SPL12 Regulates AGL6 and AGL21 to Modulate Nodulation and Root Regeneration under Osmotic Stress and Nitrate Sufficiency Conditions in Medicago sativa
Abstract
1. Introduction
2. Results
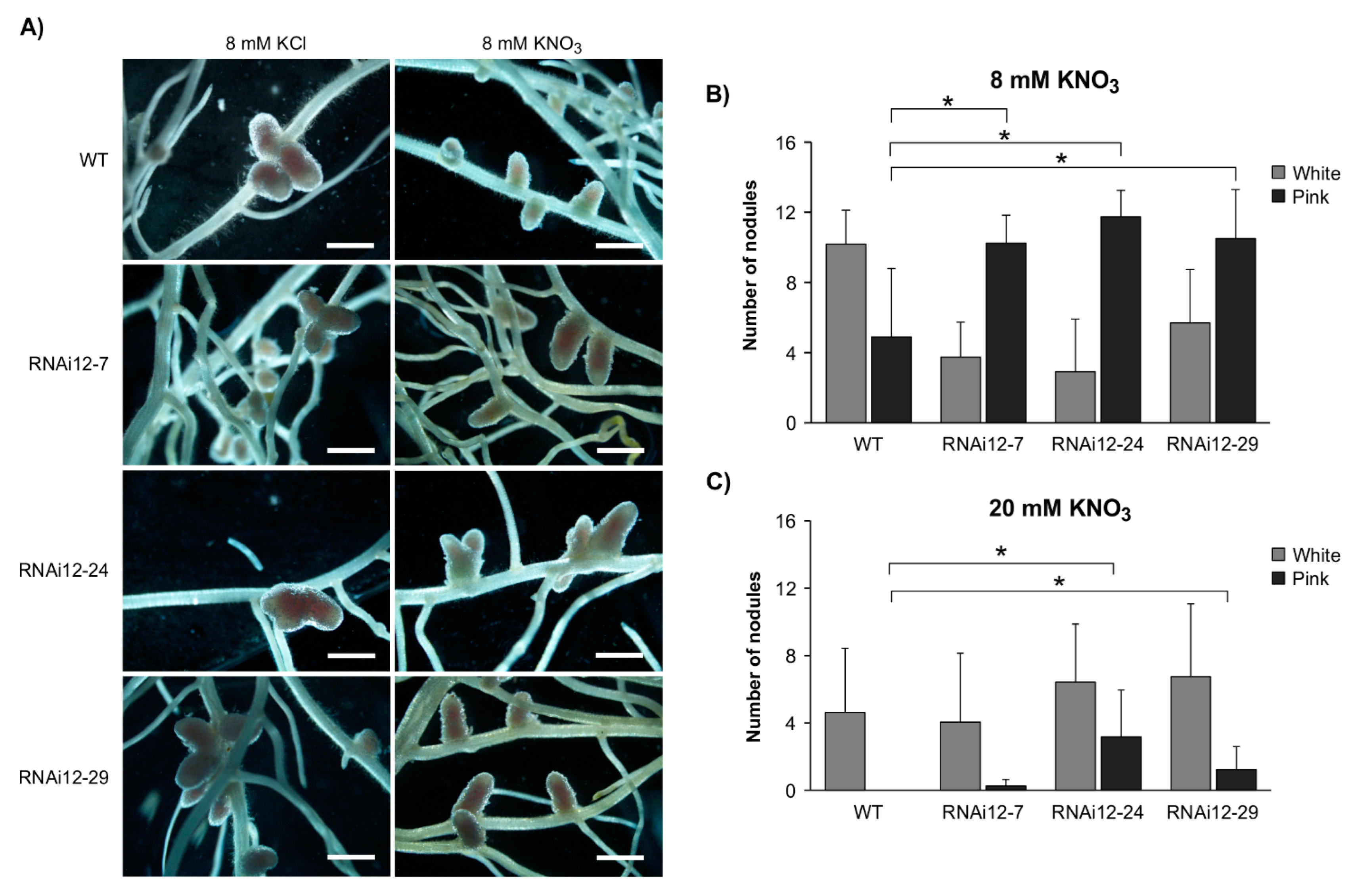
2.1. SPL12 Silencing Attenuates the Effect of Nitrate on Nodulation
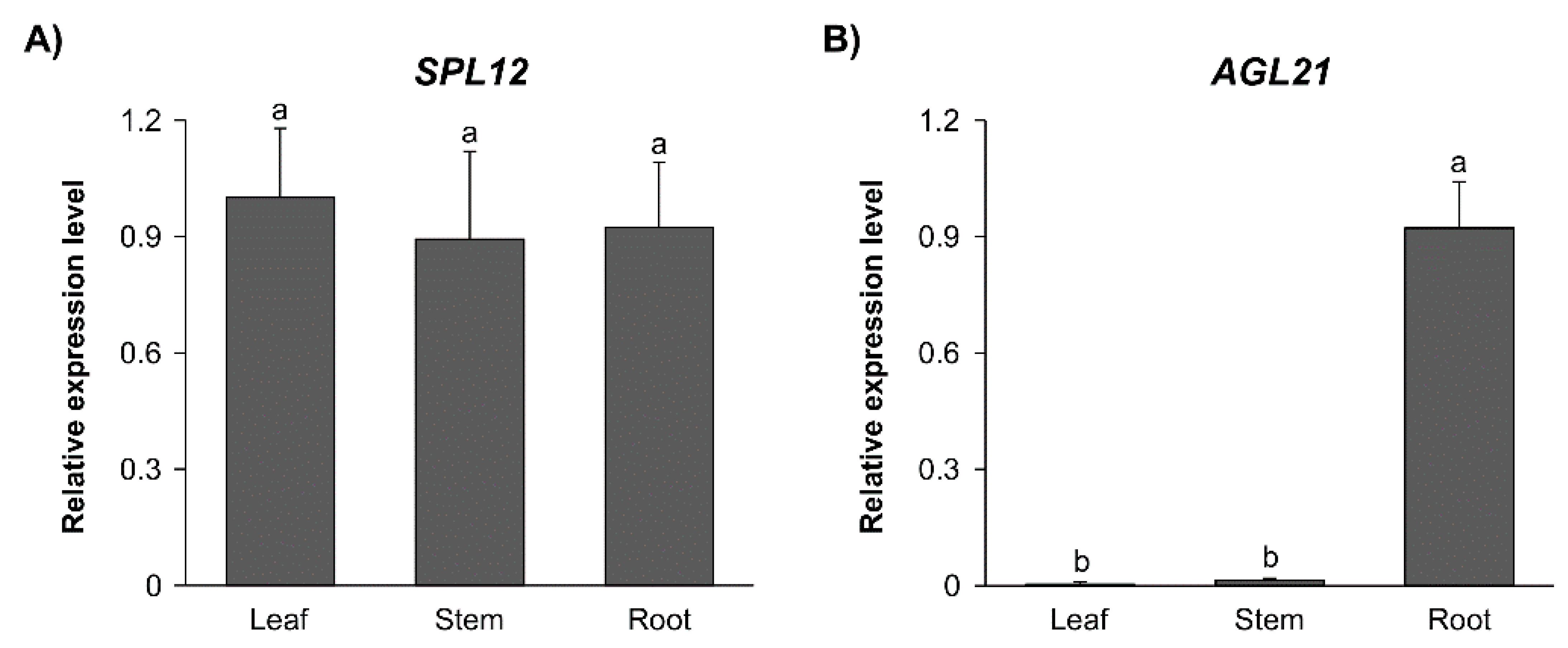
2.2. Effect of Nitrate on Expression of SPL12 and AGL21
2.3. SPL12 Is a Direct Regulator of AGL21
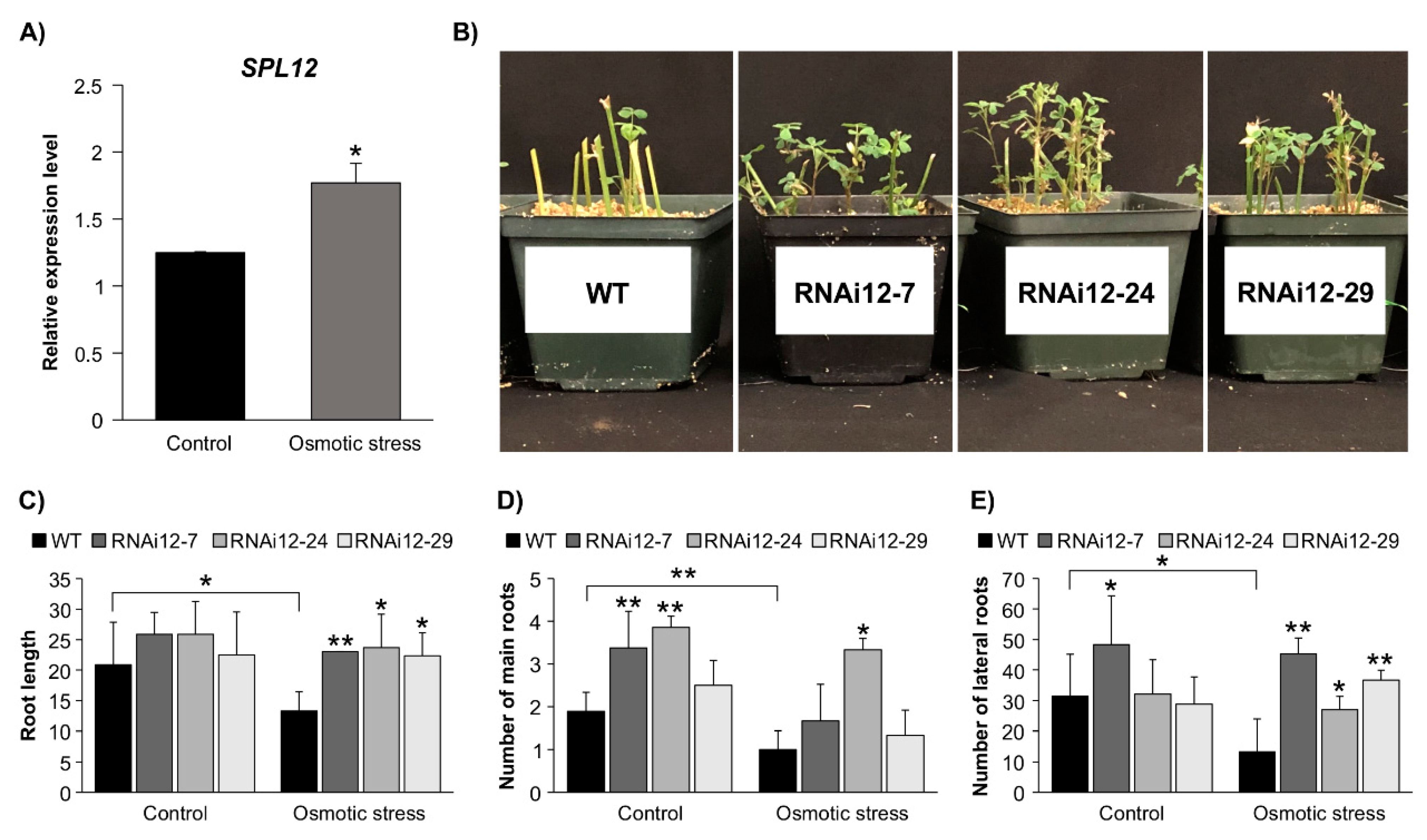
2.4. Effect of SPL12 Silencing in Response to Osmotic Stress
2.5. SPL12 Silencing Mitigates Nodulation Inhibition under Osmotic Stress
2.6. Effect of Osmotic Stress on Expression of SPL12, AGL21, and AGL6 in SPL12-RNAi Alfalfa
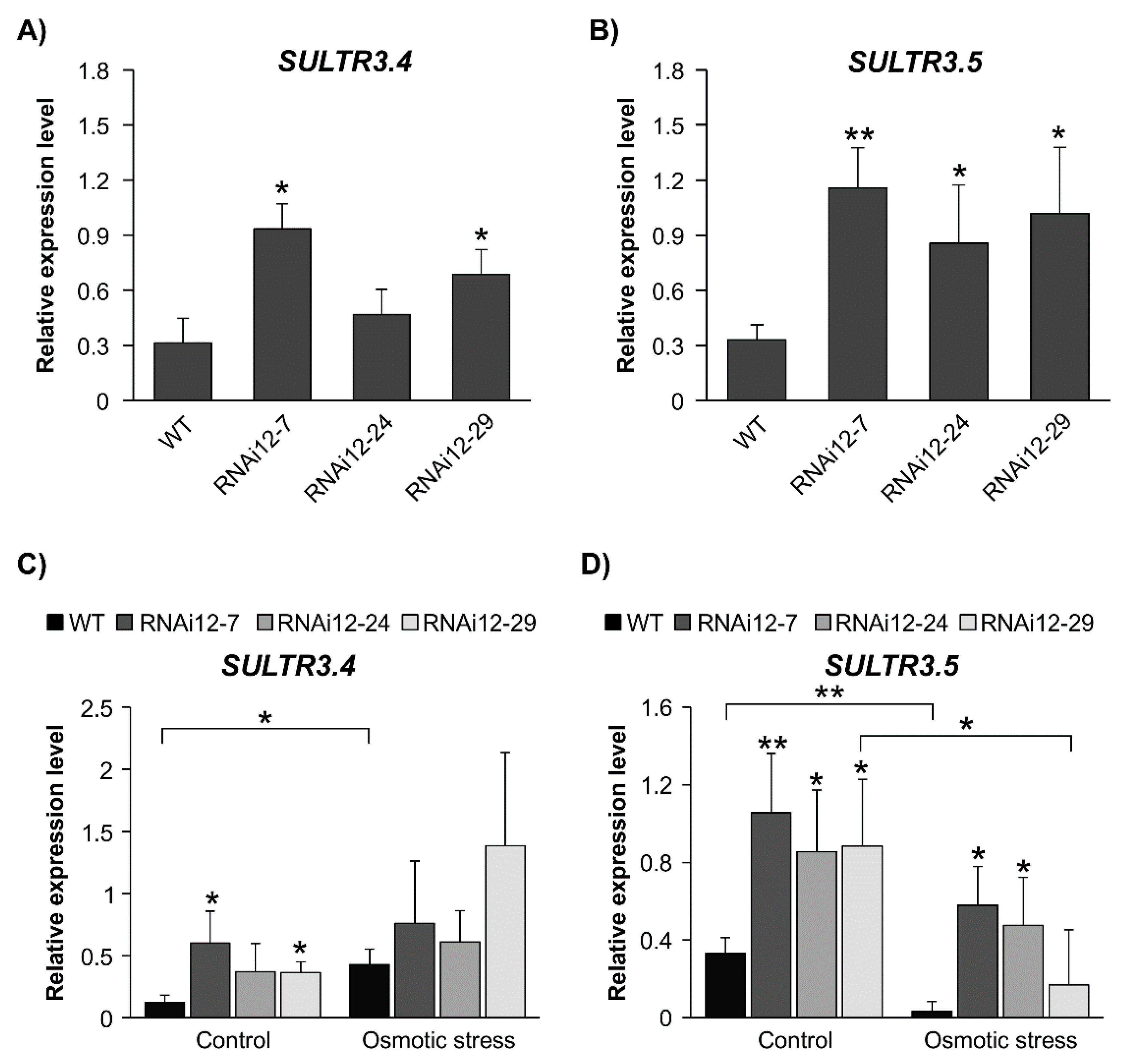
2.7. Sulfate Transporters Are Enhanced in SPL12-Silenced Plants
2.8. Effect of Mannitol Treatment on Expression of Stress-Related Genes
2.9. AGL6 Silencing Maintains Nodulation under Osmotic Stress
3. Discussion
3.1. How Nitrate Availability Affects Nodulation through the SPL12-AGL21-Regulatory Pathway
3.2. Role of SPL12 and AGL6 in Regulating Nodulation under Osmotic Stress in Alfalfa
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Phenotypic Analysis of Nodule Development
4.3. Nitrate Treatment
4.4. Mannitol Treatment
4.5. RNA Extraction, Reverse Transcription and RT-qPCR
4.6. ChIP-qPCR Analysis
4.7. Phylogenetic Tree Construction
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.; Testerink, C. Roots withstanding their environment: Exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.P.; Mattoo, A.K. Sustainable agriculture—Enhancing environmental benefits, food nutritional quality and building crop resilience to abiotic and biotic stresses. Agriculture 2018, 8, 8. [Google Scholar] [CrossRef]
- Oldroyd, G.E.; Dixon, R. Biotechnological solutions to the nitrogen problem. Curr. Opin. Biotechnol. 2014, 26, 19–24. [Google Scholar] [CrossRef]
- Davidson, E.A.; Suddick, E.C.; Rice, C.W.; Prokopy, L.S. More food, low pollution (mo fo lo po): A grand challenge for the 21st century. J. Environ. Qual. 2015, 44, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Annicchiarico, P.; Barrett, B.; Brummer, E.C.; Julier, B.; Marshall, A.H. Achievements and challenges in improving temperate perennial forage legumes. CRC Crit. Rev. Plant Sci. 2015, 34, 327–380. [Google Scholar] [CrossRef]
- Ma, Y.; Schwenke, G.; Sun, L.; Li Liu, D.; Wang, B.; Yang, B. Modeling the impact of crop rotation with legume on nitrous oxide emissions from rain-fed agricultural systems in Australia under alternative future climate scenarios. Sci. Total Environ. 2018, 630, 1544–1552. [Google Scholar] [CrossRef]
- Radović, J.; Sokolović, D.; Marković, J. Alfalfa-most important perennial forage legume in animal husbandry. Biotechnol. Anim. Husb. 2009, 25, 465–475. [Google Scholar] [CrossRef]
- Jonker, A.; Yu, P. The role of proanthocyanidins complex in structure and nutrition interaction in alfalfa forage. Int. J. Mol. Sci. 2016, 17, 793. [Google Scholar] [CrossRef]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckmann, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 2010, 1, 12. [Google Scholar] [CrossRef]
- Oldroyd, G.E.; Downie, J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef]
- Heim, H.C.; Bernhardt, T.M.; Lang, S.M.; Barnett, R.N.; Landman, U. Interaction of iron–sulfur clusters with n2: Biomimetic systems in the gas phase. J. Phys. Chem. C 2016, 120, 12549–12558. [Google Scholar] [CrossRef]
- Varin, S.; Cliquet, J.-B.; Personeni, E.; Avice, J.-C.; Lemauviel-Lavenant, S. How does sulphur availability modify n acquisition of white clover (Trifolium repens L.)? J. Exp. Bot. 2010, 61, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.; Spencer, D. Sulphur in nitrogen metabolism of legumes and non-legumes. Aust. J. Biol. Sci. 1950, 3, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Becana, M.; Wienkoop, S.; Matamoros, M.A. Sulfur transport and metabolism in legume root nodules. Front. Plant Sci. 2018, 9, 1434. [Google Scholar] [CrossRef]
- Krusell, L.; Krause, K.; Ott, T.; Desbrosses, G.; Krämer, U.; Sato, S.; Nakamura, Y.; Tabata, S.; James, E.K.; Sandal, N. The sulfate transporter sst1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 2005, 17, 1625–1636. [Google Scholar] [CrossRef]
- Roux, B.; Rodde, N.; Jardinaud, M.F.; Timmers, T.; Sauviac, L.; Cottret, L.; Carrère, S.; Sallet, E.; Courcelle, E.; Moreau, S. An integrated analysis of plant and bacterial gene expression in symbiotic root nodules using laser-capture microdissection coupled to rna sequencing. Plant J. 2014, 77, 817–837. [Google Scholar] [CrossRef]
- Gallardo, K.; Courty, P.-E.; Le Signor, C.; Wipf, D.; Vernoud, V. Sulfate transporters in the plant’s response to drought and salinity: Regulation and possible functions. Front. Plant Sci. 2014, 5, 580. [Google Scholar] [CrossRef]
- Matthews, C.; Arshad, M.; Hannoufa, A. Alfalfa response to heat stress is modulated by microrna156. Physiol. Plant. 2019, 165, 830–842. [Google Scholar] [CrossRef]
- Arshad, M.; Gruber, M.Y.; Wall, K.; Hannoufa, A. An insight into microrna156 role in salinity stress responses of alfalfa. Front. Plant Sci. 2017, 8, 356. [Google Scholar] [CrossRef]
- Arshad, M.; Feyissa, B.A.; Amyot, L.; Aung, B.; Hannoufa, A. Microrna156 improves drought stress tolerance in alfalfa (Medicago sativa) by silencing spl13. Plant Sci. 2017, 258, 122–136. [Google Scholar] [CrossRef]
- Feyissa, B.A.; Arshad, M.; Gruber, M.Y.; Kohalmi, S.E.; Hannoufa, A. The interplay between mir156/spl13 and dfr/wd40–1 regulate drought tolerance in alfalfa. BMC Plant Biol. 2019, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, B.A.; Amyot, L.; Nasrollahi, V.; Papadopoulos, Y.; Kohalmi, S.E.; Hannoufa, A. Involvement of the mir156/spl module in flooding response in Medicago sativa. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Gao, R.; Austin, R.S.; Amyot, L.; Hannoufa, A. Comparative transcriptome investigation of global gene expression changes caused by mir156 overexpression in Medicago sativa. BMC Genom. 2016, 17, 658. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.; Hileman, L. Functional evolution in the plant Squamosa-promoter binding protein-like (spl) gene family. Front. Plant Sci. 2013, 4, 80. [Google Scholar] [CrossRef]
- Gou, J.; Debnath, S.; Sun, L.; Flanagan, A.; Tang, Y.; Jiang, Q.; Wen, J.; Wang, Z.Y. From model to crop: Functional characterization of spl8 in m. Truncatula led to genetic improvement of biomass yield and abiotic stress tolerance in alfalfa. Plant Biotechnol. J. 2018, 16, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Hanly, A.; Karagiannis, J.; Lu, Q.S.M.; Tian, L.; Hannoufa, A. Characterization of the role of spl9 in drought stress tolerance in Medicago sativa. Int. J. Mol. Sci. 2020, 21, 6003. [Google Scholar] [CrossRef] [PubMed]
- Aung, B.; Gao, R.; Gruber, M.Y.; Yuan, Z.C.; Sumarah, M.; Hannoufa, A. Msmir156 affects global gene expression and promotes root regenerative capacity and nitrogen fixation activity in alfalfa. Transgenic Res. 2017, 26, 541–557. [Google Scholar] [CrossRef]
- Aung, c.; Gruber, M.Y.; Amyot, L.; Omari, K.; Bertrand, A.; Hannoufa, A. Micro rna 156 as a promising tool for alfalfa improvement. Plant Biotechnol. J. 2015, 13, 779–790. [Google Scholar] [CrossRef]
- Nasrollahi, V.; Yuan, Z.-C.; Lu, Q.; McDowell, T.; Kohalmi, S.; Hannoufa, A. Deciphering the role of spl12 and agl6 from a genetic module that functions in nodulation and root regeneration in Medicago sativa. Plant Mol. Biol. 2022, 1–19. [Google Scholar] [CrossRef]
- De Folter, S.; Shchennikova, A.V.; Franken, J.; Busscher, M.; Baskar, R.; Grossniklaus, U.; Angenent, G.C.; Immink, R.G. A bsister mads-box gene involved in ovule and seed development in petunia and Arabidopsis. Plant J. 2006, 47, 934–946. [Google Scholar] [CrossRef]
- Dong, T.; Hu, Z.; Deng, L.; Wang, Y.; Zhu, M.; Zhang, J.; Chen, G. A tomato mads-box transcription factor, slmads1, acts as a negative regulator of fruit ripening. Plant Physiol. 2013, 163, 1026–1036. [Google Scholar] [CrossRef]
- Huang, B.; Routaboul, J.-M.; Liu, M.; Deng, W.; Maza, E.; Mila, I.; Hu, G.; Zouine, M.; Frasse, P.; Vrebalov, J.T. Overexpression of the class d mads-box gene sl-agl11 impacts fleshy tissue differentiation and structure in tomato fruits. J. Exp. Bot. 2017, 68, 4869–4884. [Google Scholar] [CrossRef] [PubMed]
- Michaels, S.D.; Ditta, G.; Gustafson-Brown, C.; Pelaz, S.; Yanofsky, M.; Amasino, R.M. Agl24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. Plant J. 2003, 33, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Buylla, E.R.; García-Ponce, B.; Sánchez, M.d.l.P.; Espinosa-Soto, C.; García-Gómez, M.L.; Piñeyro-Nelson, A.; Garay-Arroyo, A. Mads-box genes underground becoming mainstream: Plant root developmental mechanisms. New Phytol. 2019, 223, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Gramzow, L.; Theissen, G. A hitchhiker’s guide to the mads world of plants. Genome Biol. 2010, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Angosto, T.; Gómez, P.; Payán, C.; Capel, J.; Huijser, P.; Salinas, J.; Martınez-Zapater, J.M. Tomato flower abnormalities induced by low temperatures are associated with changes of expression of mads-box genes. Plant Physiol. 1998, 117, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Tardif, G.; Kane, N.A.; Adam, H.; Labrie, L.; Major, G.; Gulick, P.; Sarhan, F.; Laliberté, J.-F. Interaction network of proteins associated with abiotic stress response and development in wheat. Plant Mol. Biol. 2007, 63, 703–718. [Google Scholar] [CrossRef]
- Dong, X.; Deng, H.; Ma, W.; Zhou, Q.; Liu, Z. Genome-wide identification of the mads-box transcription factor family in autotetraploid cultivated alfalfa (Medicago sativa l.) and expression analysis under abiotic stress. BMC Genom. 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Gan, Y.; Bernreiter, A.; Filleur, S.; Abram, B.; Forde, B.G. Overexpressing the anr1 mads-box gene in transgenic plants provides new insights into its role in the nitrate regulation of root development. Plant Cell Physiol. 2012, 53, 1003–1016. [Google Scholar] [CrossRef]
- Zhang, H.; Forde, B.G. An Arabidopsis mads box gene that controls nutrient-induced changes in root architecture. Science 1998, 279, 407–409. [Google Scholar] [CrossRef]
- Yu, L.-H.; Miao, Z.-Q.; Qi, G.-F.; Wu, J.; Cai, X.-T.; Mao, J.-L.; Xiang, C.-B. Mads-box transcription factor agl21 regulates lateral root development and responds to multiple external and physiological signals. Mol. Plant 2014, 7, 1653–1669. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liang, Z.; Chen, S.; Sun, H.; Fan, X.; Wang, C.; Xu, G.; Zhang, Y. A transcription factor, osmads57, regulates long-distance nitrate transport and root elongation. Plant Physiol. 2019, 180, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Gautrat, P.; Frugier, F. Nitrate-induced cle35 signaling peptides inhibit nodulation through the sunn receptor and mir2111 repression. Plant Physiol. 2021, 185, 1216–1228. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and n2 fixation by nitrate. CRC Crit Rev Plant Sci 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Burgeff, C.; Liljegren, S.J.; Tapia-López, R.; Yanofsky, M.F.; Alvarez-Buylla, E.R. Mads-box gene expression in lateral primordia, meristems and differentiated tissues of Arabidopsis thaliana roots. Planta 2002, 214, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, Y.; Gruber, M.Y.; Hannoufa, A. Mir156/spl10 modulates lateral root development, branching and leaf morphology in arabidopsis by silencing agamous-like 79. Front. Plant Sci. 2018, 8, 2226–2238. [Google Scholar] [CrossRef]
- Mortier, V.; Den Herder, G.; Whitford, R.; Van de Velde, W.; Rombauts, S.; D’haeseleer, K.; Holsters, M.; Goormachtig, S. Cle peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol. 2010, 153, 222–237. [Google Scholar] [CrossRef]
- Zhao, F.; Hawkesford, M.; McGrath, S. Sulphur assimilation and effects on yield and quality of wheat. J. Cereal Sci. 1999, 30, 1–17. [Google Scholar] [CrossRef]
- Innocenti, G.; Pucciariello, C.; Le Gleuher, M.; Hopkins, J.; de Stefano, M.; Delledonne, M.; Puppo, A.; Baudouin, E.; Frendo, P. Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta 2007, 225, 1597–1602. [Google Scholar] [CrossRef]
- Pang, Y.; Wenger, J.P.; Saathoff, K.; Peel, G.J.; Wen, J.; Huhman, D.; Allen, S.N.; Tang, Y.; Cheng, X.; Tadege, M. A wd40 repeat protein from Medicago truncatula is necessary for tissue-specific anthocyanin and proanthocyanidin biosynthesis but not for trichome development. Plant Physiol. 2009, 151, 1114–1129. [Google Scholar] [CrossRef]
- Lin, J.-s.; Li, X.; Luo, Z.; Mysore, K.S.; Wen, J.; Xie, F. Nin interacts with nlps to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat. Plants 2018, 4, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Nova-Franco, B.; Íñiguez, L.P.; Valdés-López, O.; Alvarado-Affantranger, X.; Leija, A.; Fuentes, S.I.; Ramírez, M.; Paul, S.; Reyes, J.L.; Girard, L. The micro-rna172c-apetala2-1 node as a key regulator of the common bean-rhizobium etli nitrogen fixation symbiosis. Plant Physiol. 2015, 168, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Mirowski, P.; LeCun, Y.; Shasha, D.E.; Coruzzi, G.M. Predictive network modeling of the high-resolution dynamic plant transcriptome in response to nitrate. Genome Biol. 2010, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Jin, J.F.; Lou, H.Q.; Liu, L.; Kochian, L.V.; Yang, J.L. Lespl-cnr negatively regulates cd acquisition through repressing nitrate reductase-mediated nitric oxide production in tomato. Planta 2018, 248, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Meynard, D.; Khong, G.N.; Pauluzzi, G.; Guiderdoni, E.; Gantet, P. Analysis of the expression of the agl17-like clade of mads-box transcription factors in rice. Gene Expr. Patterns 2013, 13, 160–170. [Google Scholar] [CrossRef]
- Íñiguez, L.P.; Nova-Franco, B.; Hernández, G. Novel players in the ap2-mir172 regulatory network for common bean nodulation. Plant Signal. Behav. 2015, 10, 1062957. [Google Scholar] [CrossRef]
- De Zélicourt, A.; Diet, A.; Marion, J.; Laffont, C.; Ariel, F.; Moison, M.; Zahaf, O.; Crespi, M.; Gruber, V.; Frugier, F. Dual involvement of a Medicago truncatula nac transcription factor in root abiotic stress response and symbiotic nodule senescence. Plant J. 2012, 70, 220–230. [Google Scholar] [CrossRef]
- Sindhu, S.; Dahiya, A.; Gera, R.; Sindhu, S.S. Mitigation of abiotic stress in legume-nodulating rhizobia for sustainable crop production. Agric. Res. 2020, 9, 444–459. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.; Xiong, L. A plant microrna regulates the adaptation of roots to drought stress. FEBS Lett. 2012, 586, 1742–1747. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, R.-G.; Mao, G.; Koczan, J.M. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 2006, 142, 1065–1074. [Google Scholar] [CrossRef]
- Kibido, T.; Kunert, K.; Makgopa, M.; Greve, M.; Vorster, J. Improvement of rhizobium-soybean symbiosis and nitrogen fixation under drought. Food Energy Secur. 2020, 9, e177. [Google Scholar] [CrossRef]
- Mouradi, M.; Farissi, M.; Bouizgaren, A.; Lahrizi, Y.; Qaddoury, A.; Ghoulam, C. Alfalfa and its symbiosis responses to osmotic stress. CFTM 2018, 17, 149–168. [Google Scholar]
- Ashraf, M.; Iram, A. Drought stress induced changes in some organic substances in nodules and other plant parts of two potential legumes differing in salt tolerance. Flora: Morphol. Distrib. Funct. Ecol. Plants 2005, 200, 535–546. [Google Scholar] [CrossRef]
- Sańko-Sawczenko, I.; Łotocka, B.; Mielecki, J.; Rekosz-Burlaga, H.; Czarnocka, W. Transcriptomic changes in Medicago truncatula and Lotus japonicus root nodules during drought stress. Int. J. Mol. Sci. 2019, 20, 1204. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Su, Z.; Dong, J.; Wang, T. An expression database for roots of the model legume Medicago truncatula under salt stress. BMC Genom. 2009, 10, 1–9. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cruz De Carvalho, M.H.; Torres-Jerez, I.; Kang, Y.; Allen, S.N.; Huhman, D.V.; Tang, Y.; Murray, J.; Sumner, L.W.; Udvardi, M.K.; et al. Global reprogramming of transcription and metabolism in Medicago truncatula during progressive drought and after rewatering. Plant Cell Environ. 2014, 37, 2553–2576. [Google Scholar] [CrossRef]
- Hyung, D.; Lee, C.; Kim, J.-H.; Yoo, D.; Seo, Y.-S.; Jeong, S.-C.; Lee, J.-H.; Chung, Y.; Jung, K.-H.; Cook, D.R. Cross-family translational genomics of abiotic stress-responsive genes between arabidopsis and Medicago truncatula. PLoS ONE 2014, 9, 91721–91733. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.-X.; Miao, Z.-Q.; Qi, G.-F.; Wang, Z.; Yuan, Y.; Ahmad, N.; Cao, M.-J.; Hell, R.; Wirtz, M. Sultr3s function in chloroplast sulfate uptake and affect aba biosynthesis and the stress response. Plant Physiol. 2019, 180, 593–604. [Google Scholar] [CrossRef]
- Badhan, A.; Jin, L.; Wang, Y.; Han, S.; Kowalczys, K.; Brown, D.C.; Ayala, C.J.; Latoszek-Green, M.; Miki, B.; Tsang, A.J.B.f.b. Expression of a fungal ferulic acid esterase in alfalfa modifies cell wall digestibility. Biotechnol. Biofuels 2014, 7, 39–53. [Google Scholar] [CrossRef]
- Beringer, J.E. R factor transfer in Rhizobium leguminosarum. Microbiology 1974, 84, 188–198. [Google Scholar] [CrossRef]
- Guerriero, G.; Legay, S.; Hausman, J.-F. Alfalfa cellulose synthase gene expression under abiotic stress: A hitchhiker’s guide to rt-qpcr normalization. PLoS ONE 2014, 9, e103808. [Google Scholar] [CrossRef] [PubMed]
- Castonguay, Y.; Michaud, J.; Dubé, M.-P. Reference genes for rt-qpcr analysis of environmentally and developmentally regulated gene expression in alfalfa. Am. J. Plant Sci. 2015, 6, 132–143. [Google Scholar] [CrossRef]
- Shuai, B.; Reynaga-Pena, C.G.; Springer, P.S. The lateral organ boundaries gene defines a novel, plant-specific gene family. Plant Physiol. 2002, 129, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasrollahi, V.; Yuan, Z.-C.; Kohalmi, S.E.; Hannoufa, A. SPL12 Regulates AGL6 and AGL21 to Modulate Nodulation and Root Regeneration under Osmotic Stress and Nitrate Sufficiency Conditions in Medicago sativa. Plants 2022, 11, 3071. https://doi.org/10.3390/plants11223071
Nasrollahi V, Yuan Z-C, Kohalmi SE, Hannoufa A. SPL12 Regulates AGL6 and AGL21 to Modulate Nodulation and Root Regeneration under Osmotic Stress and Nitrate Sufficiency Conditions in Medicago sativa. Plants. 2022; 11(22):3071. https://doi.org/10.3390/plants11223071
Chicago/Turabian StyleNasrollahi, Vida, Ze-Chun Yuan, Susanne E. Kohalmi, and Abdelali Hannoufa. 2022. "SPL12 Regulates AGL6 and AGL21 to Modulate Nodulation and Root Regeneration under Osmotic Stress and Nitrate Sufficiency Conditions in Medicago sativa" Plants 11, no. 22: 3071. https://doi.org/10.3390/plants11223071
APA StyleNasrollahi, V., Yuan, Z.-C., Kohalmi, S. E., & Hannoufa, A. (2022). SPL12 Regulates AGL6 and AGL21 to Modulate Nodulation and Root Regeneration under Osmotic Stress and Nitrate Sufficiency Conditions in Medicago sativa. Plants, 11(22), 3071. https://doi.org/10.3390/plants11223071






