Intelligent Recognition Algorithm of Multiple Myocardial Infarction Based on Morphological Feature Extraction
Abstract
1. Introduction
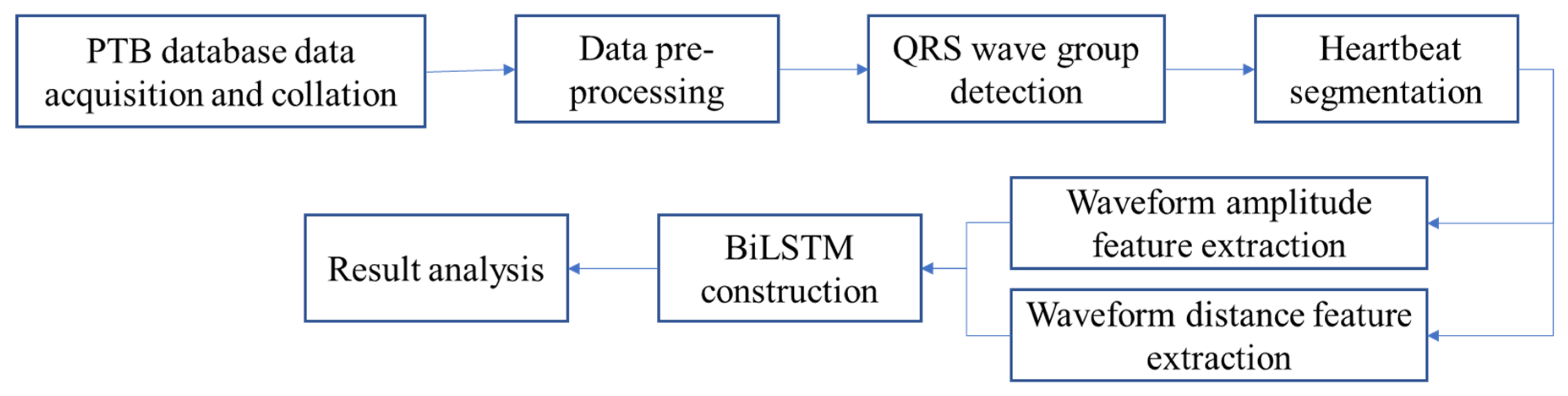
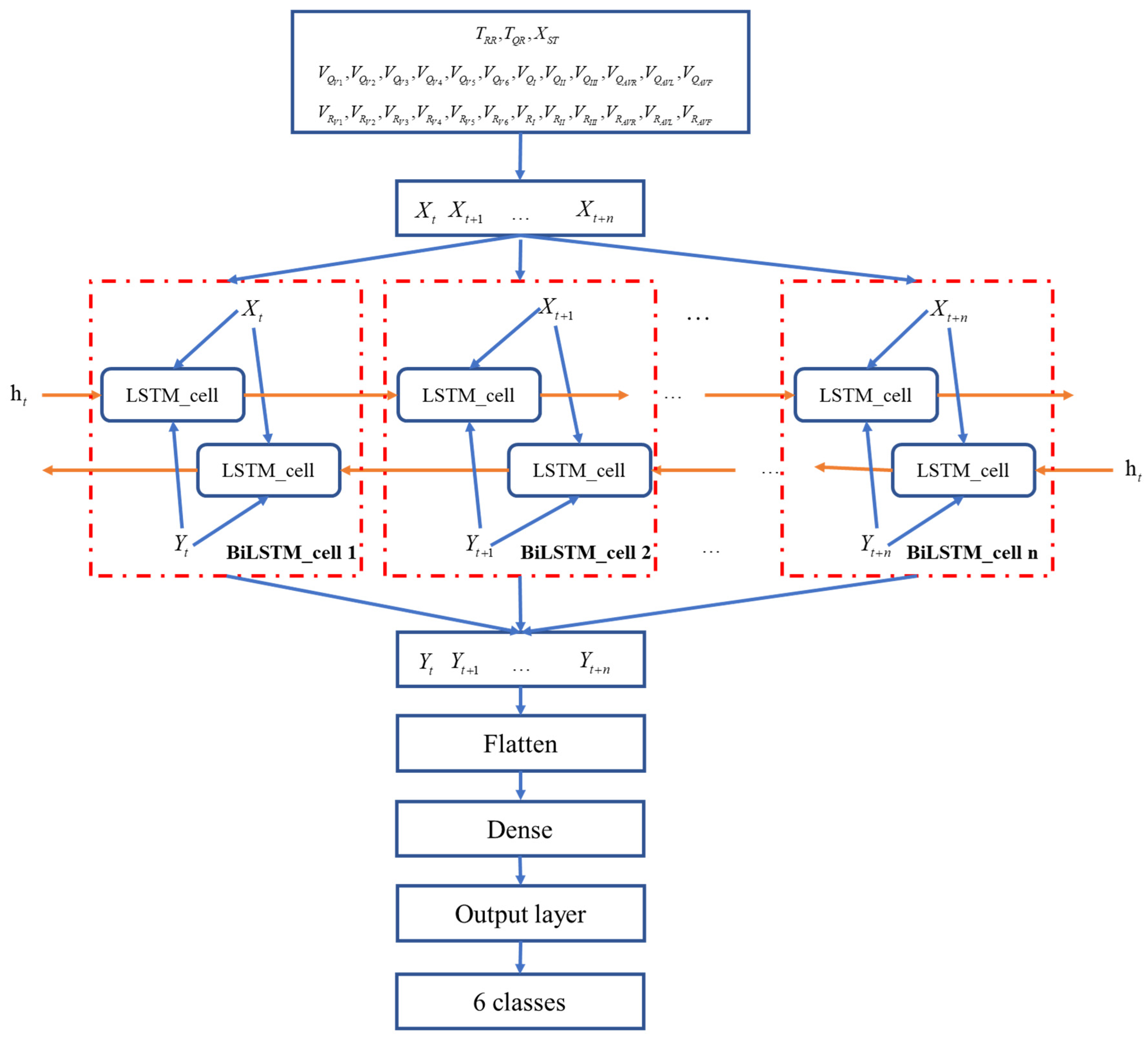
2. Method
3. Data Pre-Processing
3.1. ECG Data Acquisition
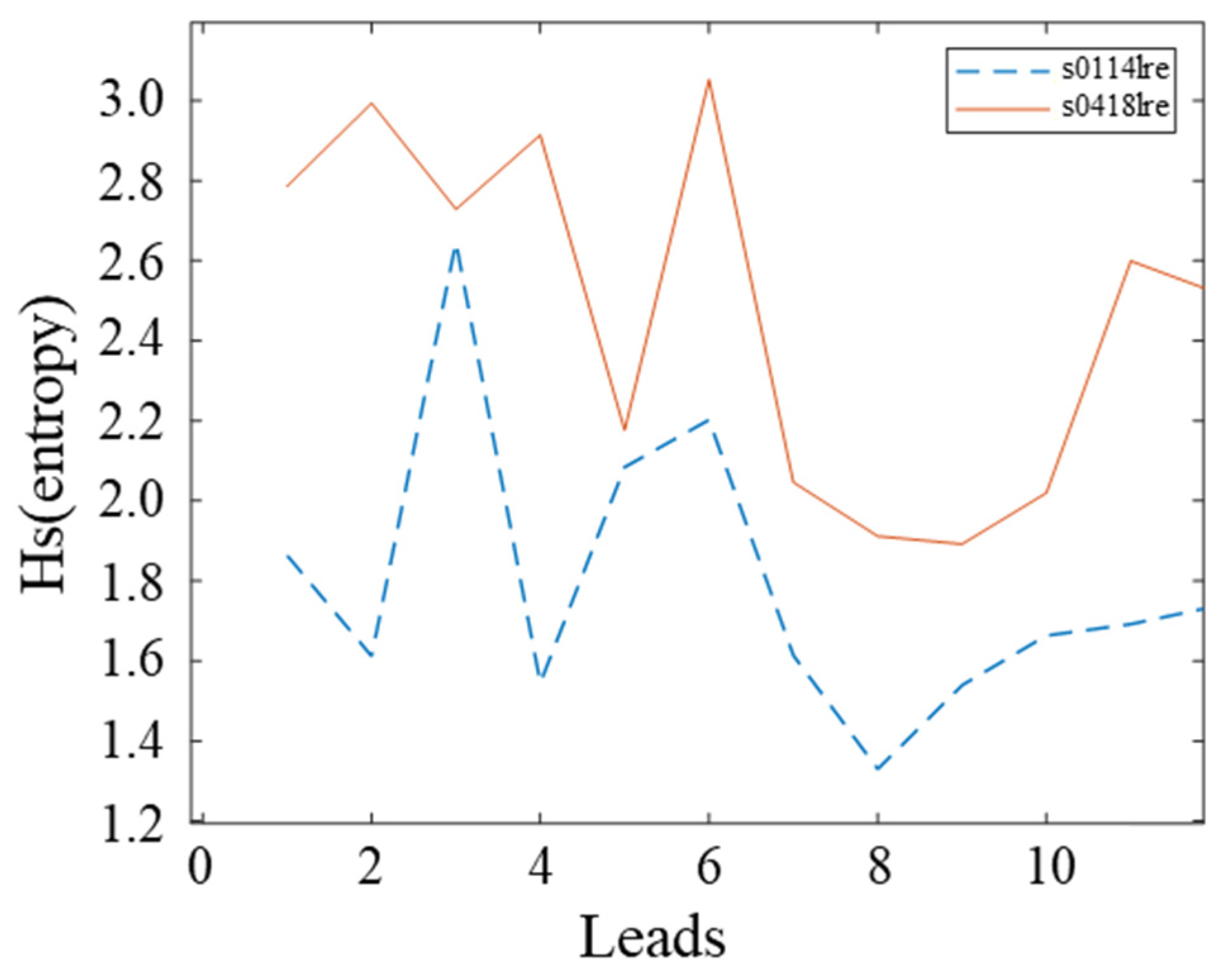
3.2. Signal Quality Analysis
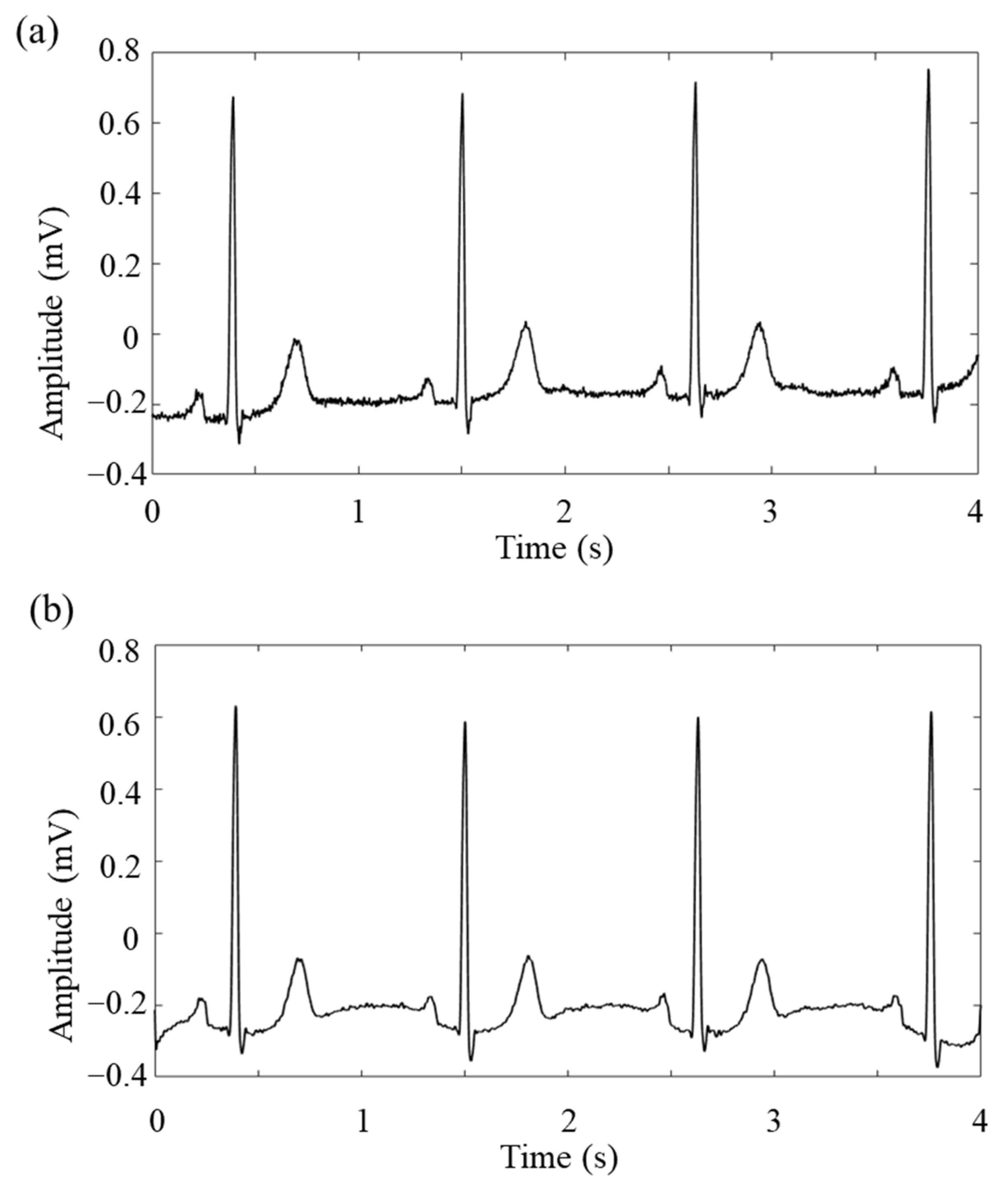
3.3. ECG Data Pre-Processing
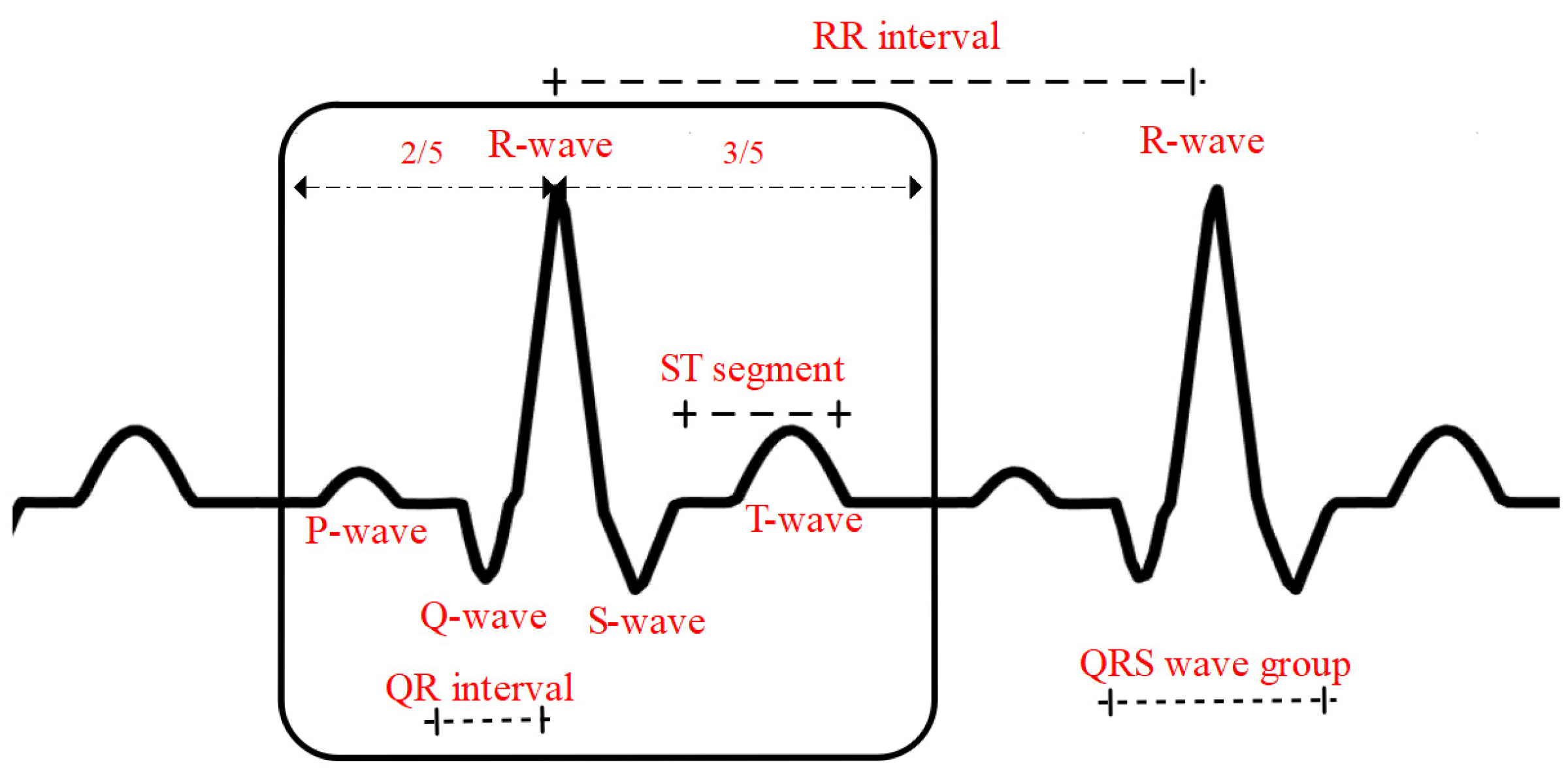
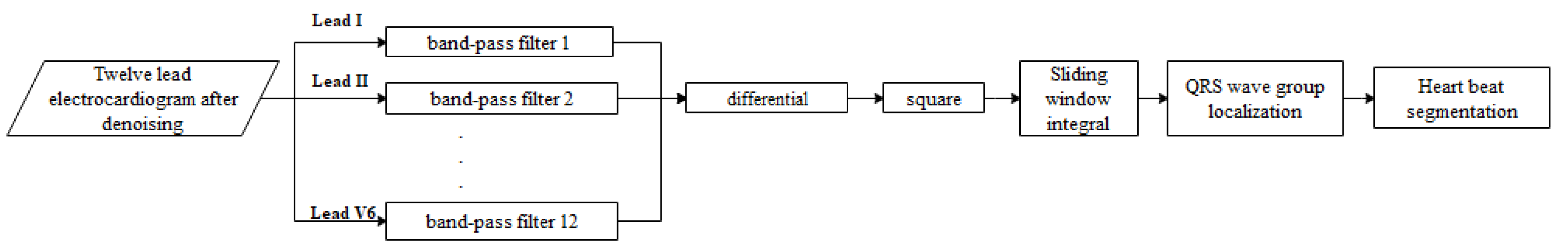
3.4. QRS Wave Group Detection and Heartbeat Segmentation
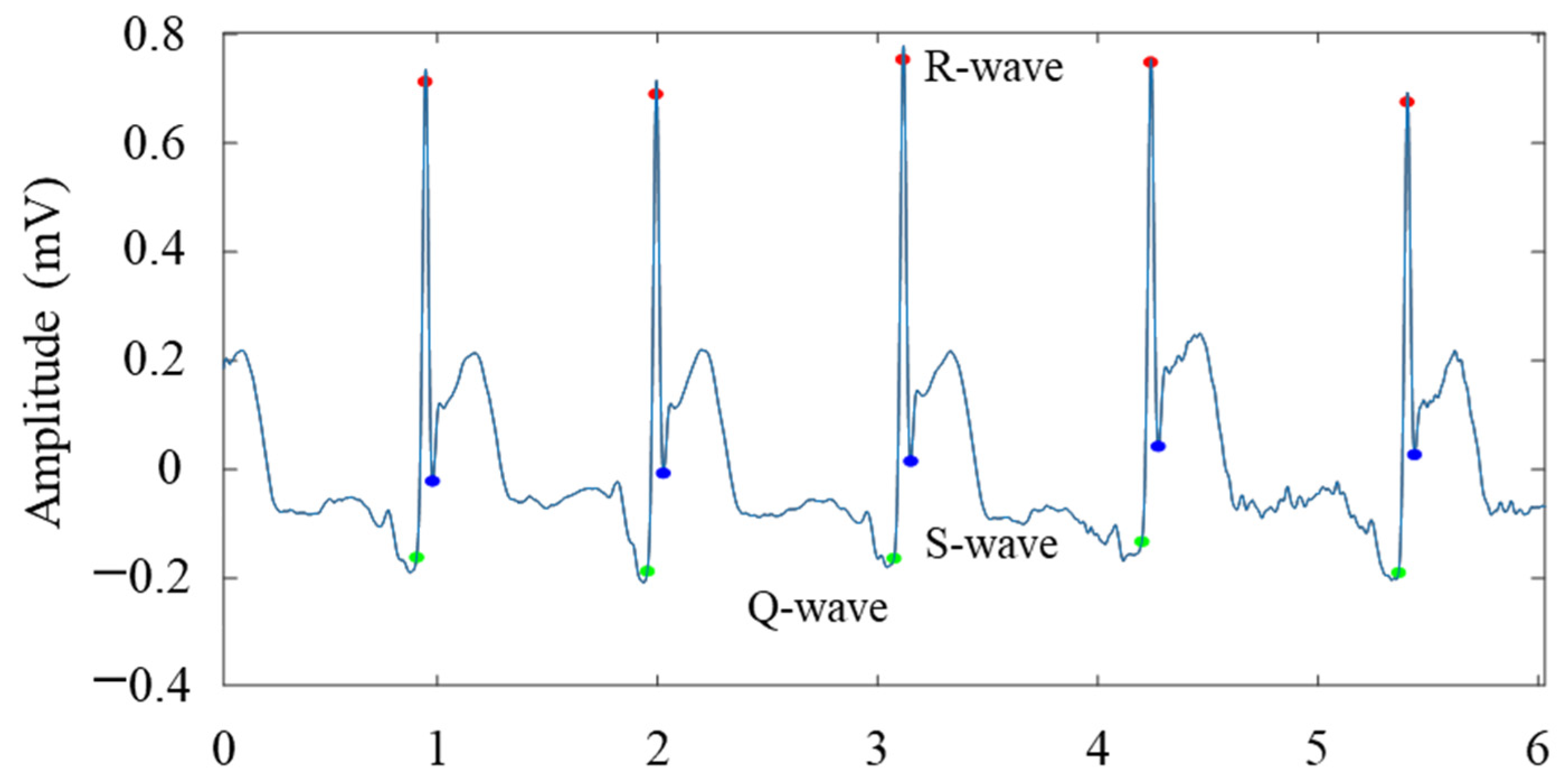
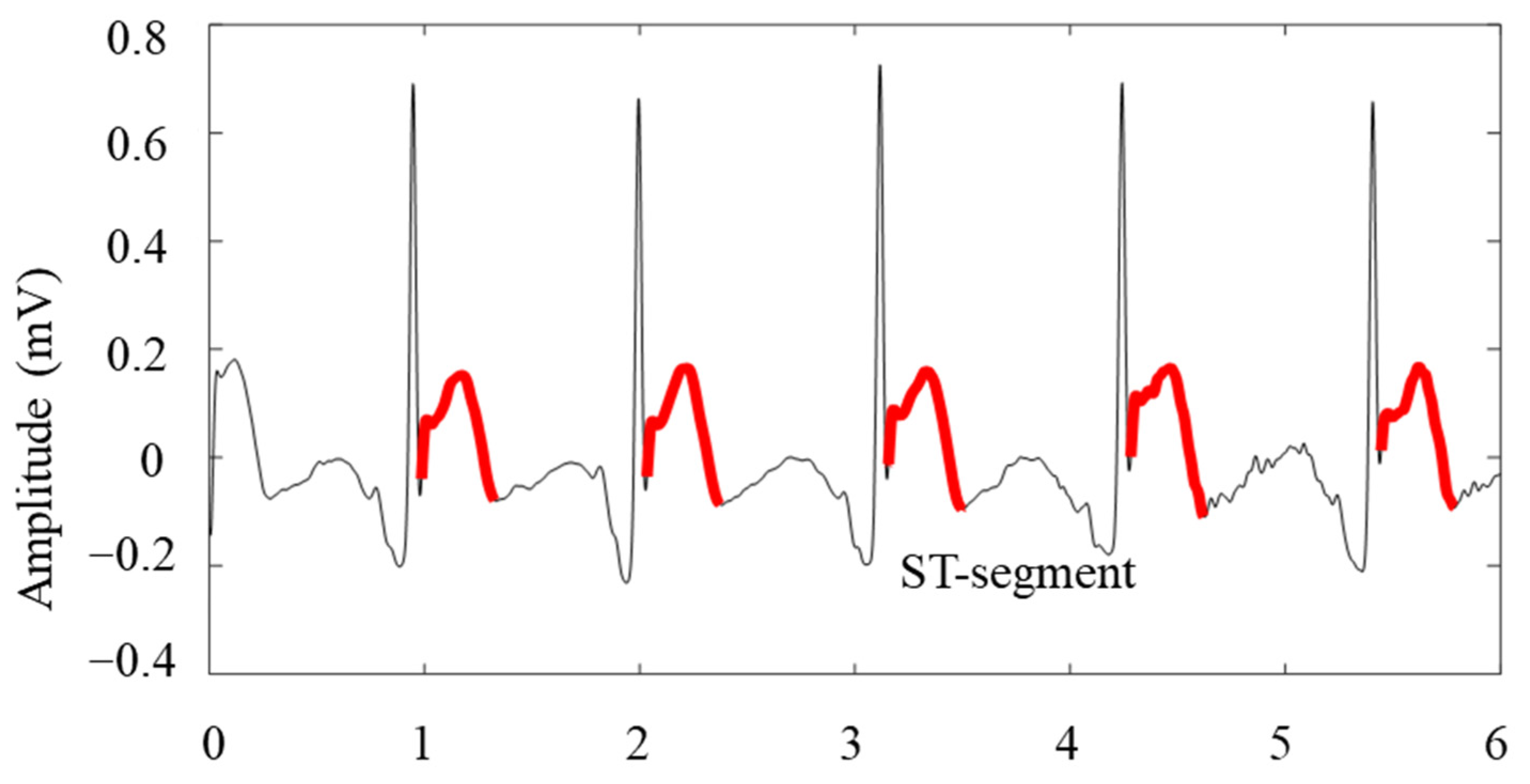
3.5. Amplitude and Distance Feature Extraction of Waveforms
- (1)
- Pathological Q waves appear on the leads oriented towards the area of myocardial necrosis;
- (2)
- Injury-type ST-segment elevation appears on leads oriented towards the area of myocardial injury surrounding the area of necrosis;
- (3)
- T-wave inversion appears on leads oriented towards the area of ischaemia surrounding the area of injury.
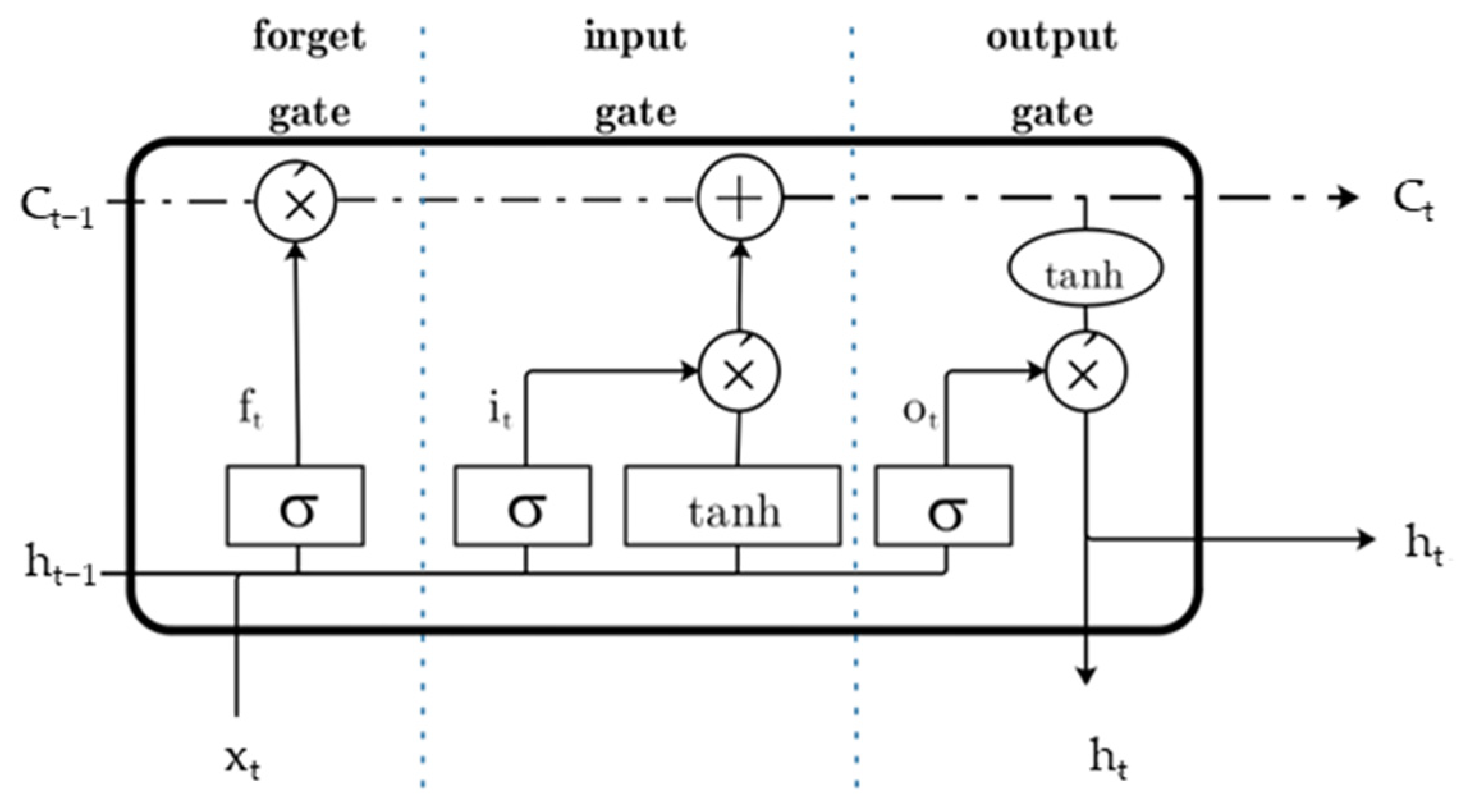
4. Network Structure Modeling
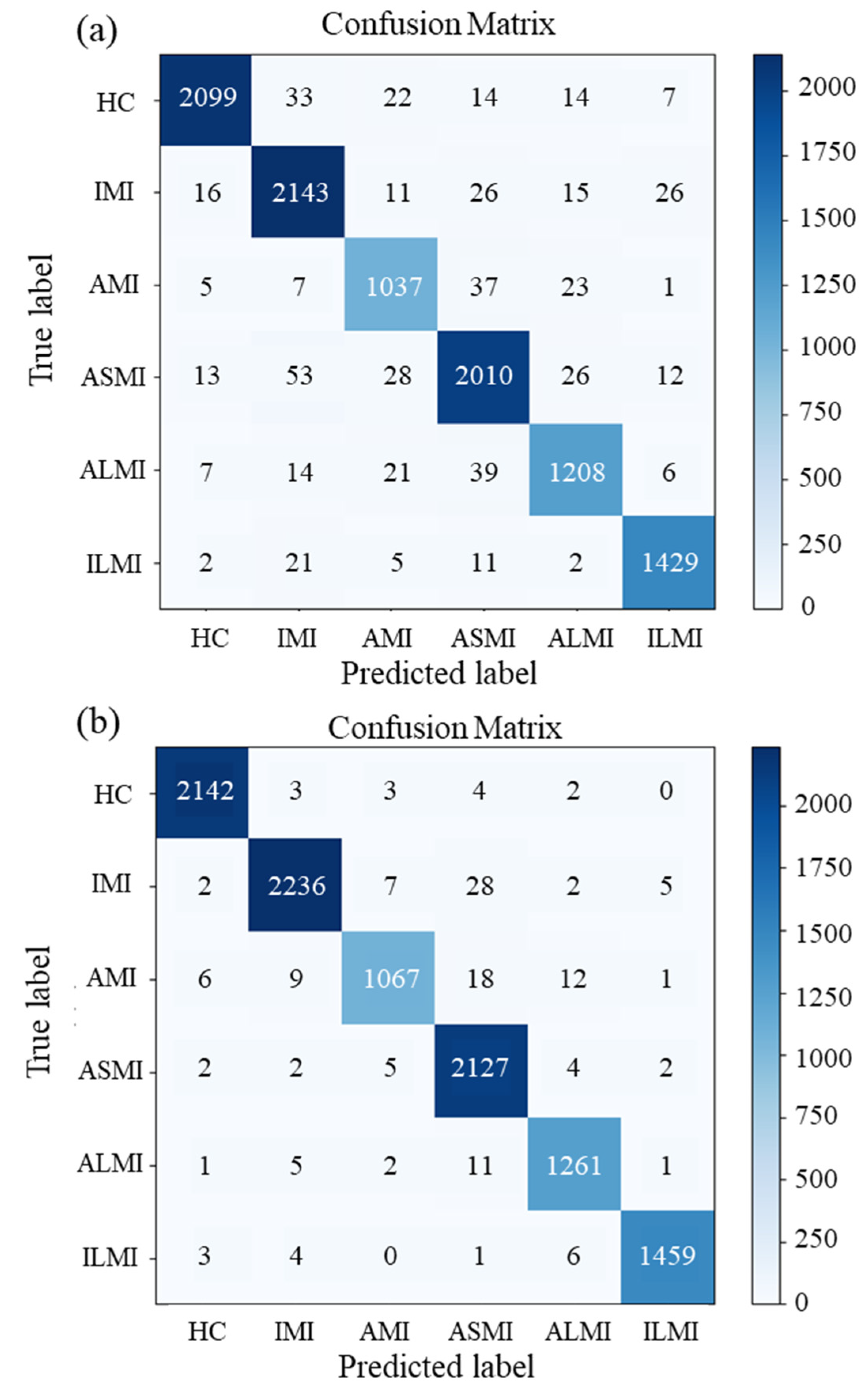
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BiLSTM | bidirectional long short-term memory network |
| ECG | electrocardiogram |
| LSTM | long short-term memory network |
| PTB | Physikalisch-Technische Bundesanstalt |
| MI | myocardial infarction |
| PPV | positive predictive value |
| NPV | negative predictive value |
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart Disease and Stroke Statistics—2013 Update. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Desai, U.; Martis, R.J.; Nayak, C.G.; Seshikala, G. Machine intelligent diagnosis of ECG for arrhythmia classification using DWT, ICA and SVM techniques. In Proceedings of the 2015 Annual IEEE India Conference (INDICON), New Delhi, India, 17–20 December 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Oh, S.L.; Ng, E.Y.K.; Tan, R.S.; Acharya, U.R. Automated beat-wise arrhythmia diagnosis using modified U-net on extended electrocardiographic recordings with heterogeneous arrhythmia types. Comput. Biol. Med. 2019, 105, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Vig, L. Anomaly detection in ECG time signals via deep long short-term memory networks. In Proceedings of the 2015 IEEE International Conference on Data Science and Advanced Analytics (DSAA), St. Etienne, France, 19–21 October 2015; pp. 1–7. [Google Scholar] [CrossRef]
- Martis, R.J.; Acharya, U.R.; Min, L.C. ECG beat classification using PCA, LDA, ICA and Discrete Wavelet Transform. Biomed. Signal Process. Control 2013, 8, 437–448. [Google Scholar] [CrossRef]
- Haraldsson, H.; Edenbrandt, L.; Ohlsson, M. Detecting acute myocardial infarction in the 12-lead ECG using Hermite expansions and neural networks. Artif. Intell. Med. 2004, 32, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Dohare, A.K.; Kumar, V.; Kumar, R. Detection of myocardial infarction in 12 lead ECG using support vector machine. Appl. Soft Comput. 2018, 64, 138–147. [Google Scholar] [CrossRef]
- Sharma, M.; Tan, R.S.; Acharya, U.R. A novel automated diagnostic system for classification of myocardial infarction ECG signals using an optimal biorthogonal filter bank. Comput. Biol. Med. 2018, 102, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Jahmunah, V.; Ng, E.Y.K.; Ru, S.; Shu, L.; Rajendra, A.U. Explainable detection of myocardial infarction using deep learning models with Grad-CAM technique on ECG signals. Comput. Biol. Med. 2022, 146, 105550. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.P.; Ying, A.; Yu, X.G.; Jian, X.W. MCA-net: A multi-task channel attention network for Myocardial infarction detection and location using 12-lead ECGs. Comput. Biol. Med. 2022, 150, 106199. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, E215–E220. [Google Scholar] [CrossRef]
- Jackson, L.B. Digital Filters and Signal Processing; Kluwer Academic Publishers: Boston, MA, USA, 1989; pp. 76–92. [Google Scholar] [CrossRef]
- Kong, D.; Zhu, J.; Wu, S.; Duan, C.; Lu, L.; Chen, D. A novel IRBF-RVM model for diagnosis of atrial fibrillation. Comput. Methods Programs Biomed. 2019, 177, 183–192. [Google Scholar] [CrossRef]
- Xu, W.; He, W.; You, B.; Guo, Y.; Hong, K.; Chen, Y.; Xu, S.; Chen, X. Acute Inferior Myocardial Infarction Detection Algorithm Based on BiLSTM Network of Morphological Feature Extraction. J. Electron. Inf. Technol. 2021, 43, 2561–2568. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of Myocardial Infarction. Med. Clin. N. Am. 2007, 91, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Padhy, S.; Dandapat, S. Third-order tensor based analysis of multilead ECG for classification of myocardial infarction. Biomed. Signal Process. 2017, 31, 71–78. [Google Scholar] [CrossRef]
- Chen, R.; Huang, Y.; Wu, J. Multi-window detection for P-wave in electrocardiograms based on bilateral accumulative area. Comput. Biol. Med. 2016, 78, 65–75. [Google Scholar] [CrossRef]
- Mincholé, A.; Jager, F.; Laguna, P. Discrimination between ischemic and artifactual ST segment events in Holter recordings. Biomed. Signal Process. 2010, 5, 21–31. [Google Scholar] [CrossRef]
- Yildirim, O.; Baloglu, U.B.; Tan, R.; Ciaccio, E.J.; Acharya, U.R. A new approach for arrhythmia classification using deep coded features and LSTM networks. Comput. Methods Programs Biomed. 2019, 176, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Salloum, R.; Kuo, C.C. ECG-based biometrics using recurrent neural networks. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), New Orleans, LA, USA, 5–9 March 2017; pp. 2062–2065. [Google Scholar] [CrossRef]
- Sharma, L.D.; Sunkaria, R.K. Myocardial infarction detection and localization using optimal features based lead specific approach. IRBM 2020, 41, 58–70. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fujita, H.; Sudarshan, V.K.; Oh, S.L.; Adam, M.; Koh, J.E.W.; Tan, J.H.; Ghista, D.N.; Martis, R.J.; Chua, C.K.; et al. Automated detection and localization of myocardial infarction using electrocardiogram: A comparative study of different leads. Knowl. Based Syst. 2016, 99, 146–156. [Google Scholar] [CrossRef]
- Safdarian, N.; Dabanloo, N.J.; Attarodi, G. A new pattern recognition method for detection and localization of myocardial infarction using T-Wave integral and total integral as extracted features from one cycle of ECG signal. J. Biomed. Sci. Eng. 2014, 2014, 818–824. [Google Scholar] [CrossRef]
- Acharya, U.R.; Fujita, H.; Oh, S.L.; Hagiwara, Y.; Tan, J.H.; Adam, M. Application of deep convolutional neural network for automated detection of myocardial infarction using ECG signals. Comput. Biol. Med. 2018, 100, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.D.; Sunkaria, R.K. Inferior myocardial infarction detection using stationary wavelet transform and machine learning approach. Signal Image Video Process. 2018, 12, 199–206. [Google Scholar] [CrossRef]

| Class | Abbreviation | Number of Samples | Number of Beats |
|---|---|---|---|
| Normal/Healthy | HC | 80 | 10,483 |
| Inferior | IMI | 83 | 11,372 |
| Anterior | AMI | 40 | 5571 |
| Anterior septal | ASMI | 72 | 10,820 |
| Anterior lateral | ALMI | 42 | 6558 |
| Inferior lateral | ILMI | 51 | 7408 |
| Total | 368 | 52,212 |
| Lead | Inferior | Anterior | Anterior Septal | Anterior Lateral | Inferior Lateral |
|---|---|---|---|---|---|
| V1 | − | ± | + | − | − |
| V2 | − | ± | + | − | − |
| V3 | − | + | ± | − | − |
| V4 | − | + | − | + | − |
| V5 | − | − | − | + | − |
| V6 | − | − | − | + | − |
| I | − | − | − | − | + |
| II | + | − | − | − | − |
| III | + | − | − | − | − |
| AVR | − | − | − | − | − |
| AVL | − | − | − | − | + |
| AVF | + | − | − | − | − |
| Characteristic Type | Characteristic Description | Characteristic Representation |
|---|---|---|
| Overall waveform distance characteristics | RR interval | |
| QR interval | ||
| ST segment potential starting point | ||
| 12-lead waveform amplitude characteristics | Q-wave amplitude: 12 leads | |
| R-wave amplitude: 12 leads |
| Classes | Number of Beats | The Total Training Set (80%) | Number of Beats in Test Set (20%) | |
|---|---|---|---|---|
| Number of Beats in Training Set (64%) | Number of Beats in Validation Set (16%) | |||
| HC | 10,483 | 6661 | 1666 | 2156 |
| IMI | 11,372 | 7290 | 1823 | 2259 |
| AMI | 5571 | 3590 | 897 | 1084 |
| ASMI | 10,820 | 6905 | 1726 | 2189 |
| ALMI | 6558 | 4217 | 1054 | 1287 |
| ILMI | 7408 | 4752 | 1188 | 1468 |
| Total | 55,212 | 33,415 | 8354 | 10,443 |
| Network Model | BiLSTM |
|---|---|
| Parameter | Epoch = 400 |
| Maxiters = 800 | |
| Learining rate = 0.001 | |
| Forget bias = 0.8 |
| Model | Label | Accuracy | Sensitivity | Specificity | PPV | NPV | F1-Score |
|---|---|---|---|---|---|---|---|
| Morphological Feature +LSTM | HC | 0.987 | 0.980 | 0.989 | 0.959 | 0.995 | 0.969 |
| IMI | 0.979 | 0.944 | 0.988 | 0.958 | 0.984 | 0.951 | |
| AMI | 0.985 | 0.923 | 0.992 | 0.934 | 0.991 | 0.928 | |
| ASMI | 0.976 | 0.941 | 0.985 | 0.938 | 0.985 | 0.939 | |
| ALMI | 0.984 | 0.938 | 0.990 | 0.933 | 0.991 | 0.935 | |
| ILMI | 0.991 | 0.965 | 0.995 | 0.972 | 0.994 | 0.968 | |
| Average | 0.984 | 0.948 | 0.990 | 0.949 | 0.990 | 0.949 | |
| MI detection (accuracy) | 0.950 | ||||||
| kappa | 0.939 | ||||||
| Morphological Feature +BiLSTM | HC | 0.998 | 0.994 | 0.999 | 0.994 | 0.998 | 0.994 |
| IMI | 0.994 | 0.990 | 0.995 | 0.981 | 0.997 | 0.985 | |
| AMI | 0.994 | 0.984 | 0.995 | 0.959 | 0.998 | 0.971 | |
| ASMI | 0.993 | 0.972 | 0.998 | 0.993 | 0.993 | 0.982 | |
| ALMI | 0.996 | 0.980 | 0.998 | 0.984 | 0.997 | 0.982 | |
| ILMI | 0.998 | 0.994 | 0.998 | 0.990 | 0.999 | 0.992 | |
| Average | 0.996 | 0.985 | 0.997 | 0.984 | 0.997 | 0.985 | |
| MI detection (accuracy) | 0.986 | ||||||
| kappa | 0.983 | ||||||
| Author, Year | Methodology | Results |
|---|---|---|
| Dohare et al., 2018 [7] | PCA+SVM | MI Detection: Acc = 96.66%; sensitivity = 96.6% |
| Safdarian N., 2014 [24] | ANN, PNN, KNN, Multilayer perceptron classification | MI Localization: Acc = 76% |
| Acharya et al., 2017 [25] | CNN | MI Detection: Acc = 95.22%; sensitivity = 95.49%, |
| Sharma L. D., 2018 [26] | T-wave feature + Naive Bayes | MI Detection: Acc = 94.74% MI Localization: Acc = 76.67% |
| Proposed method | Morphological Feature + LSTM | MI Detection: Acc = 97.4% MI Localization: Acc = 95.0%; sensitivity = 94.8% |
| Proposed method | Morphological Feature + BiLSTM | MI Detection: Acc = 99.4% MI Localization: Acc = 98.6%; sensitivity = 98.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Wang, L.; Wang, B.; Cheng, W. Intelligent Recognition Algorithm of Multiple Myocardial Infarction Based on Morphological Feature Extraction. Processes 2022, 10, 2348. https://doi.org/10.3390/pr10112348
Xu W, Wang L, Wang B, Cheng W. Intelligent Recognition Algorithm of Multiple Myocardial Infarction Based on Morphological Feature Extraction. Processes. 2022; 10(11):2348. https://doi.org/10.3390/pr10112348
Chicago/Turabian StyleXu, Wenchang, Lei Wang, Biao Wang, and Wenbo Cheng. 2022. "Intelligent Recognition Algorithm of Multiple Myocardial Infarction Based on Morphological Feature Extraction" Processes 10, no. 11: 2348. https://doi.org/10.3390/pr10112348
APA StyleXu, W., Wang, L., Wang, B., & Cheng, W. (2022). Intelligent Recognition Algorithm of Multiple Myocardial Infarction Based on Morphological Feature Extraction. Processes, 10(11), 2348. https://doi.org/10.3390/pr10112348







