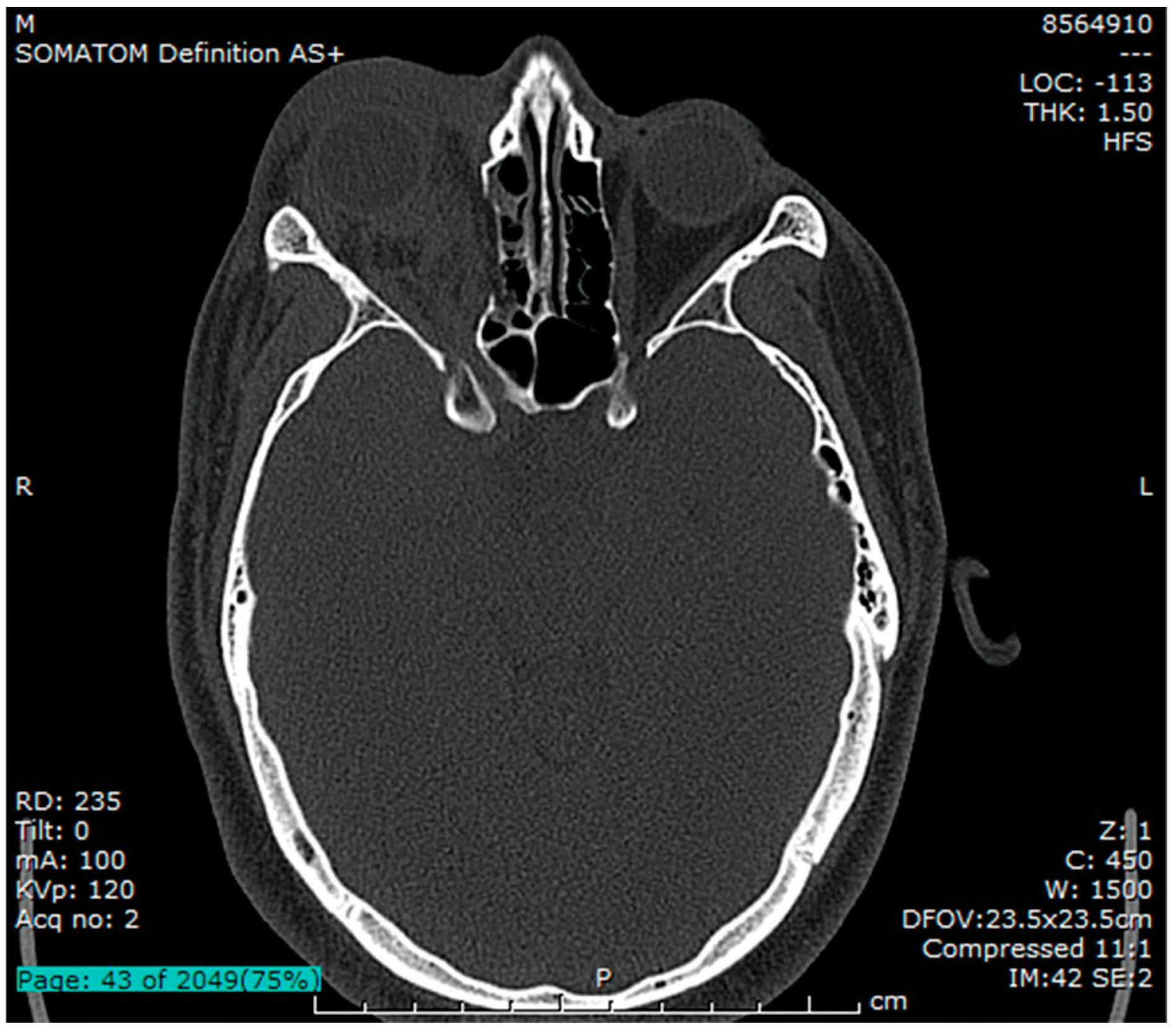
Figure 1.
Axial CT bone window at the level of the superior orbital fissure showing collapse of the medial wall, right proptosis, retro-orbital hematoma, periocular soft-tissue edema.
Figure 1.
Axial CT bone window at the level of the superior orbital fissure showing collapse of the medial wall, right proptosis, retro-orbital hematoma, periocular soft-tissue edema.
Further imaging was performed and orbital exploration was planned with release of incarcerated tissue and enophthalmic floor implant, but the patient did not attend.
Discussion
This case represents an unusual situation wherein, despite a large type III orbital-floor blow-out fracture extending from the inferior orbital groove anteriorly to the apex posteriorly (
Figure 2 and
Figure 3), there remained significant globe compression. The globe was entrapped between the orbital hemorrhage posteriorly and superiorly, the anterior orbital rim inferiorly, and behind edematous soft tissue anteriorly. The critical signs were an overlapping mix of compartment compression and compartment fracture: an orbital mass effect (proptosis; increased orbital tension and intraocular pressure; mechanical ophthalmoplegia due to hemorrhage), functional compromise (diplopia due to ophthalmoplegia; possible reduction in VA; and an afferent pupil defect due to optic nerve compromise), and signs of orbital structural compromise of wall fracture (mechanical ophthalmoplegia due to incarceration of muscle; infraorbital nerve hypoesthesia; and oculocardiac-reflex–mediated bradycardia). A mixed presentation may be clinically suspected on evaluation of clinical signs, but the diagnosis will require imaging for confirmation. This should not delay its management.
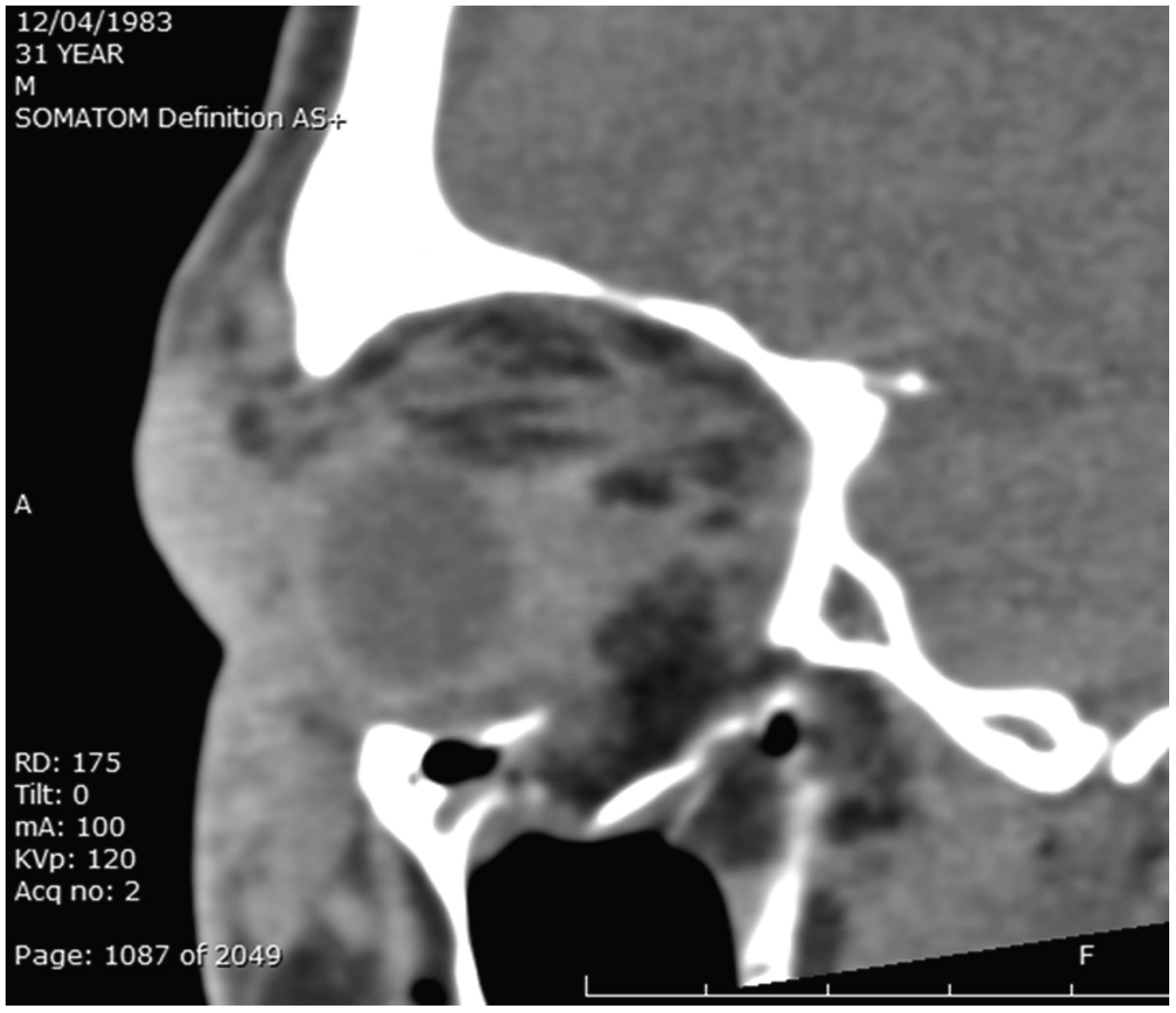
Figure 2.
Sagittal soft-tissue window CT image showing punched-out floor fracture of the orbital floor. Globe resting on the anterior orbital rim and floor. Periocular soft-tissue edema.
Figure 2.
Sagittal soft-tissue window CT image showing punched-out floor fracture of the orbital floor. Globe resting on the anterior orbital rim and floor. Periocular soft-tissue edema.
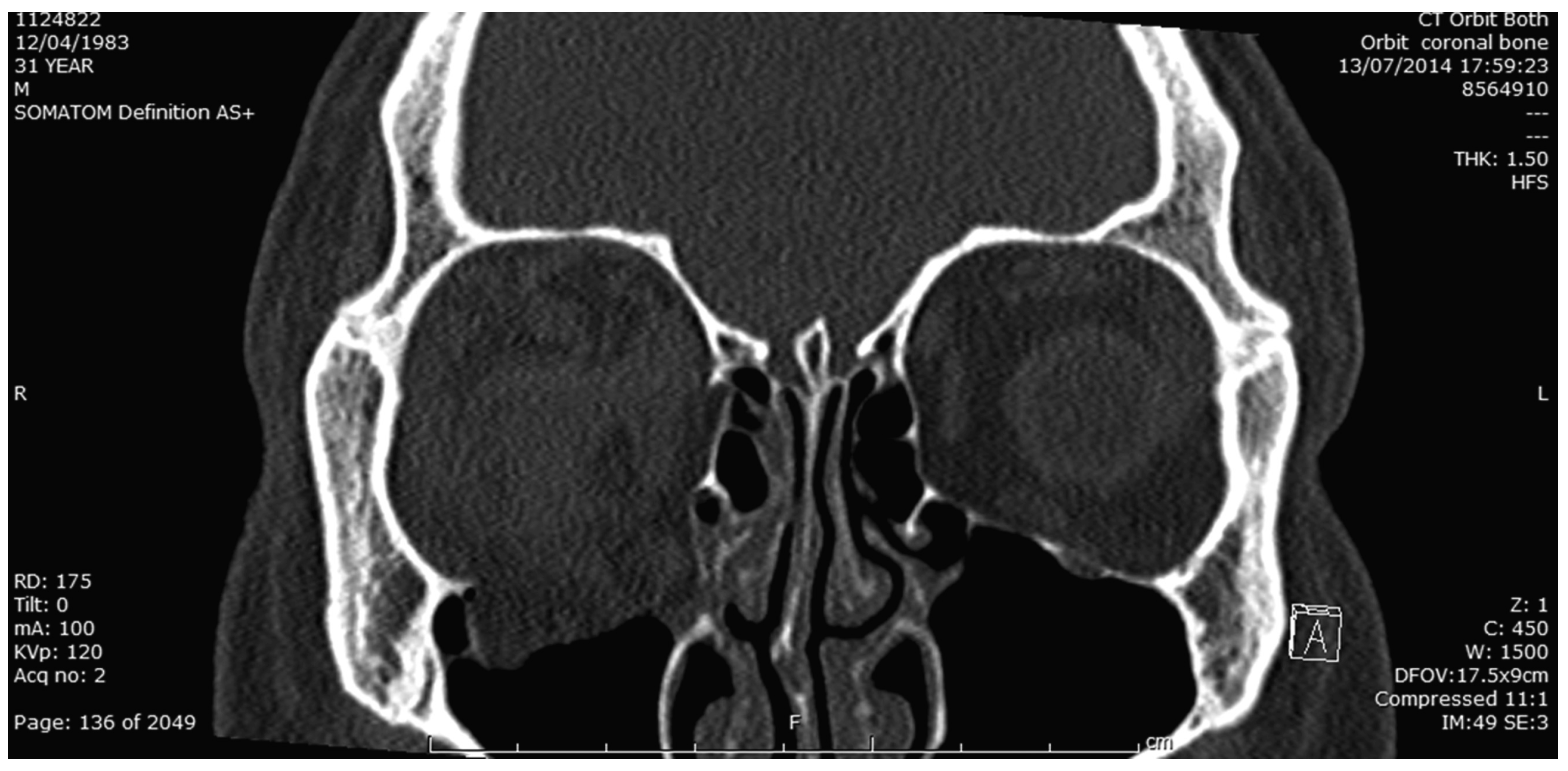
Figure 3.
Coronal bone window CT image showing floor and medial wall fracture of R orbit.
Figure 3.
Coronal bone window CT image showing floor and medial wall fracture of R orbit.
The clinical significance of this type of a combined presentation is that, in the context of periocular trauma, physicians may be misled by the signs of an orbital blow-out fracture and assume that orbital contents and any associated hemorrhage will have decompressed into the paranasal sinuses, and therefore not concern themselves with the simultaneous possibility of a sight-threatening orbital mass effect from a hemorrhage. Indeed, following failure of canthotomy and cantholysis to reduce intraorbital pressure, orbital decom-pression by deliberately fracturing the floor is a
recommended next step [
2].
Figure 4.
Day 2 post–lateral canthotomy and cantholysis.
Figure 4.
Day 2 post–lateral canthotomy and cantholysis.
This case highlights the need to actively seek and rule out signs of orbital compartment syndrome with any periocular trauma. Any clinical suggestion of orbital compartment syndrome requires decompression as a priority, regardless of the presence of a clinically suspected fracture. This decompression may be either surgical or medical, or surgical and medical combined. Other case series [
3,
4] have reported on orbital compartment syndrome with orbital wall fractures, but have not elaborated on the presenting clinical features, that is, to what extent clinical features overlapped or predominated.
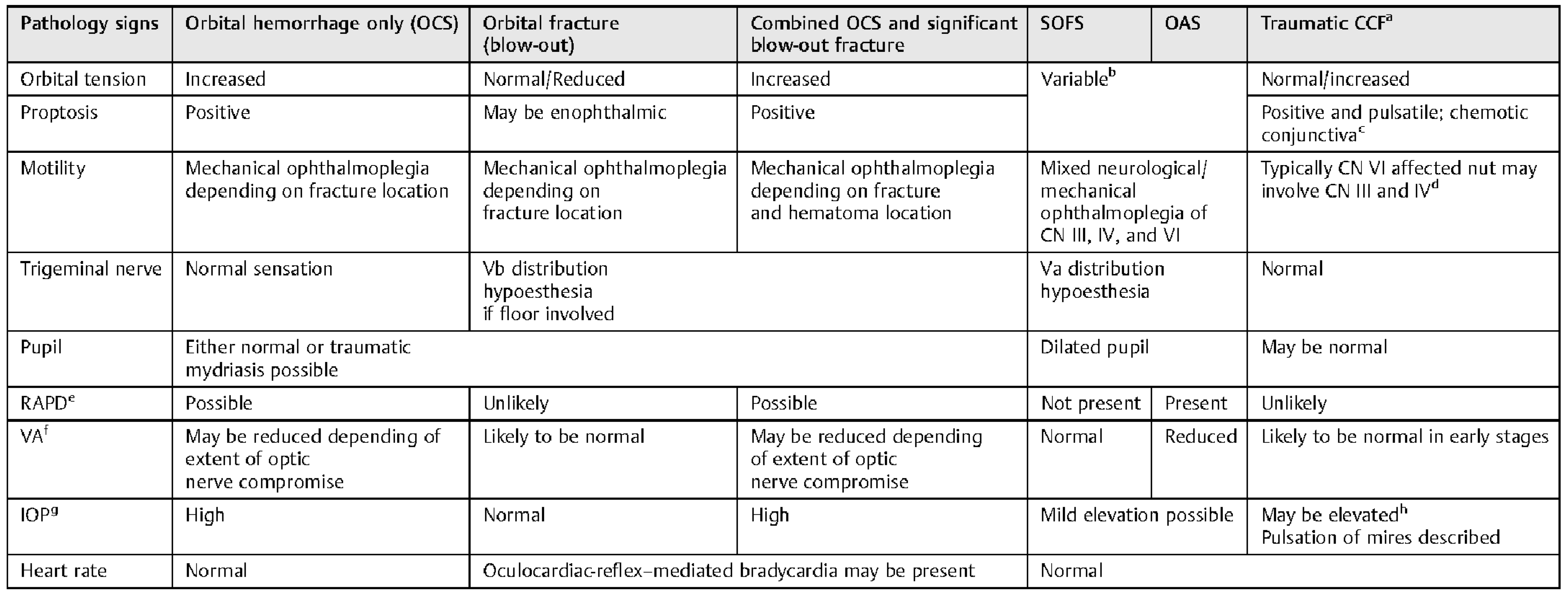
The clinical features of orbital compartment syndrome, combined compartment syndrome and fracture, and isolated orbital blow-out fractures are compared and contrasted in
Table 1. Other related trauma presentations that involve combinations of proptosis, restricted motility, are syndromes such as superior orbital fissure syndrome, orbital apex syndrome, and a carotid-cavernous fistula. A literature search of superior orbital fissure syndrome and orbital apex syndrome did not reveal any reported cases, to our knowledge, associated with an orbital hemorrhage [
5,
6,
7,
8,
9,
10,
11,
12,
13], although it remains a possibility. In these two conditions, in the absence of hema-toma, proptosis can be caused by reduced ocular muscle tone or superior ophthalmic vein compression causing soft-tissue edema. Management of these conditions typically requires steroids in the acute stage. Interestingly, a traumatic “pseudoorbital apex syndrome” has been described wherein the optic nerve dysfunction was attributable to ischemia from an internal carotid artery dissection rather than compression from bony injury [
13].
Traumatic carotid-cavernous fistula (“red eye shunt”) typically presents with a diffusely chemotic (swollen and injected) conjunctiva, pulsatile proptosis, and ophthalmoplegia (typically abducens nerve paresis, as this crosses through the cavern rather than being protected in the cavern wall such as cranial nerves III and IV, causing lateral gaze deficiency) [
14]. Bruits and headaches are also common [
14]; patients may describe pulse-synchronous tinnitus. Signs may present immediately or evolve to a classical presentation over several days [
14,
15]. A review of the literature by Takenoshita et al. [
15] of 20 case reports revealed four cases to manifest implicating signs on the day of presentation, five on the first day and the remainder later. If suspected, the conclusive diagnostic test is cerebral angiography and treatment involves occlusion of the fistula.
Table 1.
Periorbital trauma presenting with proptosis and ophthalmoplegia compared and contrasted.
Table 1.
Periorbital trauma presenting with proptosis and ophthalmoplegia compared and contrasted.
Table 1 compares and contrasts the presentations of these overlapping conditions. Physicians should be aware that they may not present in the “classical” format and signs may evolve. Clinical judgment will need to be made depending on the history and signs.
Finally, a clinical pearl that can be gleaned from this case is that the intraocular pressure and central retinal artery pulsation signs were used as
leading indicators of imminent optic nerve compromise and implied a critically elevated intraocular and intraorbital pressure. An afferent pupillary defect was not present on testing. Traditionally, reduced VA and presence of an afferent pupil defect are used to decide between medical and surgical decompression routes. VA may not be the best objective indicator of optic nerve function, as it may be difficult to get the cooperation of the patient and may also be affected by eyelid position, chemosis, lens trauma, and vitreous hemorrhage. In contrast, a positive afferent defect remains a
lagging indicator of perfusion compromise to the optic nerve head, that is, perfusion has already been compromised. There is thought to be a 90- to 120-min window before ischemia is potentially irreversible [
16].
In summary, periorbital trauma featuring proptosis and restricted motility may present initially to various medical subspecialties including accident and emergency physicians, maxillofacial surgeons, and ophthalmologists. There are many differentials to consider. Attending physicians should have a clear understanding of important clinical signs of orbital compartment syndrome versus fractures, and actively seek to rule orbital compartment syndrome out due to its sight-threatening nature. Combined fracture/compartment syndrome presentations are an entity that clinicians should be aware of, as they may mislead by presenting a confusing clinical picture to the extent that the clinician discounts the presence of a sight-threatening compartment syndrome. It is a diagnosis that may be made tentatively on clinical examination, but the management in the acute stage is as for orbital compartment syndrome. If orbital compartment syndrome is diagnosed, physicians should be prepared for its management: urgent lateral canthotomy and cantholysis. The diagnosis can only be confirmed with imaging but should not delay management.