Anaerobic Digestion of Lignocellulose Components: Challenges and Novel Approaches
Abstract
1. Introduction
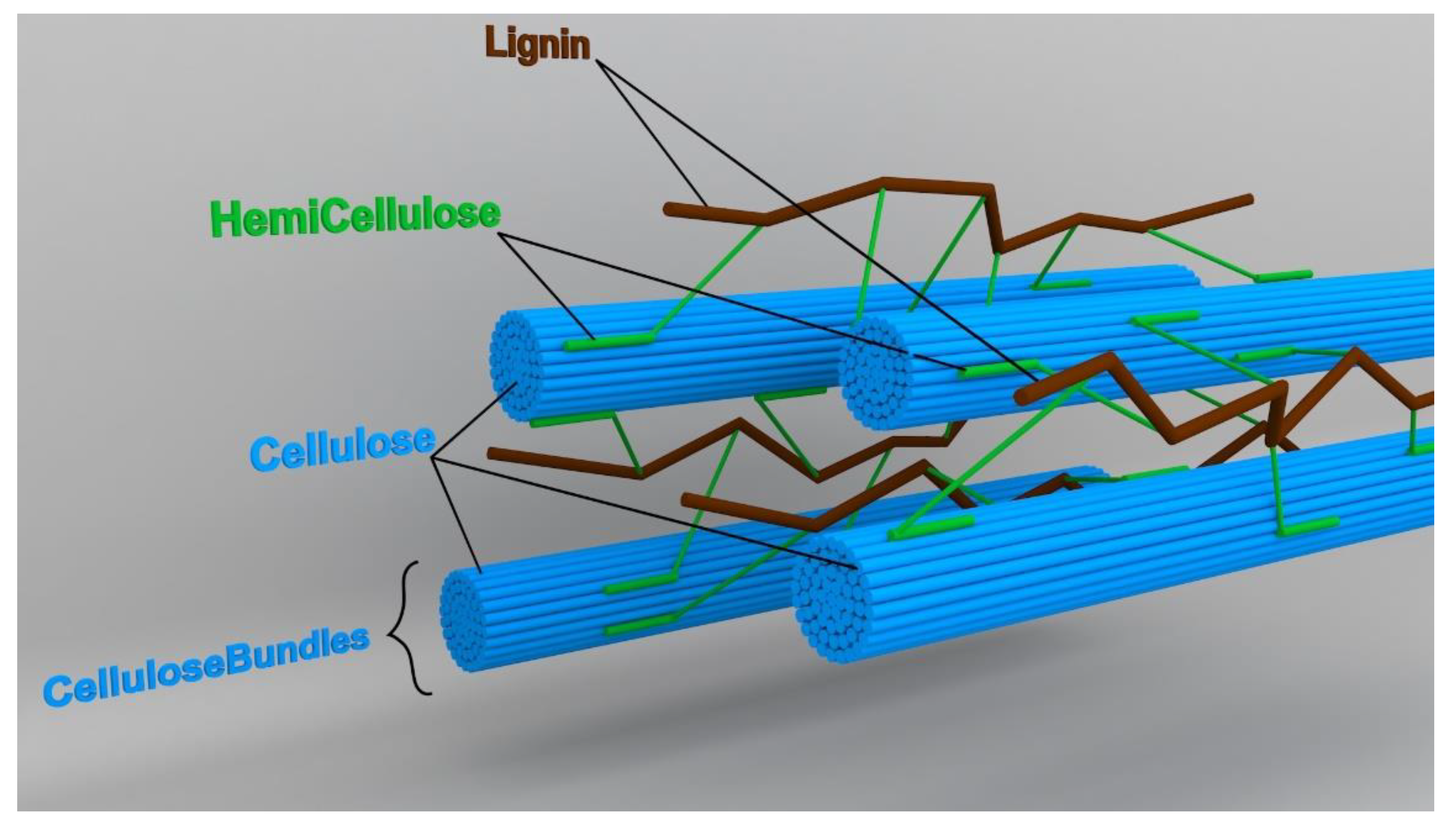
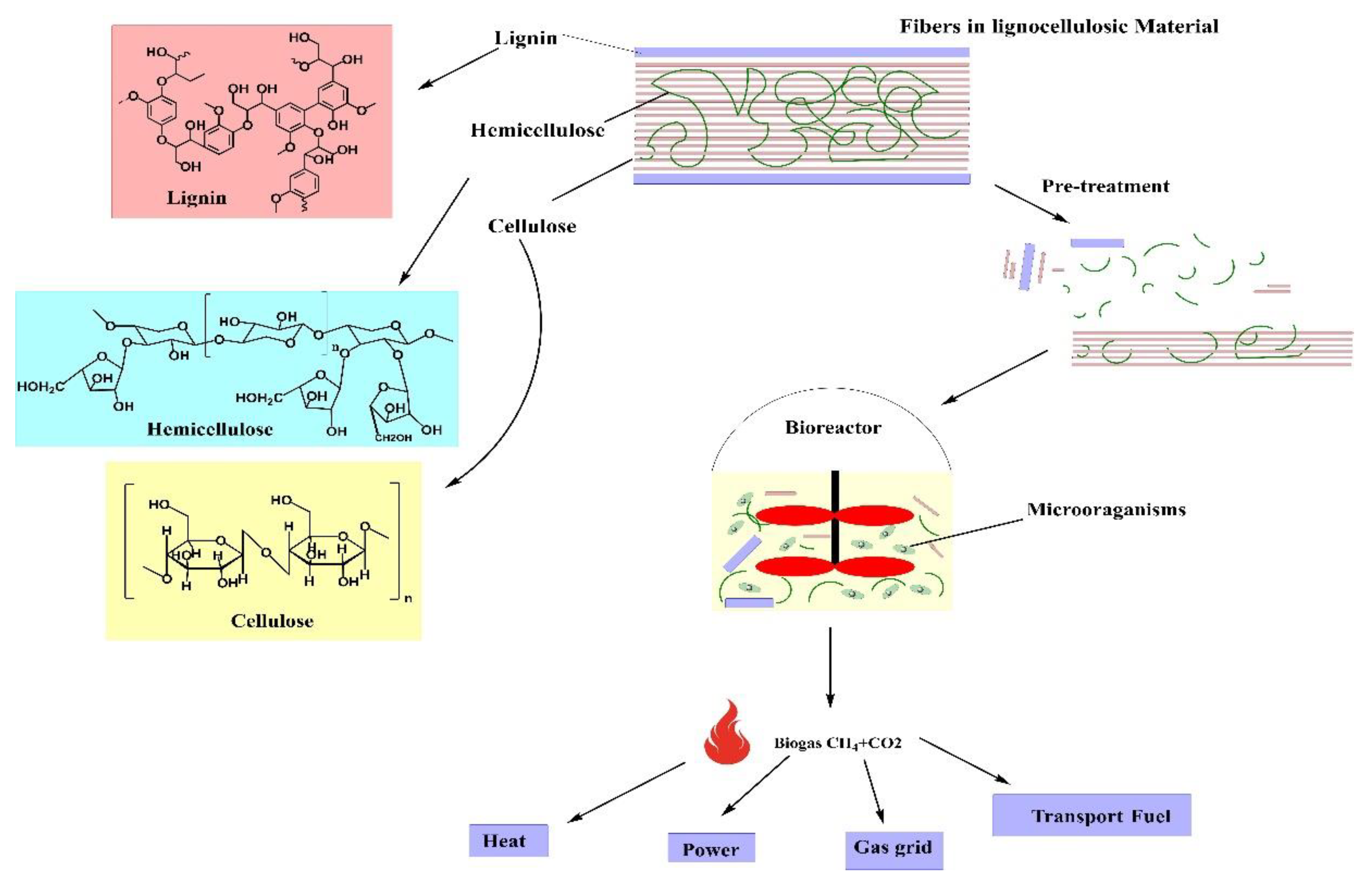
2. Structure of Lignocellulosic Materials
2.1. Cellulose
2.2. Hemicellulose
2.2.1. Xylans
2.2.2. Mannans
2.2.3. Xyloglucans
2.2.4. β–(1,3);(1,4)–glucan
2.2.5. Galactans
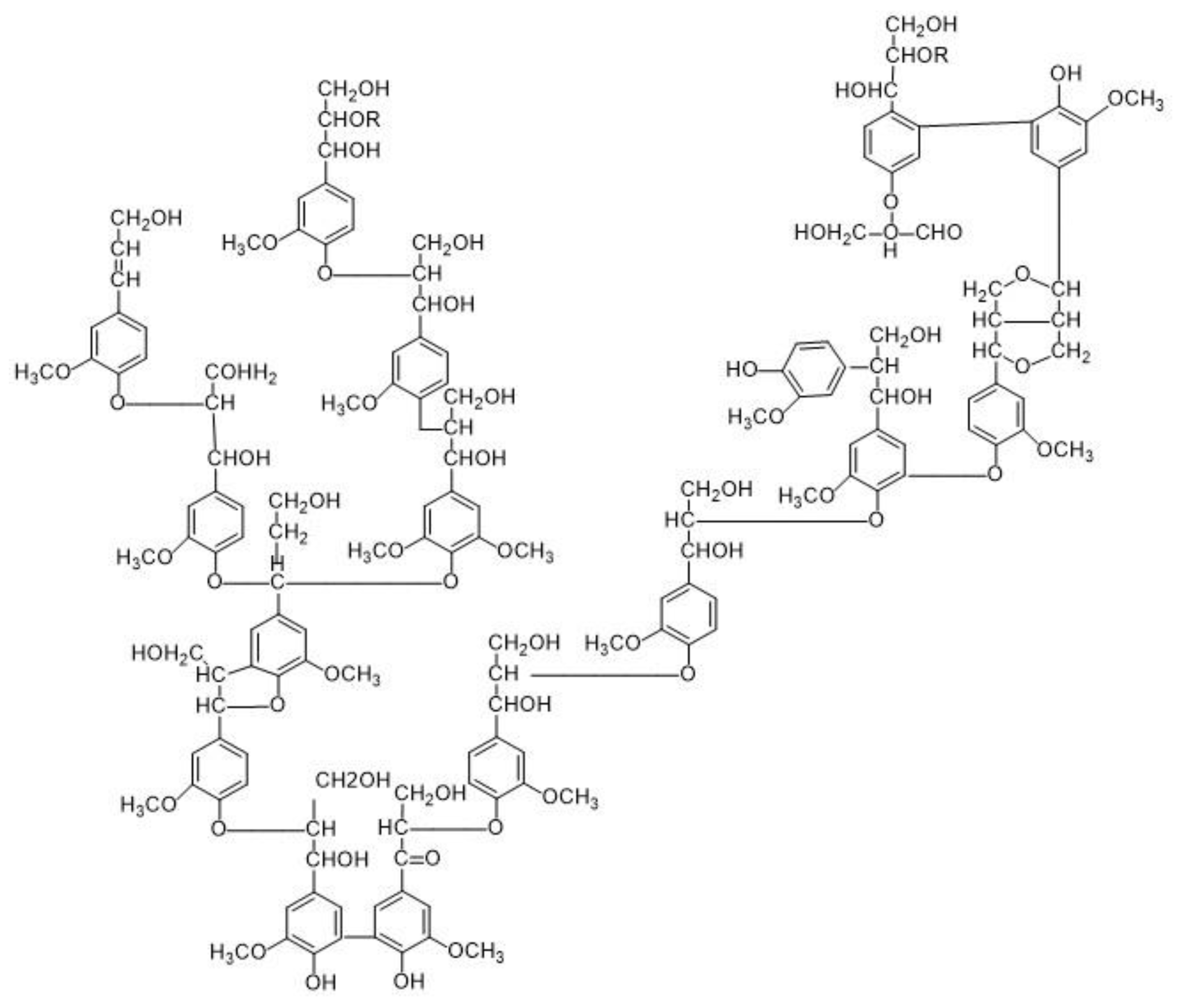
2.3. Lignin
3. AD of Lignocellulosic Materials
3.1. Pre-Treatment of Lignocellulosic Biomass Prior to AD
3.1.1. Novel and Green Pre-Treatment Methods
Steam Explosion
Wet Explosion
Ozonolysis
ILs
DESs
Organosolv Pre-Treatment
SFCs
3.2. AD of Lignocellulosic Biomass
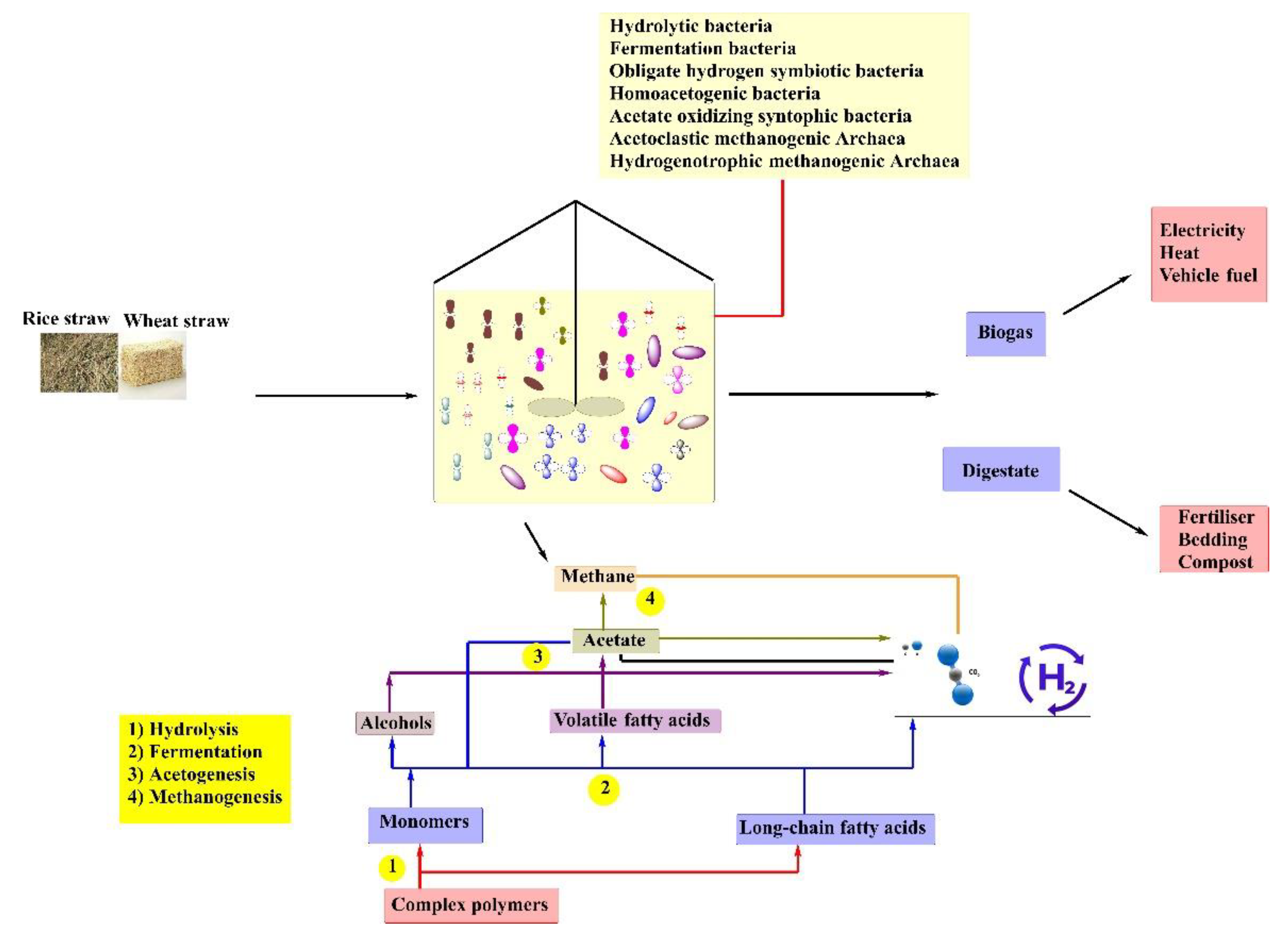
3.2.1. Concept and Process Stages
- -
- Hydrolysis
- -
- Acidogenesis
- -
- Acetogenesis
- -
- Methanogenesis
3.2.2. Future Directions and Opportunities
Genetic Engineering for Biogas Production
Impulse of Lignocellulosic Digestion by Application of a Static Magnetic Field
Co-Digestion of Various Substrates to Overcome Biodegradability Limitations
Addition of Additives to Improve Lignocellulosic Digestion
- -
- Metal elements
- -
- Carbon-based materials
Psychrophilic AD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Ge, X.; Xu, F.; Li, Y. Solid-state anaerobic digestion of lignocellulosic biomass: Recent progress and perspectives. Bioresour. Technol. 2016, 205, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. A review on enhanced biogas production from anaerobic digestion of lignocellulosic biomass by different enhancement techniques. Process Biochem. 2019, 84, 81–90. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of agricultural biomass for anaerobic digestion: Current state and challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef]
- Bajpai, P. Structure of lignocellulosic biomass. In Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer: Singapore, 2016; pp. 7–12. ISBN 978-981-10-0687-6. [Google Scholar]
- Abbas, Y.; Yun, S.; Wang, Z.; Zhang, Y.; Zhang, X.; Wang, K. Recent advances in bio-based carbon materials for anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2021, 135, 110378. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, D.; Singh, P.M.; Ragauskas, A.J. The new forestry biofuels sector. Biofuels Bioprod. Biorefining 2008, 2, 58–73. [Google Scholar] [CrossRef]
- Shahzadi, T.; Mehmood, S.; Irshad, M.; Anwar, Z.; Afroz, A.; Zeeshan, N.; Rashid, U.; Sughra, K. Advances in lignocellulosic biotechnology: A brief review on lignocellulosic biomass and cellulases. Adv. Biosci. Biotechnol. 2014, 5, 246–251. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Heinze, T. Cellulose: Structure and properties. Adv. Polym. Sci. 2015, 271, 1–52. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Pettolino, F.; Flanagan, B.; Gidley, M.J.; Gilbert, E.P. Structure of cellulose microfibrils in mature cotton fibres. Carbohydr. Polym. 2017, 175, 450–463. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A critical review on hemicellulose pyrolysis. Energy Technol. 2017, 5, 52–79. [Google Scholar] [CrossRef]
- Rennie, E.A.; Scheller, H.V. Xylan biosynthesis. Curr. Opin. Biotechnol. 2014, 26, 100–107. [Google Scholar] [CrossRef]
- Dumitriu, S. Polysaccharides, 2nd ed.; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9780429131660. [Google Scholar]
- Zhong, R.; Cui, D.; Ye, Z.H. Evolutionary origin of O-acetyltransferases responsible for glucomannan acetylation in land plants. New Phytol. 2019, 224, 466–479. [Google Scholar] [CrossRef]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and its interactions with other components of the growing cell wall. Plant Cell Physiol. 2015, 56, 180–194. [Google Scholar] [CrossRef]
- Simmons, T.J.; Uhrín, D.; Gregson, T.; Murray, L.; Sadler, I.H.; Fry, S.C. An unexpectedly lichenase-stable hexasaccharide from cereal, horsetail and lichen mixed-linkage β-glucans (MLGs): Implications for MLG subunit distribution. Phytochemistry 2013, 95, 322–332. [Google Scholar] [CrossRef]
- Xue, X.; Fry, S.C. Evolution of mixed-linkage (13, 14)-b-D-glucan (MLG) and xyloglucan in equisetum (horsetails) and other monilophytes. Ann. Bot. 2012, 109, 873–886. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Laroche, C.; Michaud, P. Galactans and its applications. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 753–794. ISBN 9783319162980. [Google Scholar]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modifications—A review. Biomass Bioenergy 2019, 128, 105325. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Mysyakina, I.S. Lignin: Chemical structure, biodegradation, and practical application (a review). Appl. Biochem. Microbiol. 2016, 52, 573–581. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Garcia, I.L.; Leiva-Candia, D.; Dorado, M.P. Valorization of food waste based on its composition through the concept of biorefinery. Curr. Opin. Green Sustain. Chem. 2018, 14, 67–79. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Valencia, C.; Franco, J.M. Lignocellulosic materials for the production of biofuels, biochemicals and biomaterials and applications of lignocellulose-based polyurethanes: A review. Polymers 2022, 14, 881. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, K.F.; Okolie, J.A. A review of biochemical process of anaerobic digestion. Adv. Biosci. Biotechnol. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- Qu, J.; Feng, Y.; Xu, G.; Zhang, M.; Zhu, Y.; Zhou, S. Design and thermodynamics analysis of marine dual fuel low speed engine with methane reforming integrated high pressure exhaust gas recirculation system. Fuel 2022, 319, 123747. [Google Scholar] [CrossRef]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef]
- Kothari, R.; Pandey, A.K.; Kumar, S.; Tyagi, V.V.; Tyagi, S.K. Different aspects of dry anaerobic digestion for bio-energy: An overview. Renew. Sustain. Energy Rev. 2014, 39, 174–195. [Google Scholar] [CrossRef]
- Khanal, S.K. Bioenergy generation from residues of biofuel industries. In Anaerobic Biotechnology for Bioenergy Production: Principles and Applications; Khanal, S.K., Ed.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2008; pp. 161–188. ISBN 9780813804545. [Google Scholar]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic digestion process: Technological aspects and recent developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- Kiyasudeen, S.K.; Ibrahim, M.H.; Quaik, S.; Ismail, S.A. An introduction to anaerobic digestion of organic wastes. In Prospects of Organic Waste Management and the Significance of Earthworms; Springer International Publishing: New York, NY, USA, 2016; pp. 23–44. ISBN 978-3-319-24708-3. [Google Scholar]
- Hobdey, S.E.; Donohoe, B.S.; Brunecky, R.; Himmel, M.E.; Bomble, Y.J. New insights into microbial strategies for biomass conversion. In Direct Microbial Conversion of Biomass to Advanced Biofuels; Himmel, M.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 111–127. ISBN 9780444595928. [Google Scholar]
- Somerville, C. Cellulose synthesis in higher plants. Annu. Rev. Cell Dev. Biol. 2006, 22, 53–78. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current understanding of the correlation of lignin structure with biomass recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Ragauskas, A.J. Lignin structural alterations in thermochemical pretreatments with limited delignification. Bioenergy Res. 2015, 8, 992–1003. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Kildegaard, G.; Balbi, M.D.P.; Salierno, G.; Cassanello, M.; De Blasio, C.; Galvagno, M. A cleaner delignification of urban leaf waste biomass for bioethanol production, optimised by experimental design. Processes 2022, 10, 943. [Google Scholar] [CrossRef]
- Ab Rasid, N.S.; Shamjuddin, A.; Abdul Rahman, A.Z.; Amin, N.A.S. Recent advances in green pre-treatment methods of lignocellulosic biomass for enhanced biofuel production. J. Clean. Prod. 2021, 321, 129038. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of Chemical Pretreatment for Bioconversion of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Weber, B.; Estrada-Maya, A.; Sandoval-Moctezuma, A.C.; Martínez-Cienfuegos, I.G. Anaerobic digestion of extracts from steam exploded agave tequilana bagasse. J. Environ. Manag. 2019, 245, 489–495. [Google Scholar] [CrossRef]
- Weber, B.; Sandoval-Moctezuma, A.C.; Estrada-Maya, A.; Martínez-Cienfuegos, I.G.; Durán-García, M.D. Agave bagasse response to steam explosion and anaerobic treatment. Biomass Convers. Biorefin. 2020, 10, 1279–1289. [Google Scholar] [CrossRef]
- Inseemeesak, B.; Areeprasert, C. Fiber extraction and energy recovery from cocos nucifera linn mesocarp residues employing steam explosion and anaerobic digestion. Ind. Crops Prod. 2020, 147, 112180. [Google Scholar] [CrossRef]
- Kaldis, F.; Cysneiros, D.; Day, J.; Karatzas, K.A.G.; Chatzifragkou, A. Anaerobic digestion of steam-exploded wheat straw and co-digestion strategies for enhanced biogas production. Appl. Sci. 2020, 10, 8284. [Google Scholar] [CrossRef]
- Bensah, E.C.; Mensah, M.Y. Emerging physico-chemical methods for biomass pretreatment. In Fuel Ethanol Production from Sugarcane; Basso, T.P., Basso, L.C., Eds.; IntechOpen: London, UK, 2019; pp. 41–62. ISBN 978-1-78984-938-7. [Google Scholar]
- Poddar, B.J.; Nakhate, S.P.; Gupta, R.K.; Chavan, A.R.; Singh, A.K.; Khardenavis, A.A.; Purohit, H.J. A comprehensive review on the pretreatment of lignocellulosic wastes for improved biogas production by anaerobic digestion. Int. J. Environ. Sci. Technol. 2022, 19, 3429–3456. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Negro, M.J.; Ballesteros, I.; González, A.; Ballesteros, M. Steam explosion for wheat straw pretreatment for sugars production. Bioethanol 2016, 2, 66–75. [Google Scholar] [CrossRef]
- Ahring, B.K.; Munck, J. Method for Treating Biomass and Organic Waste with the Purpose of Generating Desired Biologically Based Products. U.S. Patent US 8,506,716 B2, 13 August 2013. [Google Scholar]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet explosion: A universal and efficient pretreatment process for lignocellulosic biorefineries. Bioenergy Res. 2015, 8, 1101–1116. [Google Scholar] [CrossRef]
- Ahring, B.K.; Biswas, R.; Ahamed, A.; Teller, P.J.; Uellendahl, H. Making lignin accessible for anaerobic digestion by wet-explosion pretreatment. Bioresour. Technol. 2015, 175, 182–188. [Google Scholar] [CrossRef]
- Rana, D.; Laskar, D.D.; Srinivas, K.; Ahring, B.K. Wet explosion pretreatment of loblolly pine leads to an increase in methoxylation of the lignin. Bioresour. Bioprocess. 2015, 2, 26. [Google Scholar] [CrossRef]
- Dawson, B.; Spannagle, M. The Complete Guide to Climate Change; Routledge: London, UK, 2008; ISBN 9780203888469. [Google Scholar]
- Cesaro, A.; Belgiorno, V. Ozone pretreatment for the anaerobic digestion of organic solid waste. Detritus 2020, 12, 51–56. [Google Scholar] [CrossRef]
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Ozone pretreatment of biomethanated distillery wastewater in a semi batch reactor: Mapping pretreatment efficiency in terms of COD, color, toxicity and biohydrogen generation. Biofuels 2018, 11, 801–809. [Google Scholar] [CrossRef]
- Ghorbani, M.; Kianmehr, M.H.; Arabhosseini, A.; Alamouti, A.A.; Sadeghi, R. Ozonolysis pretreatment of wheat straw for enhanced delignification: Applying RSM technique for modeling and optimizing proces. Iran. J. Biosyst. Eng. 2021, 52, 37–53. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N.L. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. Ionic liquids for bioenergy production. In Ionic Liquid-Based Technologies for Environmental Sustainability; Jawaid, M., Ahmad, A., Reddy, A.V.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 235–256. ISBN 978-0-12-824545-3. [Google Scholar]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pimienta, J.A.; García-López, R.M.; Méndez-Acosta, H.O.; González-Álvarez, V.; Simmons, B.A.; Méndoza-Pérez, J.A.; Arreola-Vargas, J. Ionic liquid-water mixtures enhance pretreatment and anaerobic digestion of agave bagasse. Ind. Crops Prod. 2021, 171, 113924. [Google Scholar] [CrossRef]
- Pérez-Pimienta, J.A.; Icaza-Herrera, J.P.A.; Méndez-Acosta, H.O.; González-Álvarez, V.; Méndoza-Pérez, J.A.; Arreola-Vargas, J. Bioderived ionic liquid-based pretreatment enhances methane production from: Agave tequilana bagasse. RSC Adv. 2020, 10, 14025–14032. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Mohedano, A.F.; de la Rubia, A. Pretreatment of lignocellulosic biomass with 1-ethyl-3-methylimidazolium acetate for its eventual valorization by anaerobic digestion. Resources 2021, 10, 118. [Google Scholar] [CrossRef]
- Lima, F.; Branco, L.C.; Lapa, N.; Marrucho, I.M. Beneficial and detrimental effects of choline chloride–Oxalic acid deep eutectic solvent on biogas production. Waste Manag. 2021, 131, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Pan, M.; Zhao, G.; Ding, C.; Wu, B.; Lian, Z.; Lian, H. Physicochemical transformation of rice straw after pretreatment with a deep eutectic solvent of choline chloride/urea. Carbohydr. Polym. 2017, 176, 307–314. [Google Scholar] [CrossRef]
- Morais, E.S.; Da Costa Lopes, A.M.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of ionic liquids and deep eutectic solvents in polysaccharides dissolution and extraction processes towards sustainable biomass valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Lee, S.H. Effect of hydrogen bond donor on the choline chloride-based deep eutectic solvent-mediated extraction of lignin from pine wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef]
- Basak, B.; Patil, S.; Kumar, R.; Ha, G.S.; Park, Y.K.; Ali Khan, M.; Kumar Yadav, K.; Fallatah, A.M.; Jeon, B.H. Integrated hydrothermal and deep eutectic solvent-mediated fractionation of lignocellulosic biocomponents for enhanced accessibility and efficient conversion in anaerobic digestion. Bioresour. Technol. 2022, 351, 127034. [Google Scholar] [CrossRef] [PubMed]
- Olugbemide, A.D.; Oberlintner, A.; Novak, U.; Likozar, B. Lignocellulosic corn stover biomass pre-treatment by deep eutectic solvents (des) for biomethane production process by bioresource anaerobic digestion. Sustainability 2021, 13, 10504. [Google Scholar] [CrossRef]
- Brosse, N.; Hussin, M.H.; Rahim, A.A. Organosolv processes. In Biorefineries. Advances in Biochemical Engineering/Biotechnology; Wagemann, K., Tippkötter, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 166, pp. 153–176. ISBN 978-3-319-97117-9. [Google Scholar]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the economy of lignocellulose-based biorefineries with organosolv pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef]
- Chin, D.W.K.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels, Bioprod. Biorefining 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Oliva, A.; Tan, L.C.; Papirio, S.; Esposito, G.; Lens, P.N.L. Effect of methanol-organosolv pretreatment on anaerobic digestion of lignocellulosic materials. Renew. Energy 2021, 169, 1000–1012. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A Review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Najafi, E.; Castro, E.; Karimi, K. Biorefining for olive wastes management and efficient bioenergy production. Energy Convers. Manag. 2021, 244, 114467. [Google Scholar] [CrossRef]
- Soltaninejad, A.; Jazini, M.; Karimi, K. Sustainable bioconversion of potato peel wastes into ethanol and biogas using organosolv pretreatment. Chemosphere 2022, 291, 133003. [Google Scholar] [CrossRef] [PubMed]
- Sulbarán-Rangel, B.; Aguirre, J.S.A.; Breton-Deval, L.; del Real-Olvera, J.; Tun, K.J.G. Improvement of anaerobic digestion of hydrolysed corncob waste by organosolv pretreatment for biogas production. Appl. Sci. 2020, 10, 2785. [Google Scholar] [CrossRef]
- Knez, Ž.; Pantić, M.; Cör, D.; Novak, Z.; Knez Hrnčič, M. Are supercritical fluids solvents for the future? Chem. Eng. Process.-Process Intensif. 2019, 141, 107532. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Faik, A.; Goetz, D.J.; Gu, T. Supercritical carbon dioxide pretreatment of corn stover and switchgrass for lignocellulosic ethanol production. Bioresour. Technol. 2011, 102, 6995–7000. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Luo, P.; Xu, Q.-Q.; Wang, E.-J.; Yin, J.-Z. Investigation of the effect of supercritical carbon dioxide pretreatment on reducing sugar yield of lignocellulose hydrolysis. Cellul. Chem. Technol. 2014, 48, 89–95. [Google Scholar]
- Gao, M.; Xu, F.; Li, S.; Ji, X.; Chen, S.; Zhang, D. Effect of SC-CO2 pretreatment in increasing rice straw biomass conversion. Biosyst. Eng. 2010, 106, 470–475. [Google Scholar] [CrossRef]
- Alinia, R.; Zabihi, S.; Esmaeilzadeh, F.; Kalajahi, J.F. Pretreatment of wheat straw by supercritical CO2 and its enzymatic hydrolysis for sugar production. Biosyst. Eng. 2010, 107, 61–66. [Google Scholar] [CrossRef]
- Zhao, M.-J.; Xu, Q.-Q.; Li, G.-M.; Zhang, Q.-Z.; Zhou, D.; Yin, J.-Z.; Zhan, H.-S. Pretreatment of agricultural residues by supercritical CO2 at 50–80 °C to enhance enzymatic hydrolysis. J. Energy Chem. 2019, 31, 39–45. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Dange, R.; Bhanage, B.M. Recent advances of use of the supercritical carbon dioxide for the biomass pre-treatment and extraction: A mini-review. J. Indian Chem. Soc. 2021, 98, 100018. [Google Scholar] [CrossRef]
- Van Walsum, G.P.; Shi, H. Carbonic acid enhancement of hydrolysis in aqueous pretreatment of corn stover. Bioresour. Technol. 2004, 93, 217–226. [Google Scholar] [CrossRef]
- da Fonseca Machado, A.P.; Rezende, C.A.; Rodrigues, R.A.; Barbero, G.F.; de Tarso Vieira e Rosa, P.; Martínez, J. Encapsulation of anthocyanin-rich extract from blackberry residues by spray-drying, freeze-drying and supercritical antisolvent. Powder Technol. 2018, 340, 553–562. [Google Scholar] [CrossRef]
- Hashemi, S.; Joseph, P.; Mialon, A.; Moe, S.; Lamb, J.J.; Lien, K.M. Enzymatic pretreatment of steam-exploded birch wood for increased biogas production and lignin degradation. Bioresour. Technol. Rep. 2021, 16, 100874. [Google Scholar] [CrossRef]
- Nakamura, Y.; Asada, C. Effect of activated cow dung as inoculum on methane production of steam-exploded rice husks. Waste Biomass Valorization 2021, 12, 5019–5028. [Google Scholar] [CrossRef]
- Weber, B.; Ayala-Mercado, I.D.; Stadlbauer, E.A. Steam explosion versus hydrothermal carbonization: Evaluation of applicability for pretreatment of semi-solid waste from beverage industries to facilitate on-site biogas production. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Jomnonkhaow, U.; Sittijunda, S.; Reungsang, A. Assessment of organosolv, hydrothermal, and combined organosolv and hydrothermal with enzymatic pretreatment to increase the production of biogas from napier grass and napier silage. Renew. Energy 2022, 181, 1237–1249. [Google Scholar] [CrossRef]
- Ebrahimian, F.; Karimi, K.; Angelidaki, I. Coproduction of hydrogen, butanol, butanediol, ethanol, and biogas from the organic fraction of municipal solid waste using bacterial cocultivation followed by anaerobic digestion. Renew. Energy 2022, 194, 552–560. [Google Scholar] [CrossRef]
- Lima, D.R.S.; de Oliveira Paranhos, A.G.; Adarme, O.F.H.; Baêta, B.E.L.; Gurgel, L.V.A.; dos Santos, A.S.; de Queiroz Silva, S.; de Aquino, S.F. Integrated production of second-generation ethanol and biogas from sugarcane bagasse pretreated with ozone. Biomass Convers. Biorefinery 2022, 12, 809–825. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A.; Elkatory, M.R.; Eleryan, A.; Ragab, S.; El Sikaily, A.; Pantaleo, A. Enhancement of biogas production from macroalgae ulva latuca via ozonation pretreatment. Energies 2021, 14, 1703. [Google Scholar] [CrossRef]
- Mitraka, G.-C.; Kontogiannopoulos, K.N.; Tsivintzelis, I.; Zouboulis, A.I.; Kougias, P.G. Optimization of supercritical carbon dioxide explosion for sewage sludge pre-treatment using response surface methodology. Chemosphere 2022, 297, 133989. [Google Scholar] [CrossRef] [PubMed]
- Tossavainen, M.; Edelmann, M.; Lahti-Leikas, K.; Kivimäki, S.; Kymäläinen, M.; Piironen, V.; Lampi, A.-M.; Ojala, A.; Romantschuk, M. Chemical composition and biomethane production potential of euglena gracilis biomass and extraction residue from supercritical CO2 extraction. Bioresour. Technol. Rep. 2022, 19, 101140. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Salinas, F.; Medina, E.; Rincón, B.; Martín, M.Á.; Gutiérrez, M.C.; Cerezal-Mezquita, P. Supercritical fluid extraction of fucoxanthin from the diatom phaeodactylum tricornutum and biogas production through anaerobic digestion. Mar. Drugs 2022, 20, 127. [Google Scholar] [CrossRef]
- Abbasi, T.; Tauseef, S.M.; Abbasi, S.A. Anaerobic digestion for global warming control and energy generation—An overview. Renew. Sustain. Energy Rev. 2012, 16, 3228–3242. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D.M. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef]
- Hashemi, B.; Sarker, S.; Lamb, J.J.; Lien, K.M. Yield improvements in anaerobic digestion of lignocellulosic feedstocks. J. Clean. Prod. 2021, 288, 125447. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, Z.; Fu, P.; Yang, J.; Wang, Q. New insights into the effects of methane and oxygen on heat/mass transfer in reactive porous media. Int. Commun. Heat Mass Transf. 2021, 129, 105652. [Google Scholar] [CrossRef]
- Brandon, A.G.; Scheller, H.V. Engineering of bioenergy crops: Dominant genetic approaches to improve polysaccharide properties and composition in biomass. Front. Plant Sci. 2020, 11, 282. [Google Scholar] [CrossRef]
- Yu, P.; Chen, X.; Li, P. Enhancing microbial production of biofuels by expanding microbial metabolic pathways. Biotechnol. Appl. Biochem. 2017, 64, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Paritosh, K.; Chawade, A.; Pareek, N.; Vivekanand, V. Genetic engineering of energy crops to reduce recalcitrance and enhance biomass digestibility. Agriculture 2018, 8, 76. [Google Scholar] [CrossRef]
- Grabber, J.H.; Ralph, J.; Lapierre, C.; Barrière, Y. Genetic and molecular basis of grass cell-wall degradability. I. Lignin-cell wall matrix interactions. Comptes Rendus-Biol. 2004, 327, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Renault, H.; Werck-Reichhart, D.; Weng, J.K. Harnessing lignin evolution for biotechnological applications. Curr. Opin. Biotechnol. 2019, 56, 105–111. [Google Scholar] [CrossRef]
- Tetreault, H.M.; Gries, T.; Palmer, N.A.; Funnell-Harris, D.L.; Sato, S.; Ge, Z.; Sarath, G.; Sattler, S.E. Overexpression of ferulate 5-hydroxylase increases syringyl units in sorghum bicolor. Plant Mol. Biol. 2020, 103, 269–285. [Google Scholar] [CrossRef]
- Hodgson-Kratky, K.; Perlo, V.; Furtado, A.; Choudhary, H.; Gladden, J.M.; Simmons, B.A.; Botha, F.; Henry, R.J. Association of gene expression with syringyl to guaiacyl ratio in sugarcane lignin. Plant Mol. Biol. 2021, 106, 173–192. [Google Scholar] [CrossRef]
- Huang, J.; Xia, T.; Li, G.; Li, X.; Li, Y.; Wang, Y.; Wang, Y.; Chen, Y.; Xie, G.; Bai, F.W.; et al. Overproduction of native endo-β-1,4-glucanases leads to largely enhanced biomass saccharification and bioethanol production by specific modification of cellulose features in transgenic rice 06 biological sciences 0601 biochemistry and cell biology 06 biological sciences 0607 plant biology. Biotechnol. Biofuels 2019, 12, 11. [Google Scholar] [CrossRef]
- Li, F.; Liu, S.; Xu, H.; Xu, Q. A novel FC17/CESA4 mutation causes increased biomass saccharification and lodging resistance by remodeling cell wall in rice. Biotechnol. Biofuels 2018, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Sato-Izawa, K.; Nakamura, S.-I.; Matsumoto, T. Mutation of rice Bc1 gene affects internode elongation and induces delayed cell wall deposition in developing internodes. Plant Signal. Behav. 2020, 15, e1749786. [Google Scholar] [CrossRef] [PubMed]
- Vandenbrink, J.P.; Hilten, R.N.; Das, K.C.; Paterson, A.H.; Feltus, F.A. Analysis of crystallinity index and hydrolysis rates in the bioenergy crop sorghum bicolor. Bioenergy Res. 2012, 5, 387–397. [Google Scholar] [CrossRef]
- Dadwal, A.; Sharma, S.; Satyanarayana, T. Progress in ameliorating beneficial characteristics of microbial cellulases by genetic engineering approaches for cellulose saccharification. Front. Microbiol. 2020, 11, 1387. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Williamson, J.J.; Alberti, F. Microbial hosts for metabolic engineering of lignin bioconversion to renewable chemicals. Renew. Sustain. Energy Rev. 2021, 152, 111674. [Google Scholar] [CrossRef]
- Lee, S.; Sohn, J.H.; Bae, J.H.; Kim, S.C.; Sung, B.H. Current status of pseudomonas putida engineering for lignin valorization. Biotechnol. Bioprocess Eng. 2020, 25, 862–871. [Google Scholar] [CrossRef]
- Paul, M.; Mohapatra, S.; Kumar Das Mohapatra, P.; Thatoi, H. Microbial Cellulases—An update towards its surface chemistry, genetic engineering and recovery for its biotechnological potential. Bioresour. Technol. 2021, 340, 125710. [Google Scholar] [CrossRef]
- Jia, B.; Yun, S.; Shi, J.; Han, F.; Wang, Z.; Chen, J.; Abbas, Y.; Xu, H.; Wang, K.; Xing, T. Enhanced anaerobic mono- and co-digestion under mesophilic condition: Focusing on the magnetic field and ti-sphere core–shell structured additives. Bioresour. Technol. 2020, 310, 123450. [Google Scholar] [CrossRef]
- Zieliński, M.; Zielińska, M.; Cydzik-Kwiatkowska, A.; Rusanowska, P.; Dębowski, M. Effect of static magnetic field on microbial community during anaerobic digestion. Bioresour. Technol. 2021, 323, 124600. [Google Scholar] [CrossRef]
- Lyu, W.; Song, Q.; Shi, J.; Wang, H.; Wang, B.; Hu, X. Weak magnetic field affected microbial communities and function in the A/O/A sequencing batch reactors for enhanced aerobic granulation. Sep. Purif. Technol. 2021, 266, 118537. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Ni, S.Q.; Zhang, J.; Zhang, X.; Ahmad, H.A.; Gao, B. Weak magnetic field: A powerful strategy to enhance partial nitrification. Water Res. 2017, 120, 190–198. [Google Scholar] [CrossRef]
- Beretta, G.; Mastorgio, A.F.; Pedrali, L.; Saponaro, S.; Sezenna, E. The effects of electric, magnetic and electromagnetic fields on microorganisms in the perspective of bioremediation. Rev. Environ. Sci. Biotechnol. 2019, 18, 29–75. [Google Scholar] [CrossRef]
- Zhang, X.; Yarema, K.; Xu, A. Impact of static magnetic field (SMF) on microorganisms, plants and animals. In Biological Effects of Static Magnetic Fields; Springer: Singapore, 2017; pp. 133–172. ISBN 978-981-10-3577-7. [Google Scholar]
- Guo, L.; Azam, S.M.R.; Guo, Y.; Liu, D.; Ma, H. Germicidal efficacy of the pulsed magnetic field against pathogens and spoilage microorganisms in food processing: An overview. Food Control 2022, 136, 108496. [Google Scholar] [CrossRef]
- Zhao, B.; Sha, H.; Li, J.; Cao, S.; Wang, G.; Yang, Y. Static magnetic field enhanced methane production via stimulating the growth and composition of microbial community. J. Clean. Prod. 2020, 271, 122664. [Google Scholar] [CrossRef]
- Li, W.; Ma, H.; He, R.; Ren, X.; Zhou, C. Prospects and application of ultrasound and magnetic fields in the fermentation of rare edible fungi. Ultrason. Sonochem. 2021, 76, 105613. [Google Scholar] [CrossRef] [PubMed]
- Kunatsa, T.; Xia, X. A review on anaerobic digestion with focus on the role of biomass co-digestion, modelling and optimisation on biogas production and enhancement. Bioresour. Technol. 2022, 344, 126311. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Passos, F.; Romero-Güiza, M.S.; Ferrer, I.; Astals, S. Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Khanal, S.K. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef]
- Kunatsa, T.; Zhang, L.; Xia, X. Biogas potential determination and production optimisation through optimal substrate ratio feeding in co-digestion of water hyacinth, municipal solid waste and cow dung. Biofuels 2020. [Google Scholar] [CrossRef]
- Velásquez Piñas, J.A.; Venturini, O.J.; Silva Lora, E.E.; Calle Roalcaba, O.D. Technical assessment of mono-digestion and co-digestion systems for the production of biogas from anaerobic digestion in Brazil. Renew. Energy 2018, 117, 447–458. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, F.; Yu, J.; Cai, Y.; Luo, X.; Cui, Z.; Hu, Y.; Wang, X. Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation. Bioresour. Technol. 2018, 269, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zahan, Z.; Othman, M.Z.; Muster, T.H. Anaerobic digestion/co-digestion kinetic potentials of different agro-industrial wastes: A comparative batch study for C/N optimisation. Waste Manag. 2018, 71, 663–674. [Google Scholar] [CrossRef]
- Liu, M.; Wei, Y.; Leng, X. Improving biogas production using additives in anaerobic digestion: A review. J. Clean. Prod. 2021, 297, 126666. [Google Scholar] [CrossRef]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Du, N.; Li, M.; Zhang, Q.; Ulsido, M.D.; Xu, R.; Huang, W. Study on the biogas potential of anaerobic digestion of coffee husks wastes in Ethiopia. Waste Manag. Res. 2021, 39, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Janke, L.; Zheng, Z.; Wang, X.; Pröter, J.; Schäfer, F. Enhancing anaerobic digestion of chicken manure leachate: Effects of trace elements supplementation on methane production. Bioresour. Technol. Rep. 2021, 14, 100662. [Google Scholar] [CrossRef]
- Fermoso, F.G.; Van Hullebusch, E.D.; Guibaud, G.; Collins, G.; Svensson, B.H.; Carliell-Marquet, C.; Vink, J.P.M.; Esposito, G.; Frunzo, L. Fate of trace metals in anaerobic digestion. Adv. Biochem. Eng. Biotechnol. 2015, 151, 171–195. [Google Scholar] [CrossRef]
- Wu, B.; Lin, R.; Kang, X.; Deng, C.; Xia, A.; Dobson, A.D.W.; Murphy, J.D. Graphene addition to digestion of thin stillage can alleviate acidic shock and improve biomethane production. ACS Sustain. Chem. Eng. 2020, 8, 13248–13260. [Google Scholar] [CrossRef]
- Qi, Q.; Sun, C.; Zhang, J.; He, Y.; Tong, Y.W. Internal enhancement mechanism of biochar with graphene structure in anaerobic digestion: The bioavailability of trace elements and potential direct interspecies electron transfer. Chem. Eng. J. 2021, 406, 126833. [Google Scholar] [CrossRef]
- Nabi, M.; Liang, H.; Cheng, L.; Yang, W.; Gao, D. A comprehensive review on the use of conductive materials to improve anaerobic digestion: Focusing on landfill leachate treatment. J. Environ. Manag. 2022, 309, 114540. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, T.; Si, B.; Watson, J.; Zhang, Y. Accelerating anaerobic digestion for methane production: Potential role of direct interspecies electron transfer. Renew. Sustain. Energy Rev. 2021, 145, 111069. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Ma, C.; White, J.C.; Zhao, Z.; Duan, C.; Zhang, Y.; Adeel, M.; Rui, Y.; Li, G.; et al. Carbon nanomaterials induce residue degradation and increase methane production from livestock manure in an anaerobic digestion system. J. Clean. Prod. 2019, 240, 118257. [Google Scholar] [CrossRef]
- Chen, F.; Ma, J.; Zhu, Y.; Li, X.; Yu, H.; Sun, Y. Biodegradation performance and anti-fouling mechanism of an ICME/Electro-biocarriers-MBR system in livestock wastewater (antibiotic-containing) treatment. J. Hazard. Mater. 2022, 426, 128064. [Google Scholar] [CrossRef] [PubMed]
- Baniamerian, H.; Isfahani, P.G.; Tsapekos, P.; Alvarado-Morales, M.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Application of nano-structured materials in anaerobic digestion: Current status and perspectives. Chemosphere 2019, 229, 188–199. [Google Scholar] [CrossRef]
- Chiappero, M.; Norouzi, O.; Hu, M.; Demichelis, F.; Berruti, F.; Di Maria, F.; Mašek, O.; Fiore, S. Review of biochar role as additive in anaerobic digestion processes. Renew. Sustain. Energy Rev. 2020, 131, 110037. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.-B.; Liu, Y.-G.; Gu, Y.-L.; Zeng, G.-M.; Hu, X.-J.; Wang, X.; Liu, S.-H.; Jiang, L.-H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Chen, M.; Liu, S.; Yuan, X.; Li, Q.X.; Wang, F.; Xin, F.; Wen, B. Methane production and characteristics of the microbial community in the co-digestion of potato pulp waste and dairy manure amended with biochar. Renew. Energy 2021, 163, 357–367. [Google Scholar] [CrossRef]
- Ma, H.; Hu, Y.; Kobayashi, T.; Xu, K.Q. The role of rice husk biochar addition in anaerobic digestion for sweet sorghum under high loading condition. Biotechnol. Rep. 2020, 27, e00515. [Google Scholar] [CrossRef] [PubMed]
- Saif, I.; Salama, E.S.; Usman, M.; Lee, D.S.; Malik, K.; Liu, P.; Li, X. Improved digestibility and biogas production from lignocellulosic biomass: Biochar addition and microbial response. Ind. Crops Prod. 2021, 171, 113851. [Google Scholar] [CrossRef]
- Bin Khalid, Z.; Siddique, N.I.; Nayeem, A.; Adyel, T.M.; Bin Ismail, S.; Ibrahim, M.Z. Biochar application as sustainable precursors for enhanced anaerobic digestion: A systematic review. J. Environ. Chem. Eng. 2021, 9, 105489. [Google Scholar] [CrossRef]
- Xie, Z.; Cao, Q.; Chen, Y.; Luo, Y.; Liu, X.; Li, D. The biological and abiotic effects of powdered activated carbon on the anaerobic digestion performance of cornstalk. Bioresour. Technol. 2022, 343, 126072. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.; Fazzino, F.; Folino, A.; Paone, E.; Komilis, D. Semi-continuous anaerobic digestion of orange peel waste: Effect of activated carbon addition and alkaline pretreatment on the process. Sustainability 2019, 11, 3386. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; Gonzalez, R.; Otero, M.; Gomez, X. Enhancing anaerobic digestion of poultry blood using activated carbon enhancing anaerobic digestion of poultry blood. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Madigou, C.; Bouchez, T.; Chapleur, O. Improving anaerobic digestion with support media: Mitigation of ammonia inhibition and effect on microbial communities. Bioresour. Technol. 2017, 235, 229–239. [Google Scholar] [CrossRef]
- Hassan, M.F.; Sabri, M.A.; Fazal, H.; Hafeez, A.; Shezad, N.; Hussain, M. Recent trends in activated carbon fibers production from various precursors and applications—A comparative review. J. Anal. Appl. Pyrolysis 2020, 145, 104715. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, T.; Liu, Y.; Liu, P.; Zhang, J.; Fang, L.; Sun, D. Enhancement of desulfurization by hydroxyl ammonium ionic liquid supported on active carbon. Environ. Res. 2022, 213, 113637. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, G.; An, C.; Chen, X.; Zhang, P.; Xin, X.; Shen, J.; Agnew, J. Anaerobic digestion of livestock manure in cold regions: Technological advancements and global impacts. Renew. Sustain. Energy Rev. 2020, 119, 109494. [Google Scholar] [CrossRef]
- Yadvika; Santosh; Sreekrishnan, T.R.; Kohli, S.; Rana, V. Enhancement of biogas production from solid substrates using different techniques—A review. Bioresour. Technol. 2004, 95, 1–10. [Google Scholar] [CrossRef]
- Akindolire, M.A.; Rama, H.; Roopnarain, A. Psychrophilic anaerobic digestion: A critical evaluation of microorganisms and enzymes to drive the process. Renew. Sustain. Energy Rev. 2022, 161, 112394. [Google Scholar] [CrossRef]
- Rajagopal, R.; Bellavance, D.; Rahaman, M.S. Psychrophilic anaerobic digestion of semi-dry mixed municipal food waste: For north american context. Process Saf. Environ. Prot. 2017, 105, 101–108. [Google Scholar] [CrossRef]
- Xu, X.; Sun, Y.; Sun, Y.; Li, Y. Bioaugmentation improves batch psychrophilic anaerobic co-digestion of cattle manure and corn straw. Bioresour. Technol. 2022, 343, 126118. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.R.; Rouissi, T.; Brar, S.K.; Surampalli, R.Y. Critical insights into psychrophilic anaerobic digestion: Novel strategies for improving biogas production. Waste Manag. 2021, 131, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Meng, Q.; Dong, G.; Qi, N.; Zhao, H.; Shi, S. Metabolic engineering of threonine catabolism enables saccharomyces cerevisiae to produce propionate under aerobic conditions. Biotechnol. J. 2022, 17, 2100579. [Google Scholar] [CrossRef]
- Boboua, S.Y.B.; Zhou, C.; Li, J.; Bi, W.; Wang, R.; Chen, S.; Zheng, G. Augmentation characteristics and microbial community dynamics of low temperature resistant composite strains LTF-27. Environ. Sci. Pollut. Res. 2022, 29, 35338–35349. [Google Scholar] [CrossRef]
| Biomaterial | Chemical Composition (% DW) | ||
|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | |
| Fruits | |||
| Black currant | 12 | 25.3 | 59.3 |
| Grape | 30.3 | 21 | 17.4 |
| Olive | 36.4 | 26.8 | 26 |
| Prickly pear | 27 | – | 2.5 |
| Cherry pomace | 12 | 10.7 | – |
| Pear pomace | 34.5 | 25.3 | 59 |
| Tamarind kernel | 10–15 | 55–65 | – |
| Apple waste | 7.2 | 24.4 | 23.5 |
| Banana waste | 13 | 15 | 24 |
| Cereals | |||
| Barley hull | 24 | 36 | 19 |
| Barley Straw | 36–43 | 24–33 | 6.3–9.8 |
| Corncob | 32.3–45.6 | 39.8 | 6.7–13.9 |
| Corn stover | 35.1–39.5 | 20.7–24.6 | 11.0–19.1 |
| Oat husks | 23 | 35 | 25 |
| Oat Straw | 31–35 | 20–26 | 10–15 |
| Nut shells | 25–30 | 22–28 | 30–40 |
| Rice husk | 28.7–35.6 | 11.96–29.3 | 15.4–20 |
| Rice Straw | 29.2–34.7 | 23–25.9 | 17–19 |
| Sorghum Straw | 32–35 | 24–27 | 15–21 |
| Wheat bran | 10.5–14.8 | 35.5–39.2 | 8.3–12.5 |
| Wheat Straw | 35–39 | 22–30 | 12–16 |
| Winter rye | 29–30 | 22–26 | 16.1 |
| Other biomaterials | |||
| Bamboo | 49–50 | 18–20 | 23 |
| Coffee pulp | 33.7–36.9 | 44.2–47.5 | 15.6–19.1 |
| Cotton | 85–95 | 5–15 | 0 |
| Cotton stalk | 31 | 11 | 30 |
| Oilseed rape | 27.3 | 20.5 | 14.2 |
| Sugarcane bagasse | 25–45 | 28–32 | 15–25 |
| Sugar beet pulp | 27.4 | 28.1 | 3.1 |
| Sugarcane tops | 35 | 32 | 14 |
| Feedstock | Chemical Used | Pretreatment Conditions | Methane Yield | Reference |
|---|---|---|---|---|
| Ionic Liquids Pretreatment | ||||
| Agave bagasse (AB) | 1-ethyl-3-methylimidazolium acetate [Emim][OAc] | 119 °C, 142 min | - | [62] |
| Agave bagasse (AB) | choline lysinate [Ch][Lys] | 160 °C, 205 min | 0.28 L CH4/g CODfed | [62] |
| Agave bagasse (AB) | ethanolamine acetate [EOA][OAc] | 160 °C, 90 min | - | [62] |
| Agave bagasse (AB) | choline lysinate [Ch][Lys] | 0.26 L CH4/g CODfed | [63] | |
| Steam Explosion Pretreatment | ||||
| Coffee husks (CH) | - | 210 °C, 15 min | 292 NmL CH4 | [88] |
| Birch wood | - | 220 °C, 10 min, 18–20 bar | 566 mL/g VS | [89] |
| Rice Husk | - | 224 °C, 2.53 MPa, 5–7 min | 199 mL/g | [90] |
| Agave bagasse | - | 240 °C, 0.68–0.98 MPa, 22 min | 316–362 mL g CODfed−1 | [91] |
| Wet Explosion Pretreatment | ||||
| Biorefinery lignin. | 0–2% NaOH | 220 °C with 4% oxygen | 195.4 ± 2.3 mL/gVS/day | [21] |
| Feedlot manure | - | 170 °C for 25 min, 4 bars oxygen | 320 ± 36 L/kg-VS/Day | [51] |
| Organosolv Pretreatment | ||||
| Potato peel wastes (PPW) | 50–75% (v/v) Ethanol | 120–180 °C | 57.9 L biomethane/kg waste | [78] |
| Napier grass | 50% (v/v) aqueous ethanol | 190 °C for 15–60 min | 410 mL/g-VS | [92] |
| Hazelnut skin (HS) | 50% (v/v) water-methanol solution | 130–200 °C | 310.6 mL CH4/g VS | [75] |
| Biodegradable fraction of municipal solid waste (BFMSW) | 85% aqueous ethanol | 120 °C for 30 min | 31.7 L methane/kg | [93] |
| Ozonolysis | ||||
| Sugarcane bagasse | 7.5 mgO3 gSB−1 | - | 252.1 NmL gSV−1 | [94] |
| Macroalgae Ulva latuca | 8.3 mg O3 min–1 | - | 498.75 mL/g VS | [95] |
| Supercritical CO2 explosion | ||||
| Sewage Sludge | - | 115 °C and time of 13 min | 300 mL CH4/g VS | [96] |
| Euglena gracilis | - | 30–50 °C, 300 bar, 2 h | 456 mL g−1 VS | [97] |
| Phaeodactylum tricornutum | 30 MPa, 30 °C, and 30% ethanol | 56.7 L CH4/kg VS | [98] | |
| Deep eutectic solvents | ||||
| Organic Fraction of Municipal Solid Wastes (OFMSW) | ChCl:OA (1:1) | 60 °C | 593 mL | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agregán, R.; Lorenzo, J.M.; Kumar, M.; Shariati, M.A.; Khan, M.U.; Sarwar, A.; Sultan, M.; Rebezov, M.; Usman, M. Anaerobic Digestion of Lignocellulose Components: Challenges and Novel Approaches. Energies 2022, 15, 8413. https://doi.org/10.3390/en15228413
Agregán R, Lorenzo JM, Kumar M, Shariati MA, Khan MU, Sarwar A, Sultan M, Rebezov M, Usman M. Anaerobic Digestion of Lignocellulose Components: Challenges and Novel Approaches. Energies. 2022; 15(22):8413. https://doi.org/10.3390/en15228413
Chicago/Turabian StyleAgregán, Rubén, José M. Lorenzo, Manoj Kumar, Mohammad Ali Shariati, Muhammad Usman Khan, Abid Sarwar, Muhammad Sultan, Maksim Rebezov, and Muhammad Usman. 2022. "Anaerobic Digestion of Lignocellulose Components: Challenges and Novel Approaches" Energies 15, no. 22: 8413. https://doi.org/10.3390/en15228413
APA StyleAgregán, R., Lorenzo, J. M., Kumar, M., Shariati, M. A., Khan, M. U., Sarwar, A., Sultan, M., Rebezov, M., & Usman, M. (2022). Anaerobic Digestion of Lignocellulose Components: Challenges and Novel Approaches. Energies, 15(22), 8413. https://doi.org/10.3390/en15228413











