Abstract
Vegetable consumption is considered as an important part of the human diet as it serves as an essential source of vitamins, nutrients, and minerals. In this regard, the demand for new technologies and ideas in the agricultural sector has grown steadily to help expand the production of vegetable crops. The uptake and accumulation of trace elements (TEs) and pharmaceuticals and personal care products (PPCPs) as contaminants in vegetables have been accelerated by man-made activities. The dietary intake of these contaminated vegetables often poses significant human health risks. To counteract this, mitigation strategies in the form of environmental amendments have received increasing attention in the last decade. The incorporation of amendments in the form of biochar has been shown to reduce the uptake of contaminants in the soil and their accumulation in vegetables. The present review is organized to offer an overview of the occurrence and sources of important contaminants of concern particularly associated with vegetable plants. The factors influencing their uptake and accumulation in the edible parts of vegetable plants are discussed briefly along with the human health risk imposed via the consumption of contaminated vegetables. Furthermore, this review also explores feasible mitigation strategies through the use of biochar for these contaminants, along with future perspectives for addressing this issue of food contamination.
1. Introduction
The contamination of soil and agricultural produce has become a serious problem for food security and the environment due to an increase in anthropogenic activities [1]. Recent studies have reported an exponential rise in vegetable consumption in the urban population [2,3]. These studies also emphasized that vegetables grown in urban areas were exposed to higher contaminants than those grown in rural areas. A large fraction of such contamination can be accounted for by the presence of well-known traditional pollutants, namely trace elements (TEs), (such as Cd, Cu, Pb, As, Si, Co, Hg, and Cr) and new emerging contaminants (ECs), such as synthetic chemicals (e.g., pharmaceuticals and personal care products (PPCPs), cosmetics, industrial solvents, artificial sweeteners, microplastics, polycyclic aromatic hydrocarbons, engineered nanomaterials, perfluorinated chemicals, and pesticides) in the food chain. Among these pollutants, the uptake of TEs in the soil–plant system has been reported in various studies [2,4,5,6]. Moreover, in recent years, the accumulation of ECs (the most prominent being PPCPs in vegetables) have also attracted the attention of various researchers [7,8,9]. The major sources of such rapid contamination include industrial activities, wastewater irrigation, waste disposal, or manure amendments [10]. Reusing reclaimed wastewater for irrigation and agricultural practices through treatment plants has led to the deposition of such contaminants in the soil [11]. These contaminants are transferred from the soil via the plant roots and then later accumulate in the edible tissues of vegetable plants [12]. In this regard, the consumption of TEs and PPCPs through contaminated vegetables may pose potential health risks, such as gastrointestinal disorders, cancer, central nervous system disorders, and kidney or liver disorders in the human body [13]. In order to reduce the risk effect of such contaminants of concern several mitigation strategies are often applied in the soil. Adding amendments is one of the common mitigation methods gaining interest in the last decade. Biochar is one such amendment that is receiving increasing attention more recently. Biochar is known to improve soil fertility and reduce the uptake of contaminants from the soil [14]. It is formed by pyrolysis of any biomass in an anaerobic condition [15]. Biochar is known to improve the soil physical, chemical, and biological characteristics as well as reduce the bioavailability of pollutants in vegetables leading to a decrease in risks for humans [16]. Biochar contains a high cation exchange capacity, a large surface area and pore size, an excellent aromatic structure, carbon content, and functional groups which help in the efficient adsorption of TEs and PPCPs, as found in recent studies [17,18,19,20].
The contamination of agricultural products is a concern for our food safety and human health. Although many review articles have already presented the uptake and accumulation of TEs in plants, few reviews on the co-occurrence on the uptake of TEs and PPCPs, particularly in vegetables, exist [3,13]. Therefore, the occurrence of mixed contaminants in food has become a research interest. Moreover, the database for PPCPs accumulation in the soil–vegetable system is not sufficient enough to develop proper protections and conclusions about their effects on human health through the consumption of contaminated food. In the light of this limitation, it is worth discussing and collating the information about the potential uptake and accumulation of various soil contaminants especially in the food contained in the daily food baskets of common people. Therefore, the present review aims to give a brief overview of the contaminants of concern in the soil–vegetable interface.
This review initially focuses on the outline of TEs and PPCPs, mainly determining the uptake in vegetable plants. It then elaborates on the various sources and factors influencing the uptake of contaminants. In addition, the review highlights the concentration levels and accumulation patterns of TEs and PPCPs in the edible parts of vegetables and their associated health risk through consumption. Moreover, the recent mitigation strategies through biochar are described along with future perspectives in this research field.
2. Contaminants of Soil–Vegetable Interface
2.1. TEs
Vegetables contain essential minerals that comprise an important part of the human diet [21]. Minerals contain groups of metals and non-metals that play an important role in the growth and metabolism of plants. They can be categorized into macro/major or micro/trace elements [13]. Macro-elements, such as Ca, Mg, N, and P, are required in plants at high concentrations (>0.1% dry weight). In contrast, TEs are required in low amounts in plants and can be further subdivided into essential and non-essential TEs. Zn, Mo, Fe, Ni, B, Cr, and Cu are examples of essential TEs required for plant metabolism and development [22]. However, as the concentration of essential TEs exceeds 0.1% of dry weight, they can turn into toxic and harmful substances in the environment.
In contrast, non-essential TEs, such as Cd, As, Pb, and F, are not required for plant growth or development and are highly toxic. High concentrations of TEs can affect soil, plants, food crops, human health, and the surrounding environment in a negative manner [23]. TEs, such as Cd, Pb, As, Cu, and Cr, tend to exhibit high accumulation rates in the soil–vegetable interface. Such TEs are translocated in much greater amounts in plants and vegetables due to similar physio–chemical properties [24,25]. According to the United States Environmental Protection Agency, Pb is an extremely toxic metal present in the environment [26]. Noxious concentrations of Pb can inhibit the enzymatic activities of plants while decreasing the total protein content required for proper functioning in plant tissues [27]. Furthermore, Cd toxicity can decrease the seed germination process and affect the nutrient content in plants [28]. Pb and Cd can show intense mobility and translocation rates from the soil to plant roots [29,30]. In addition to Pb and Cd, As is also found predominantly in the environment and is carcinogenic in nature [31]. Increases in the concentration of As can result in the absence of seed germination, stunted growth, and a decrease in the dry weight of plants [32]. Moreover, Cu is known as an essential TE required for plant metabolic functions, such as counterbalancing redox reactions. However, Cu has shown a potential risk of toxicity in plants [33]. Excess Cu can induce reactive oxygen species (ROS) in plants and affect the photosystems in the photosynthesis process. Furthermore, Cu toxicity can lead to chlorosis and reduction of the total chlorophyll content in plants [34]. Likewise, Cr can decrease the biomass of plant cells, alter the soil-microbial population, and eliminate the nutrient assimilation process [35]. Cr can cause detrimental effects on the environment by accumulating easily even at low doses.
2.2. PPCPs
ECs can be defined as any chemicals or products of chemical origin used widely by humans. They are normally present in the environment at a low concentration range (ng L−1 to µg L−1), but can pose detrimental effects on living organisms. The accumulation of ECs in food crops has been studied under different (experimental/field) conditions [11,36,37]. The major ECs classes include PPCPs, pesticides, food preservatives, polyaromatic hydrocarbons, microplastics, and nanomaterials. In this section, we focus on the most prominent EC class found to have accumulated in vegetables, that is PPCPs. These ECs have shown significant uptake and translocation from soil to vegetable plants as documented in the literature [8,38,39]. PPCPs as mentioned above include pharmaceuticals and personal care products (PCPs). Pharmaceuticals are medicinal compounds of chemical origin used for the treatment and curing of diseases. Pharmaceutical drugs can inhibit soil microbial activity, soil respiration, seed germination, and the growth of plants [40]. They can target ion channels and may inhibit the transport of the essential enzymes and nutrients required for plant growth [12]. Li et al. [41] stated that pharmaceutical compounds can transfer into plants through contaminated soil. Pharmaceuticals include a broad range of products including antibiotics, cytostatic drugs, anti-inflammatory drugs, hormones, stimulant drugs (e.g., caffeine), β-blockers, and antiepileptic drugs. Antibiotics such as tetracycline, sulfadimidine, oxytetracycline, chloramphenicol, trimethoprim, tylosin, and erythromycin are biologically active compounds used for treating bacterial infections [10]. Anti-epileptic drugs such as carbamazepine, dilantin, and primidone, have also been widely reported in the environment due to their inordinate use. Carbamazepine is one of the most thoroughly investigated pharmaceutical drugs in plant uptake of PPCPs [42]. Furthermore, anti-inflammatory drugs including diclofenac, ibuprofen, naproxen, acetaminophen, and β-blockers such as atenolol and propranolol, have been found to have accumulated in vegetable plants [10].
Besides pharmaceuticals, PCPs include daily life and household items such as plasticizers widely used in plastic bottles, gels, shampoo, and other beautification/cosmetics products, synthetic musk including galaxolide used in fragrances, skin care products, preservatives (such as parabens), ultraviolet filters (such as oxybenzone), and antimicrobial products (such as triclosan and triclocarban) [43]. Triclosan can increase fungal diversity, decrease the soil respiration process, and reduce plant growth [44]. Triclosan and triclocarban are the most widely used PCPs that have been identified in plants and generally found in the concentration range of 10–40 mg kg−1 [36]. The potential uptake of PPCPs through soil and vegetable plants has paved the way for its presence in humans.
3. Sources of TEs and PPCPs in Vegetables
There are various sources of TEs and PPCPs, which can broadly be categorized into natural or anthropogenic sources. Although soil contamination by natural phenomena (e.g., weathering of rocks, volcanic eruptions, and soil erosion, etc.) is unavoidable, the increase in soil contamination by anthropogenic sources (Figure 1) can at least partially be controlled and managed. A summary of the various sources of TEs and PPCPs is discussed in this section.
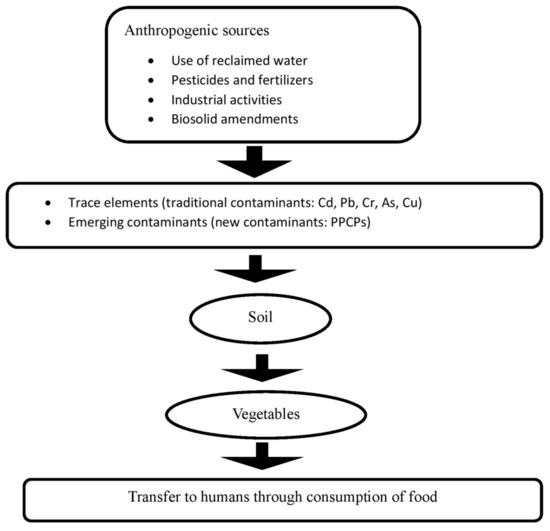
Figure 1.
Anthropogenic sources of TEs and PPCPs in the soil–vegetable interface.
3.1. Industrial Activities
TEs can accumulate in the environment due to industrial activities. Industrial discharge either in the form of contaminated gases or waste effluents can contribute to TE-associated contamination [45]. In this respect, anthropogenic sources of TEs and PPCPs in vegetables reported from different studies are summarized along with their geographical area in Table 1. TE uptake in soil can occur from industrial wastes released via direct or indirect means [46]. For instance, foliar uptake of TEs was investigated in cabbage and spinach grown near a smelter in relation to industrial activity [47]. Similarly, in a field experiment by Naser et al. [48] Cd and Pb concentrations were observed in spinach and tomatoes grown near a polluted industrial area. TE (Pb, As, Cu, and Cd) accumulation was also seen in leafy vegetables (e.g., lettuce, cabbage, spinach, and edible amaranth) grown near a mining site in China [49].

Table 1.
The different types of TEs and PPCPs studied in vegetable crops.
3.2. Agricultural Practices
Agricultural practices include the use of pesticides, herbicides, or inorganic/organic fertilizers which can contribute to TE contamination [13]. Fertilizers are added in soil to improve growth of plants as well as provide N, P, and K in plants [69]. Trace amounts of Pb, Cd, As, Cr, and other chemicals are already present during manufacturing of fertilizers. Their indiscriminate use in plants can easily translocate TEs into the soil. Cd is one such contaminant of concern due to its high mobility and translocation capability in soil and plants [13]. In the same manner, pesticide application to control pests and increase crop yield can also induce TE accumulation [70]. Once pesticides are sprayed on the soil, they can translocate into the edible portions of vegetable crops through the roots [71]. For example, excessive usage of DELVAP 100EC, a pesticide residue, increased TE concentrations in spinach leaves [72].
3.3. Wastewater Irrigation
Reclaimed/recycled water such as sewage or any wastewater is widely used as an alternative to typical irrigation water in order to conserve water resources [73]. However, this alternative can induce or increase both TE and PPCP toxicity. The accumulation of TEs in soils and vegetable crops cultivated with various sources of reclaimed/recycled water irrigation has been documented in many studies [4,6,74]. For example, elevated levels of TEs (i.e., Cd, Pb, Cu, and Cr) were found in tomatoes cultivated with treated sewage water in a field study [50]. Another study detected TEs, such as Cr, Cu, and Pb in vegetables (onion, garlic, eggplant, and tomato) irrigated with wastewater [53].
Reclaimed/recycled water not only induces the contamination of TEs in vegetable crops, but also increases the PPCP concentrations via agricultural irrigation. Incremental contamination in reclaimed/recycled water applied to crops has become one of the prime sources for their uptake in the soil–plant system [12]. Recycled water from treated plants releases micro-contaminants into the soil–plant system that penetrates the tissues of edible crops [60,75]. A study reported PPCPs (carbamazepine and diclofenac) in the leaves of iceberg lettuces which was irrigated with treated wastewater [76]. Similarly, the potential uptake of PPCPs was found in carrots due to wastewater irrigation in a study [60]. Likewise, Azanu et al. [58] explored the uptake of tetracycline by carrot and lettuce plants with the introduction of treated wastewater
3.4. Biosolid Amendments
Biosolids are solid organic matters produced from sewage treatment plants. Sludge obtained from sewage treatment plants is often used as fertilizer and manure in agriculture, which has become a source of TEs and PPCPs being transferred into the soil and vegetables [59]. Studies have shown that biosolids have the potential to accumulate TEs and PPCPs in the soil–vegetable system leading to health risks [36,61,77]. For instance, amendments of soybean plants with biosolids led to the uptake of diclofenac and ibuprofen in a study by Cortes et al. [59]. Similarly, PPCPs (i.e., carbamazepine, diphenhydramine, and triclocarbon) were reported in vegetables (e.g., tomato, lettuce, and radish plants) treated with biosolids [78]. Another study found the accumulation of 15 PPCPS (i.e., carbamazepine, sulfamethoxazole, acetaminophen, estrone, sulfadiazine, caffeine, lincomycin lamotrigine, carbadox, triclosan, 17 β-estradiol, trimethoprim, monensin, oxytetracycline, and tylosin) in the soil–radish system due to biosolid amendments [20]. Biosolid amendments have also been reported to accumulate TEs such as Pb, Cd, Cu, As, and Cr in the soil, which can transfer into vegetable plants easily [69]. For instance, the health risk assessment was analyzed for Cu and Cd in vegetables amended with biosolids [79]. The authors concluded that there was a potential risk of exposure due to biosolid amendment in the soil. In another study, the uptake of TEs (i.e., Cd, Pb, and Cu) was found in lettuce grown in soil amended with biosolids [80].
4. Factors Influencing the Uptake of Contaminants
4.1. Soil Factors
The interaction between the soil and plants determines the uptake of TEs and PPCPs. Soil plays an essential role in plant growth and development; however, it can also accumulate or regulate the toxic contaminants. Various physio–chemical and biological properties govern the functioning of the soil, such as pH, soil organic content, soil moisture, CEC, redox potential, enzymes, temperature, texture, mobility, and aging [8,13,81]. Soil pH is considered important in determining the solubility/mobility of the soil contaminants. An inverse relationship has been observed between soil pH and contaminant solubility [82]. The solubility of TEs and PPCPs decreases at high pH levels and increases at low pH levels due to surface charge variation and adsorption of solutes with charged soil components [82]. However, TEs often tend to show differences in uptake with respect to each other. For example, in the case of As, its solubility increases with increasing pH levels [83]. Due to ionic interaction present between the elements, another factor influencing the TE and PPCP movement in the soil is the redox potential (Eh) [84]. The Eh of soil decreases with the addition of organic fertilizers. The bioavailability also decreased for Cd and increased for As in soil with a decreasing Eh with organic amendments [83]. CEC is another significant factor present in the uptake of contaminants and depends purely on the soil content. For instance, soil with a high clay content increases the CEC of soil and decreases the availability of contaminants [13]. In contrast, sand decreases the CEC and increases the mobility of contaminants in the soil. Organic amendments like biochar or peat also increase the CEC of soil [85].
Furthermore, soil organic matter also influences the uptake of TEs and PPCPs. The organic matter of soil contains humic substances, such as humic and fulvic acids. Humic acid is insoluble at high pH levels, while fulvic acid is soluble in all pH conditions. As such, fulvic acid shows an anionic nature with active functional groups. Therefore, due to the presence of these humic substances, soil shows anionic properties and can bind strongly with cations through the ion-exchange reaction [86]. The main mechanisms involved in the retention of TEs and PPCPs in soil are adsorption and ion-exchange reactions. Thus, humic substances are determinants for the retention of TEs and PPCPs in soil [8,13].
Soil temperature is also a critical factor in determining the uptake of TEs and PPCPs. High temperatures enhance the uptake process for the contaminants. For instance, it was reported in a study that Cd translocation from soil to plants increased with an increase in soil temperature [87]. In addition, an increase in soil organic content may also lead to an increase in soil temperature which enhances the bioavailability of these contaminants in the soil. Soil moisture content also influences the availability of different soil contaminants. For instance, in a study by Stafford et al. [88], Cd solubility was affected by a regular increase in soil moisture and remained unaffected by irregular changes in the duration of soil saturation.
4.2. Plant Factors
In addition to soil factors, plant mediated pathways also influence the uptake of contaminants. The uptake of TEs and PPCPs mainly occurs with the help of the roots and leaves of the plants [89]. Root uptake is affected by the hydrophobicity and cell wall permeability [86] since the cell wall restricts the movement of the pollutant molecules based on size. The molecular weight plays an important role in determining the contaminant uptake through the roots. Pollutants with low molecular weights can easily move through the cell wall pores while high molecular weight compounds have low uptake rates in plants [86]. Moreover, active and passive transport are the major pathways helping in the uptake of contaminants into the plants. Active transport initiates the movement of contaminants with the help of carrier proteins through the plasma membrane. In addition, passive transport occurs along the intercellular spaces of the plant root cells with the help of diffusion [90]. Soil contaminants enter the aerial parts of the plant through the xylem with the help of transpiration and accumulate in the vegetative tissue via the phloem [91]. All these processes are regulated with the help of binding compounds and transport proteins, such as yellow stripe (YS), heavy metal transporting ATPases (HMAs), phytochelatins, ferroportin (FPN), copper transporter (COPT), and cation exchanger (CAX), for the uptake [13].
In contrast to root uptake from soil, foliar uptake of TEs and PPCPs depends on the morphology of plants, such as leaf area, leaf inclination angle, size of the stomata, and cuticular movements [92]. The stomatal system serves as the major regulator in the infiltration process for contaminants due to the presence of apertures. The opening and closing of the stomatal aperture depends on external and internal factors, such as light, humidity, temperature, partial pressure of CO2 in the intracellular space, and ionic condition of the plants [86]. Due to these factors, aperture diameters are not fixed and can be modified with the variation of the aforementioned factors. It has been observed that vegetable plant uptake of contaminants is inconsistent due to the differences in anatomical and morphological characteristics of the plants [81]. It has also been reported that leafy vegetables tend to accumulate more TEs and PPCPs than other varieties of vegetables due to their greater transpiration rate [13].
5. Accumulation Concentration of TEs and PPCPs in Vegetables
5.1. TEs
Many studies have documented the accumulation of TEs in vegetable crops [13,25,93]. The concentration level of TEs in vegetables reported by various researchers is summarized in Table 2 and some are discussed below. TE (i.e., Cu and Cr) accumulation was reported in lettuce grown near a waste-dumping and uncontrolled burning area in the Campania lands in Italy [94]. The study reported accumulation at 0.898 and 0.085 mg kg−1 for Cu and Cr, respectively. In another study conducted in China, the uptake of TEs, such as Pb, Cd, As, and Cr was investigated in the underground portion of Chinese cabbages grown in pots under greenhouse conditions [95]. These authors found average concentrations for Pb, Cd, As, and Cr of 1.62, 0.67, 1.36, and 0.73 mg kg−1, respectively, in the cabbages. These values were above the permissible limits for TEs given by various regulatory agencies (Table 3). Similarly, the accumulation capacities of Cu, Cd, and Cr were studied in onions consumed and distributed in the local markets of Ghana [96]. Among the studied TEs, the concentrations ranged from 0.03–0.06 mg kg−1, 0.13–0.52 mg kg−1, and 0.01-0.07 mg kg−1 for Cd, Cr, and Cu, respectively, in the onions. Furthermore, the levels of TEs were analyzed in dietary surveys for vegetables cultivated near a mining complex in Armenia [97]. This study determined the uptake levels for As, Cu, and Pb were at 0.03, 12.01, and 0.54 mg kg−1, respectively, in potatoes. However, in carrots, the uptake levels for Cu and Pb were found to be 5.78 and 0.54 mg kg−1, respectively. Another recent study reported TE (Pb, Cr, Cu, and Cd) accumulation in vegetables (cabbage, tomato, pepper, carrot, and lettuce) grown in soils of Ethiopia. The results found high mean concentrations of Cd in all vegetables within the range of 0.20–0.38 mg kg−1 [98].

Table 2.
Concentration of TEs (mg kg−1) studied in different vegetables.

Table 3.
Permissible limits by various organizations for TEs in vegetables (mg kg−1).
5.2. PPCPs
Accumulation of PPCPs in plants is often expressed using the bio-concentration factor (BCF), root concentration factor (RCF), and translocation factor (TF). BCF is defined as the ratio of the concentration in plant tissues to that of the spike concentration given in the media [36]. RCF is evaluated precisely for PPCP accumulation in roots and is elucidated as the ratio of the concentration in the roots to that of the spiked concentration. Likewise, TF is expressed as the ratio of the concentration in the aerial plant parts to that in the roots [12]. A number of studies have been performed regarding the accumulation of PPCPs under greenhouse conditions (i.e., hydroponic and pot experiments) [75,109,110,111] and few under field conditions [11,63,77]. For example, in a field study, the accumulation of PPCPs such as trimethoprim, sulfamethoxazole, and diclofenac, was reported at 0.572, 0.406, and 3.863 µg kg−1 respectively, in tomatoes irrigated with wastewater [11]. In another study PPCP uptake in eggplants grown in wastewater was investigated [63]. The study reported the occurrence of triclosan, chloramphenicol, ibuprofen, and trimethoprim in the range of 0.02–28.1 µg kg−1, respectively, in the eggplants. PPCP (i.e., carbamazepine, caffeine, and ibuprofen, triclosan, and bisphenol-A) accumulation was also reported in lettuces with varying spike concentrations (0–40 µg L−1) [112]. The authors reported a concentration in lettuces at the range of 0.93–2054 ng g−1. However, of all the studied PPCPs, carbamazepine showed the highest accumulation and maximum translocation in the lettuce leaves. The concentration of carbamazepine at different spiked concentrations (i.e., 0–40 µg L−1) was in the range of 233–2054 ng g−1 in the leaves of lettuces. In another study, the uptake of PPCPs (i.e., caffeine, carbamazepine, meprobamate, and primidone) was investigated in celery plants irrigated with wastewater [68]. These authors reported the concentrations of caffeine, carbamazepine, meprobamate, and primidone in celery plants at 0.17, 0.50, 0.20, and 0.45 ng g−1, respectively, when determined on a dry weight basis. The concentrations of various PPCPs reported by several researchers in the edible parts of vegetables are shown in Table 4.

Table 4.
Concentration of PPCPs (ng g−1/µg kg−1) studied in different vegetables.
6. Effect of TEs and PPCPs on the Growth of Vegetables
6.1. TEs
TEs can be accumulated in almost all vegetable parts including the root, stem, leaves, and tubers. It is reported that leaves tend to absorb a maximum amount of TEs followed by the root and stem [13]. TE toxicity can inhibit enzymatic activity and induce oxidative stress in the vegetables [115]. Pb and Cd can interfere and halt the transportation and absorption of other essential elements in vegetables, which can disrupt the photosynthesis process, electron transport chain, respiration rate, and growth in vegetables [116]. TE toxicity can also result in the reduction in leaf area, biomass, dry weight, shoot growth, and yield in vegetables [117]. Other prominent effects on the growth of vegetables includes an increase in oxidative and peroxidase enzymes along with a decrease in anti-oxidant activities [13]. TEs are independent and show different effects on different vegetables. For instance, it was found that Cd toxicity can alter root growth in potato and decrease protein content in carrot leaves [118] and can reduce fruit production in tomatoes [119]. Similarly, As can cause a reduction in photosynthetic pigments as reported for carrots, lettuces, and spinach [120]. Cr toxicity can impair seed germination and inhibit plant growth, can cause chlorosis in young leaves as investigated in onions, and induce nutrient imbalance and injury as studied in tomatoes [121]. In a study by Hamid et al. [122], it was found that Pb toxicity can increase the formation of the harmful chemical compound lead acetate in beans, and reduce chlorophyll content in peas. Similarly, TEs such as Cu may alter root development and inhibit the micronutrients activity [13].
6.2. PPCPs
The effect of PPCPs on vegetables depends on the specific class type of PPCPs, the concentration, and the vegetable species exposed [39]. The prominent effect on vegetables due to PPCP toxicity includes decreases in reproduction, reduction in root length, chlorosis, decreased biomass, increases in oxidative stress, and inhibition of growth [39,123]. For example, carbamazepine toxicity can result in burnt edges, decreased photosynthesis pigments, and white spots in vegetable plants [124]. The effect of industrial pharmaceuticals on the growth and germination of spinach through wastewater irrigation was studied by Islam et al. [125]. The results showed 92% germination rate in control conditions in contrast to PPCP contamination at only a 21% germination. Similarly, the roots and shoot length were significantly decreased in the PPCP-contaminated spinach. The authors concluded that PPCP contamination has deleterious effects on vegetables, which lowers the yield, growth, and biomass. Therefore, to avoid PPCP contamination, the proper treatment of effluent is critical before using it for irrigation. The effect of ten antibiotics was studied in carrots and lettuces to investigate their influence on seed germination and root and shoot length [126]. The results showed a negative impact on seed germination with a decrease in root vegetable growth. Goldstein et al. [127], stated that PPCPs such as carbamazepine have the maximum tendency to accumulate more in the leaves than in other parts of plants.
7. Health Risk through Consumption of Contaminated Vegetables
7.1. TEs
Consumption of contaminated vegetables can affect human health. The list of adverse effects on human health due to consumption of TE and PPCP-contaminated vegetables is presented in Table 5. Common symptoms due to TE toxicity on human health include mental retardation in children, malnutrition, central nervous disorders, decreased intra-uterine growth, dementia, insomnia, depression, liver and kidney disorders, weak immune systems, instability, decreased vision, gastrointestinal disorders, cancer, and death [13]. Regular exposure to Cd can lead to bronchiolitis, alveolitis, emphysema, and other respiratory diseases [128]. Certain neurological and mental illnesses can be induced especially in children due to Pb toxicity [129]. Moreover, Pb and Cd can readily accumulate in bone matrices and tissues and induce fractures and bone deformities. It can also cause malfunctioning of the lungs and liver and can affect other metabolic functions of the human body [49,130]. The toxicity range depends on the dietary intake of the contaminated vegetables and is assessed using health risk parameters. The various indices and parameters used in the assessment of the health risks posed by TEs and PPCPs is shown in Table 6. For TE-contaminated vegetables, the health risk assessment for humans is determined by parameters such as the daily intake of metals (DIM), hazard quotient (HQ), target hazard quotient (THQ), and health risk index (HRI) [13]. THQ measures the health risk posed by TEs through vegetable consumption. HRI represents the toxicity index, where a value <1 shows it is safe for the population and a value >1 shows detrimental effects on the population. For instance, a high HRI value was reported in a study on vegetables grown near Pb mines upon regular consumption of leafy vegetables. The risk assessment study revealed an HRI value >1 indicating a risk to consumers for several health problems, such as Alzheimer’s disease, due to excessive Pb intake through vegetables [129].

Table 5.
Risk effects of TEs and PPCPs on human health.

Table 6.
Toxicity assessment parameters for TEs and PPCPs.
In addition, high levels of Cu exposure can cause anemia, and liver and kidney damage [137]. It is also associated with a genetic disorder called Wilson disease when it accumulates in the liver [142]. Cr in the form of Cr+6 is considered to be detrimental for human health due to its toxicity and carcinogenic nature [143]. Furthermore, Smith et al. [144] stated that the consumption of As-contaminated vegetables even at low concentrations can lead to irregularity in heartbeat, nausea, vomiting, and low red-blood cells (RBCs), and white-blood cells (WBCs) counts. In addition, high concentrations of As in vegetables can cause diabetes, cardiovascular disease, an increase in blood pressure, and neurological and pulmonary disorders as well as cancers when consumed regularly.
7.2. PPCPs
Imperceptible amounts of PPCPs in dietary intakes can cause allergies, especially in children. The health risks associated with the consumption of PPCP-contaminated vegetables are shown in Table 5. Long-term consumption of PPCP-contaminated vegetables, especially antibiotics, can lead to the development of resistance against natural human antibiotic activity, which can cause illnesses that are difficult to cure and can lead to death [109]. Likewise, Stuart et al. [43] reported in a review regarding the risk assessment of ECs that paraben toxicity can decrease estrogen activity and increase hypersensitivity reactions.
However, many laboratory studies have suggested a low risk of PPCPs on human health through vegetable consumption [7,10]. The annual human exposure value for PPCPs (i.e., carbamazepine, diclofenac, triclosan, and parabens) is acceptable in the range of 20–200 mg [10]. For instance, in a greenhouse experiment, a low annual exposure of PPCPs was reported for spinach in the range of 0.04 to 3.5 × 102 µg and for lettuce in the range of 0.08 to 1.5 × 102 µg for an average 70 kg individual [111]. These estimates in the study were much lower than the acceptable range (i.e., 20–200 mg) and showed a low risk of exposure through vegetable consumption. Similarly, triclosan exposure was found to cause minimal risk to human health in a study evaluating the health risk assessment [145]. To date, except for the laboratory-based calculations, there is not enough data under realistic field conditions to make conclusions for human safety regarding exposure to PPCPs via contaminated vegetable consumption [146]. Furthermore, the risks associated with PPC-contaminated vegetables are measured in terms of the risk quotient (RQ) and health hazard index (HHI) (refer to Table 6). RQ is defined as the ratio of the estimated daily intake (EDI) to the accepted daily intake (ADI) and HHI is calculated by the summation of the RQ of each of the PPCPs. When the RQ and HHI value is <0.01, PPCP-exposure risk to humans is negligible, while when the RQ and HHI value is >0.01 it shows the possibility of risk; when this value is >0.05 it shows a high risk for humans [10]. For example, in a study on the accumulation of pharmaceuticals in peanut kernels, their daily intake was explored with respect to their potential risks to human health [147]. The study reported a high risk of exposure with HHI >0.05 for enrofloxacin. However, for norfloxacin, it showed the possibility of a health risk with an HHI > 0.01. To conclude, more studies are required in the near future for a better understanding of the health risk imposed by PPCP-contaminated vegetables.
8. Mitigation Strategies of TEs and PPCPs through Biochar
Soil clean-up is a challenging task due to technical and financial difficulties. Various efforts have been employed for the remediation of soil to reduce the impact of pollutants from harming human health and the environment. These include physical remediation (isolation, replacement, vitrification, and electrokinetic approaches), chemical remediation (such as ion exchange, immobilization techniques, photocatalysis, and chemical precipitation), and biological remediation (microorganism-based remediation or plant-based (called phytoremediation) approaches). However, due to their respective advantages and disadvantages, these methods are not always effective [148]. Recent approaches for mitigation have been shifted to the application of amendments to the soil due to the feasibility and productive results: a low cost and effective timeframe. Biochar is such a soil amendment and has been used widely in recent years [149]. Biochar is defined as solid carbonized material which is eco-friendly and cost effective [150]. It is porous in nature and obtained from the anaerobic pyrolysis of biomass such as plant, animal, and sludge waste at a temperature in the range of 300–1000 °C [151]. Biochar has been found to be effective in the remediation of soil from both TEs and PPCPs and has gained interest recently [152]. In addition, biochar has also proved to be beneficial in improving soil nutrient quality, and plant growth, yield, and productivity [151].
8.1. TEs
Biochar amendment for TE remediation has been used widely in recent years and is estimated to be of prime importance in near future for sustainable agriculture produce [153]. Various techniques used in the characterization and analysis of biochar such as X-ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier transform infrared spectroscopy (FTIR), etc., have shown high sorption capacity for TEs [154]. Application of biochar for mitigating TEs in the soil–vegetable interface has been found in various studies 122,123,124,125 [121,122,123,124]. For instance, in a study by Hmid et al. [155], Pb uptake was decreased in soil and beans due to biochar application. Moreover, it was also found that TE uptake decreased with increasing doses of biochar. Likewise, in another study it was found that the application of rice husk-derived biochar decreased the TE (Pb, Cd, Cu) concentration in lettuces [156]. In another study, the concentration of TEs (Cd, As, Cu) decreased in tomatoes after the application of biochar [157]. Important findings on biochar remediation for the TE-contaminated soil–vegetable interface is given in Table 7. Biochar contains organic functional groups, a high pH, and an effective surface area and pore structure which makes it a good adsorbent for TEs [158]. Moreover, a high pH leads to low TE mobility in soil leading to a decrease in TEs uptake in the soil and vegetables [158]. A high pH may also alter the redox state of TEs in the soil resulting in their immobilization [153]. The mechanism involved in the adsorption of TEs includes surface sorption, precipitation, complexation, ion exchange, and electrostatic interaction [154]. It was also found that the remediation of TEs by biochar depends on the type of biomass used, pyrolysis temperature, soil organic content, application of different doses, and the TEs [159]. However, more research is still required before coming to any conclusion.

Table 7.
The impact of biochar amendment on vegetables contaminated with TEs and PPCPs.
8.2. PPCPs
Biochar has shown good to excellent results in removing PPCP contamination due to the presence of organic and aromatic functional groups found on its surface [170]. Biochar mitigates the PPCPs by altering the pH, electrical conductivity, and total organic content of the soil which helps in its immobilization [171]. The mechanism involved for PPCP adsorption is little different from that of TE mechanisms. These include pore filling, partitioning, hydrophobic interaction, electrostatic interaction, and electron donar and acceptor interaction [154]. So far few studies regarding the amendment from biochar on the PPCP-contaminated soils–vegetable system are available. For example, biochar application on sulphamethazine-contaminated soil decreased the uptake by 86% as well as improved the lettuce yield and biomass [169]. In another study, the PPCPs such as sulfadiazine and sulfamethazine were significantly reduced with the amendment from wheat straw biochar [172]. Similarly, Williams et al. [173] found a reduction in the PPCPs (carbamazepine and propranolol) in contaminated soil with the addition of mallee eucalyptus oil and wheat chaff-derived biochar. Table 7 lists the important findings of studies on biochar remediation on PPCPs in different vegetables; more studies are encouraged involving the interaction of biochar and PPCPs in different vegetables in the near future.
8.3. The Impact of Biochar on the Growth of Vegetables in soil Contaminated with TE and PPCP Contaminants
The growth performance of vegetables has shown a significant increase in yield and biomass after the addition of biochar [174]. For instance, the effect of biochar on the growth and yield of tomatoes grown in TE-contaminated soil was investigated in a study [175]. The maize stalk-derived biochar showed a decrease in the uptake of Cu, Cd, and Pb in tomatoes. Moreover, it also resulted in a significant rise in the quality, yields, and photosynthetic pigments of the tomatoes. The authors concluded that the amendment with biochar showed a positive impact on the growth and productivity of the tomato along with a reduction in the TEs concentration in a two-year field study [175]. The impact of biochar was investigated in another study in lettuces which were grown in Cr-contaminated soil [176]. The results showed an increase in the nutrient uptake and a decrease in the Cr of the lettuces. Li et al. [177] also found a considerable rise in the biomass and yield, by 30–65% more than controls, for pakchoi cabbages grown on multi-element-contaminated soil with As, Cd, and Cu after amendment with different doses of biochar. Similar results were found in different vegetable species such as spinach [178], eggplant [179], lettuce [180], tomato [181], and carrot [182]. In addition, the effect of biochar application on vegetables grown in PPCP-contaminated soils have also shown similar results. For instance, in a study by Li et al. [20], biochar amendment showed a positive effect on the reduction and yield of radishes contaminated with PPCPs. Biochar application has shown to increase root length, and plant biomass and yield with the correct or appropriate quantity of biochar dose; however, it may vary between the contaminant type, variety of vegetable, and the biochar dose applied. For example, the effect of walnut shell-derived biochar was studied in lettuces and carrots grown in PPCP-contaminated soil [167]. An increase in the root and shoot biomass of the lettuce was observed after application of the walnut shell-derived biochar. In contrast no difference in root and shoot biomass was observed for carrots grown in ciprofloxacin, triclosan, and triclocarbon-contaminated soil [167].
9. Conclusions and Future Outlook
Contamination by TEs and ECs such as PPCPs is a matter of concern for soil–vegetable systems. The exponential growth of the human population, industrialization, and urbanization have introduced large varieties of chemicals into the soil and surrounding environment. Therefore, the peculiar rise of such contamination is considered to be a risk. This article reviewed the potential contaminants within the traditional and emerging contaminants in soil–vegetable systems, and their sources, accumulation, uptake factors, health risks, and mitigation strategies using biochar. TEs (As, Pb, Cu, Cr, Cd) and PPCPs are the major groups of contaminants in soil which affect the soil–vegetable interface adversely. Adequate measures should be taken for mitigating TE and PPCP contamination in soil in order to reduce the potential risk in humans and the environment. Some recommended strategies are as follows:
- 1.
- Vegetable crops should be grown in a contaminant-free environment with limited/regulated fertilizers and pesticides;
- 2.
- Better initiatives and awareness programs should be initiated frequently by the local community and government for addressing soil contaminants and their associated health risk among the common man and the farmers;
- 3.
- VTE and PPCP discharges that are released from individual, industrial, and agricultural activities should be regulated and monitored accurately;
- 4.
- More field trials involving the application of biochar for soil remediation should be carried out;
- 5.
- VMonitoring the production of contaminant-free biochar is also necessary before applying biochar for soil remediation.
Author Contributions
Conceptualization, M.P.; writing—original draft preparation, M.P.; writing—editing, A.T. (Astha Tirkey) and A.T. (Ankesh Tiwari); writing—review, S.S.L. and R.D.; writing-editing; K.H.K. and S.K.P.; supervision, S.K.P. All authors have read and agreed to the published version of the manuscript.
Funding
The first author M.P. acknowledges the University Grants Commission (UGC), New Delhi, India, for financial support in the form of JRF (NTA Ref No- 201610031349). The second author A.T. acknowledges the Council of Scientific & Industrial Research (CSIR), New Delhi, India, for financial assistance in the form of SRF (File no- 09/1041(0018)/2019-EMR-I). The third author A.T. acknowledges UGC, New Delhi, and Guru Ghasidas Vishwavidyalaya, Bilaspur C.G. for financial assistance. K.H.-K. acknowledges support through a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (Grant No: 2016R1E1A1A01940995).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| TEs | trace elements |
| ECs | emerging contaminants |
| PPCPs | pharmaceuticals and personal care products |
| Cd | Cadmium |
| Cu | Copper |
| Pb | Lead |
| As | Arsenic |
| Si | Silicon |
| Hg | Mercury |
| Co | Cobalt |
| Cr | Chromium |
| CEC | cation exchange capacity |
| Ca | Calcium |
| Mg | Magnesium |
| N | Nitrogen |
| P | Phosphorous |
| Zn | Zinc |
| Mo | Molybdenum |
| Fe | Iron |
| Ni | Nickel |
| F | Fluorine |
| ROS | reactive oxygen species |
| Eh | redox potential |
| YS | yellow stripe |
| HMAs | heavy metal transporting ATPase |
| FPN | Ferroportin |
| COPT | copper transporter |
| CAX | cation exchanger |
| CO2 | carbon di oxide |
| BCF | bio concentration factor |
| RCF | root concentration factor |
| TF | translocation factor |
| HQ | hazard quotient |
| THQ | target hazard quotient |
| HRI | health risk index |
| DIM | daily intake of metal |
| WBCs | white blood cells |
| RQ | risk quotient |
| HHI | health hazard index |
| EDI | estimated daily intake |
| ADI | accepted daily intake |
| XRD | X-ray diffraction |
| SEM | scanning electron microscopy |
| FTIR | Fourier transform infrared |
References
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Hadayat, N.; De Oliveira, L.M.; Da Silva, E.; Han, L.; Hussain, M.; Liu, X.; Ma, L.Q. Assessment of trace metals in five most-consumed vegetables in the US: Conventional vs. organic. Environ. Pollut. 2018, 243, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Margenat, A.; Matamoros, V.; Díez, S.; Cañameras, N.; Comas, J.; Bayona, J.M. Occurrence and human health implications of chemical contaminants in vegetables grown in peri-urban agriculture. Environ. Int. 2019, 124, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Chabukdhara, M.; Munjal, A.; Nema, A.K.; Gupta, S.K.; Kaushal, R.K. Heavy metal contamination in vegetables grown around peri-urban and urban-industrial clusters in Ghaziabad, India. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 736–752. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Hussain, M.I.; Ismail, S.; Khan, Q.M. Evaluating heavy metal accumulation and potential health risks in vegetables irrigated with treated wastewater. Chemosphere 2016, 163, 54–61. [Google Scholar] [CrossRef]
- Mehmood, A.; Mirza, M.A.; Choudhary, M.A.; Kim, K.-H.; Raza, W.; Raza, N.; Lee, S.S.; Zhang, M.; Lee, J.-H.; Sarfraz, M. Spatial distribution of heavy metals in crops in a wastewater irrigated zone and health risk assessment. Environ. Res. 2019, 168, 382–388. [Google Scholar] [CrossRef]
- Carter, L.J.; Harris, E.; Williams, M.; Ryan, J.J.; Kookana, R.S.; Boxall, A.B.A. Fate and Uptake of Pharmaceuticals in Soil—Plant Systems. J. Agric. Food Chem. 2014, 62, 816–825. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Rawat, S.; Adisa, I.O.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Plant uptake and translocation of contaminants of emerging concern in soil. Sci. Total Environ. 2018, 636, 1585–1596. [Google Scholar] [CrossRef]
- de Santiago-Martín, A.; Meffe, R.; Teijón, G.; Hernández, V.M.; Lopez-Heras, I.; Alonso, C.A.; de Bustamante, I. Pharmaceu-ticals and trace metals in the surface water used for crop irrigation: Risk to health or natural attenuation? Sci. Total Environ. 2020, 705, 135825. [Google Scholar] [CrossRef]
- Keerthanan, S.; Jayasinghe, C.; Biswas, J.K.; Vithanage, M. Pharmaceutical and Personal Care Products (PPCPs) in the envi-ronment: Plant uptake, translocation, bioaccumulation, and human health risks. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1221–1258. [Google Scholar] [CrossRef]
- Christou, A.; Karaolia, P.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Long-term wastewater irrigation of vegetables in real agricultural systems: Concentration of pharmaceuticals in soil, uptake and bioaccumulation in tomato fruits and human health risk assessment. Water Res. 2017, 109, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, R.S.; Ahmed, M.; Al-Busaidi, A.; Choudri, B. Translocation of pharmaceuticals and personal care products (PPCPs) into plant tissues: A review. Emerg. Contam. 2017, 3, 132–137. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, K.K.; Kumar, V.; Kumar, S.; Chadd, R.P.; Kumar, A. Trace elements in soil-vegetables interface: Transloca-tion, bioaccumulation, toxicity and amelioration—A review. Sci. Total Environ. 2019, 651, 2927–2942. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Tirkey, A.; Tiwari, A.; Pandey, S.K.; Khan, M.L. Microbial interaction of biochar and its application in soil, water and air. In Microbes and Microbial Biotechnology for Green Remediation; Malik, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 185–203. [Google Scholar]
- Vista, S.P.; Khadka, A. Determining appropriate dose of biochar for vegetables. J. Pharmacogn. Phytochem. 2017, 1, 673–677. [Google Scholar]
- Anae, J.; Ahmad, N.; Kumar, V.; Thakur, V.K.; Gutierrez, T.; Yang, X.J.; Cai, C.; Yang, Z.; Coulon, F. Recent advances in biochar engineering for soil contaminated with complex chemical mixtures: Remediation strategies and future perspectives. Sci. Total Environ. 2021, 767, 144351. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Marcinkowska, K.; Gruszka, D.; Kluczek, K. The Effects of Rabbit-Manure-Derived Biochar Co-Application with Compost on the Availability and Heavy Metal Uptake by Green Leafy Vegetables. Agronomy 2022, 12, 2552. [Google Scholar] [CrossRef]
- Shen, Y.; Song, S.; Thian, B.W.Y.; Fong, S.L.; Ee, A.W.L.; Arora, S.; Ghosh, S.; Li, S.F.; Tan, H.T.; Dai, Y.; et al. Impacts of biochar concentration on the growth performance of a leafy vegetable in a tropical city and its global warming potential. J. Clean. Prod. 2020, 264, 121678. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Q.; Ma, J.; Cui, L.; Quan, G.; Yan, J.; Wu, L.; Hina, K.; Abdul, B.; Wang, H. Effects of biochar on cadmium (Cd) uptake in vegetables and its natural downward movement in saline-alkali soil. Environ. Pollut. Bioavailab. 2020, 32, 36–46. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Qi, H.; Li, H.; Boyd, S.A.; Zhang, W. Impact of biochar amendment on the uptake, fate and bioavailability of pharmaceuticals in soil-radish systems. J. Hazard. Mater. 2020, 398, 122852. [Google Scholar] [CrossRef]
- Jehan, S.; Muhammad, S.; Ali, W.; Hussain, M.L. Potential risks assessment of heavy metal(loid)s contaminated vegetables in Pakistan: A review. Geocarto Int. 2022, 37, 7287–7302. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in hor-ticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Oves, M.; Khan, M.S.; Zaidi, A.; Ahmad, E. Soil Contamination, Nutritive Value, and Human Health Risk Assessment of Heavy Metals: An Overview. In Toxicity of Heavy Metals to Legumes and Bioremediation; Zaldi, A., Wanl, P.A., Khan, M.S., Eds.; Springer: New York, NY, USA, 2012; pp. 1–27. [Google Scholar] [CrossRef]
- Pan, X.-D.; Wu, P.-G.; Jiang, X.-G. Levels and potential health risk of heavy metals in marketed vegetables in Zhejiang, China. Sci. Rep. 2016, 6, 20317. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Zhou, J.; Zhang, Z.; Zhang, Y.; Wei, Y.; Jiang, Z. Effects of lead stress on the growth, physiology, and cellular structure of privet seedlings. PLoS ONE 2018, 13, e0191139. [Google Scholar] [CrossRef] [PubMed]
- Loi, N.N.; Sanzharova, N.I.; Shchagina, N.I.; Mironova, M.P. The Effect of Cadmium Toxicity on the Development of Lettuce Plants on Contaminated Sod-Podzolic Soil. Russ. Agric. Sci. 2018, 44, 49–52. [Google Scholar] [CrossRef]
- Roba, C.; Roşu, C.; Piştea, I.; Ozunu, A.; Baciu, C. Heavy metal content in vegetables and fruits cultivated in Baia Mare mining area (Romania) and health risk assessment. Environ. Sci. Pollut. Res. 2016, 23, 6062–6073. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, S.; Khan, M.A.; Aamir, M.; Ullah, H.; Nawab, J.; Rehman, I.U.; Shah, J. Heavy metals effects on plant growth and dietary intake of trace metals in vegetables cultivated in contaminated soil. Int. J. Environ. Sci. Technol. 2019, 16, 2295–2304. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Vioque, J.; Navarrete-Muñoz, E.M.; Carey, M.; García-Villarino, M.; Fernández-Somoano, A.; Tardón, A.; Santa-Marina, L.; Irizar, A.; Casas, M.; et al. Inorganic arsenic exposure and neuropsychological development of children of 4–5 years of age living in Spain. Environ. Res. 2019, 174, 135–142. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Chiou, W.-Y.; Hsu, F.-C. Copper Toxicity and Prediction Models of Copper Content in Leafy Vegetables. Sustainability 2019, 11, 6215. [Google Scholar] [CrossRef]
- Medda, S.; Mondal, N.K. Chromium toxicity and ultrastructural deformation of Cicer arietinum with special reference of root elongation and coleoptile growth. Ann. Agrar. Sci. 2017, 15, 396–401. [Google Scholar] [CrossRef]
- Wu, X.; Dodgen, L.K.; Conkle, J.L.; Gan, J. Plant uptake of pharmaceutical and personal care products from recycled water and biosolids: A review. Sci. Total Environ. 2015, 536, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.L.; Nason, S.L.; Karthikeyan, K.G.; Pedersen, J.A. Root uptake of pharmaceuticals and personal care product ingre-dients. Environ. Sci. Technol. 2016, 50, 525–541. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, Y.; Liu, Y.-W.; Chang, H.-Q.; Li, Z.-J.; Xue, J.-M. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 2017, 16, 1659–1668. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Uptake of pharmaceuticals by plants grown under hydroponic conditions and natural occurring plant species: A review. Sci. Total Environ. 2018, 636, 477–486. [Google Scholar] [CrossRef]
- Du, L.; Liu, W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef]
- Li, Y.; Sallac, J.B.; Zhang, W.; Boyd, S.A.; Li, H. Insight into the distribution of pharmaceuticals in soil-water-plant systems. Water Res. 2019, 152, 38–46. [Google Scholar] [CrossRef]
- Klampfl, C.W. Metabolization of pharmaceuticals by plants after uptake from water and soil: A review. TrAC Trends Anal. Chem. 2019, 111, 13–26. [Google Scholar] [CrossRef]
- Stuart, M.; Lapworth, D.; Crane, E.; Hart, A. Review of risk from potential emerging contaminants in UK groundwater. Sci. Total Environ. 2012, 416, 1–21. [Google Scholar] [CrossRef]
- Butler, E.; Whelan, M.; Ritz, K.; Sakrabani, R.; van Egmond, R. The effect of triclosan on microbial community structure in three soils. Chemosphere 2012, 89, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gabarrón, M.; Faz, A.; Acosta, J.A. Effect of different industrial activities on heavy metal concentrations and chemical distribution in topsoil and road dust. Environ. Earth Sci. 2017, 76, 129. [Google Scholar] [CrossRef]
- Jiao, X.; Teng, Y.; Zhan, Y.; Wu, J.; Lin, X. Soil Heavy Metal Pollution and Risk Assessment in Shenyang Industrial District, Northeast China. PLoS ONE 2015, 10, e0127736. [Google Scholar] [CrossRef]
- Xiong, T.-T.; Leveque, T.; Austruy, A.; Goix, S.; Schreck, E.; Dappe, V.; Sobanska, S.; Foucault, Y.; Dumat, C. Foliar uptake and metal(loid) bioaccessibility in vegetables exposed to particulate matter. Environ. Geochem. Health 2014, 36, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Naser, H.M.; Shil, N.; Mahmud, N.; Rashid, M.; Hossain, K. Lead, cadmium and nickel contents of vegetables grown in industrially polluted and non-polluted areas of Bangladesh. Bangladesh J. Agric. Res. 2009, 34, 545–554. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.-T.; Zhou, X.; Liu, L.; Gu, J.-F.; Wang, W.-L.; Zou, J.-L.; Tian, T.; Peng, P.-Q.; Liao, B.-H. Accumulation of Heavy Metals in Vegetable Species Planted in Contaminated Soils and the Health Risk Assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef]
- Alghobar, M.A.; Suresha, S. Evaluation of metal accumulation in soil and tomatoes irrigated with sewage water from Mysore city, Karnataka, India. J. Saudi Soc. Agric. Sci. 2017, 16, 49–59. [Google Scholar] [CrossRef]
- Paltseva, A.; Cheng, Z.; Deeb, M.; Groffman, P.M.; Shaw, R.K.; Maddaloni, M. Accumulation of arsenic and lead in gar-den-grown vegetables: Factors and mitigation strategies. Sci. Total Environ. 2018, 640, 273–283. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Wang, P.; Wang, Z.; Li, M.; Zhao, F.-J. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef]
- Amin, N.-U.; Hussain, A.; Alamzeb, S.; Begum, S. Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, District Mardan, Pakistan. Food Chem. 2013, 136, 1515–1523. [Google Scholar] [CrossRef]
- Bhatia, A.; Singh, S.; Kumar, A. Heavy Metal Contamination of Soil, Irrigation Water and Vegetables in Perieriia Agricultural Areas and Markets of Delhi. Water Environ. Res. 2015, 87, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Dumat, C.; Niazi, N.K.; Bibi, I.; Gul, B.H.F.S.; Javeed, H.M.R. Influence of groundwater and wastewater irrigation on lead accumulation in soil and vegetables: Implications for health risk assessment and phytoremediation. Int. J. Phytoremediat. 2017, 19, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Alimohammadi, M.; Younesian, M.; Madihi-Bidgoli, S.; Nabizadeh-Nodehi, R.; Jahed Khaniki, G.R.; Hadi, M.; Ghanbari, F. Heavy metal(oid)s concentration in Tehran supermarket vegetables: Carcinogenic and non-carcinogenic health risk assess-ment. Toxin Rev. 2020, 39, 303–310. [Google Scholar] [CrossRef]
- Taghipour, H.; Mosaferi, M. Heavy Metals in the Vegetables Collected from Production Sites. Health Promot. Perspect. 2013, 3, 185. [Google Scholar] [CrossRef] [PubMed]
- Azanu, D.; Mortey, C.; Darko, G.; Weisser, J.J.; Styrishave, B.; Abaidoo, R.C. Uptake of antibiotics from irrigation water by plants. Chemosphere 2016, 157, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.M.; Larsson, E.; Jönsson, J.Å. Study of the uptake of non-steroid anti-inflammatory drugs in wheat and soybean after application of sewage sludge as a fertilizer. Sci. Total Environ. 2013, 449, 385–389. [Google Scholar] [CrossRef]
- Malchi, T.; Maor, Y.; Tadmor, G.; Shenker, M.; Chefetz, B. Irrigation of Root Vegetables with Treated Wastewater: Evaluating Uptake of Pharmaceuticals and the Associated Human Health Risks. Environ. Sci. Technol. 2014, 48, 9325–9333. [Google Scholar] [CrossRef]
- Holling, C.S.; Bailey, J.L.; Heuvel, B.V.; Kinney, C.A. Uptake of human pharmaceuticals and personal care products by cabbage (Brassica campestris) from fortified and biosolids-amended soils. J. Environ. Monit. 2012, 14, 3029–3036. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Xu, S.; Hua, R. Uptake of three sulfonamides from contaminated soil by pakchoi cabbage. Ecotoxicol. Environ. Saf. 2013, 92, 297–302. [Google Scholar] [CrossRef]
- Liu, X.; Liang, C.; Liu, X.; Zhao, F.; Han, C. Occurrence and human health risk assessment of pharmaceuticals and personal care products in real agricultural systems with long-term reclaimed wastewater irrigation in Beijing, China. Ecotoxicol. Environ. Saf. 2020, 190, 110022. [Google Scholar] [CrossRef]
- Pannu, M.W.; Toor, G.S.; O’Connor, G.A.; Wilson, P.C. Toxicity and bioaccumulation of biosolidsioso, triclosan in food crops. Environ. Toxicol. Chem. 2012, 31, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Prosser, R.S.; Lissemore, L.; Topp, E.; Sibley, P.K. Bioaccumulation of triclosan and triclocarban in plants grown in soils amended with municipal dewatered biosolids. Environ. Toxicol. Chem. 2014, 33, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Macherius, A.; Eggen, T.; Lorenz, W.G.; Reemtsma, T.; Winkler, U.; Moeder, M. Uptake of galaxolide, tonalide, and triclosan by carrot, barley, and meadow fescue plants. J. Agric. Food Chem. 2020, 60, 7785–7791. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, J.; Stoffella, P.J.; Wilson, P.C. Uptake and distribution of bisphenol A and nonylphenol in vegetable crops irrigated with reclaimed water. J. Hazard. Mater. 2015, 283, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Conkle, J.L.; Ernst, F.; Gan, J. Treated Wastewater Irrigation: Uptake of Pharmaceutical and Personal Care Products by Common Vegetables under Field Conditions. Environ. Sci. Technol. 2014, 48, 11286–11293. [Google Scholar] [CrossRef]
- Bahiru, D.B. Accumulation of Toxic and Trace Metals in Agricultural Soil: A Review of Source and Chemistry in Ethiopia. Int. J. Environ. Chem. 2021, 5, 17–22. [Google Scholar] [CrossRef]
- Zhang, M.-K.; Liu, Z.-Y.; Wang, H. Use of Single Extraction Methods to Predict Bioavailability of Heavy Metals in Polluted Soils to Rice. Commun. Soil Sci. Plant Anal. 2010, 41, 820–831. [Google Scholar] [CrossRef]
- Wang, W.; Wan, Q.; Li, Y.; Xu, W.; Yu, X. Uptake, translocation and subcellular distribution of pesticides in Chinese cabbage (Brassica rapa var. chinensis). Ecotoxicol. Environ. Saf. 2019, 183, 109488. [Google Scholar] [CrossRef]
- Chiroma, T.M.; Abdulkarim, B.I.; Kefas, H.M. The impact of pesticide application on heavy metal (Cd, Pb and Cu) levels in spinach. Leonardo Electron. J. Pract. Technol. 2007, 11, 117–122. [Google Scholar]
- Arora, M.; Kiran, B.; Rani, S.; Rani, A.; Kaur, B.; Mittal, N. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008, 111, 811–815. [Google Scholar] [CrossRef]
- Ratul, A.K.; Hassan, M.; Uddin, M.K.; Sultana, M.S.; Akbor, M.A.; Ahsan, M.A. Potential health risk of heavy metals accumulation in vegetables irrigated with polluted river water. Int. Food Res. J. 2016, 25, 329–338. [Google Scholar]
- Calderón-Preciado, D.; Jiménez-Cartagena, C.; Matamoros, V.; Bayona, J. Screening of 47 organic microcontaminants in agricultural irrigation waters and their soil loading. Water Res. 2011, 45, 221–231. [Google Scholar] [CrossRef] [PubMed]
- García, M.G.; Fernández-López, C.; Pedrero-Salcedo, F.; Alarcón, J.J. Absorption of carbamazepine and diclofenac in hydro-ponically cultivated lettuces and human health risk assessment. Agric. Water Manag. 2018, 206, 42–47. [Google Scholar] [CrossRef]
- Sabourin, L.; Duenk, P.; Bonte-Gelok, S.; Payne, M.; Lapen, D.R.; Topp, E. Uptake of pharmaceuticals, hormones and parabens into vegetables grown in soil fertilized with municipal biosolids. Sci. Total Environ. 2012, 431, 233–236. [Google Scholar] [CrossRef]
- Wu, C.; Spongberg, A.L.; Witter, J.D.; Sridhar, B.M. Transfer of wastewater associated pharmaceuticals and personal care products to crop plants from biosolids treated soil. Ecotoxicol. Environ. Saf. 2012, 85, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Koupaie, E.H.; Eskicioglu, C. Health risk assessment of heavy metals through the consumption of food crops fertilized by biosolids: A probabilistic-based analysis. J. Hazard. Mater. 2015, 300, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Hirpassa, W.D.; Codling, E.E. Growth and Metal Uptake of Lettuce [Lactuca sativa L.] on Soil Amended with Biosolids and Gypsum. Commun. Soil Sci. Plant Anal. 2019, 50, 2033–2040. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Factors Affecting Phytoextraction: A Review. Pedosphere 2016, 26, 148–166. [Google Scholar] [CrossRef]
- Shen, B.; Wang, X.; Zhang, Y.; Zhang, M.; Wang, K.; Xie, P.; Ji, H. The optimum pH and Eh for simultaneously minimizing bioavailable cadmium and arsenic contents in soils under the organic fertilizer application. Sci. Total Environ. 2020, 711, 135229. [Google Scholar] [CrossRef]
- Fulda, B.; Voegelin, A.; Kretzschmar, R. Redox-Controlled Changes in Cadmium Solubility and Solid-Phase Speciation in a Paddy Soil as Affected by Reducible Sulfate and Copper. Environ. Sci. Technol. 2013, 47, 12775–12783. [Google Scholar] [CrossRef] [PubMed]
- Egene, C.; Van Poucke, R.; Ok, Y.; Meers, E.; Tack, F. Impact of organic amendments (biochar, compost and peat) on Cd and Zn mobility and solubility in contaminated soil of the Campine region after three years. Sci. Total Environ. 2018, 626, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kvesitadze, G.; Khatisashvili, G.; Sadunishvili, T.; Kvesitadze, E. Plants for remediation: Uptake, translocation and transformation of organic pollutants. In Plants, Pollutants and Remediation; Öztürk, M., Ashraf, M., Aksoy, A., Ahmad, M.S.A., Hakeem, K.R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 241–305. [Google Scholar]
- Cornu, J.-Y.; Denaix, L.; Lacoste, J.; Sappin-Didier, V.; Nguyen, C.; Schneider, A. Impact of temperature on the dynamics of organic matter and on the soil-to-plant transfer of Cd, Zn and Pb in a contaminated agricultural soil. Environ. Sci. Pollut. Res. 2016, 23, 2997–3007. [Google Scholar] [CrossRef]
- Stafford, A.; Paramsothy, J.; Michael, H.; Christopher, A. Influence of soil moisture status on soil cadmium phytoavailability and accumulation in plantain (Plantago lanceolata). Soil. Syst. 2018, 2, 9. [Google Scholar] [CrossRef]
- Fantke, P.; Jolliet, O. Life cycle human health impacts of 875 pesticides. Int. J. Life Cycle Assess. 2016, 21, 722–733. [Google Scholar] [CrossRef]
- Barberon, M.; Geldner, N. Radial Transport of Nutrients: The Plant Root as a Polarized Epithelium. Plant Physiol. 2014, 166, 528–537. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Heavy Metals in Crop Plants: Transport and Redistribution Processes on the Whole Plant Level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Hu, W.; Huang, B.; Tian, K.; Holm, P.E.; Zhang, Y. Heavy metals in intensive greenhouse vegetable production systems along Yellow Sea of China: Levels, transfer and health risk. Chemosphere 2017, 167, 82–90. [Google Scholar] [CrossRef]
- Esposito, M.; De Roma, A.; Cavallo, S.; Miedico, O.; Chiaravalle, E.; Soprano, V.; Baldi, L.; Gallo, P. Trace elements in vegetables and fruits cultivated in Southern Italy. J. Food Compos. Anal. 2019, 84, 103302. [Google Scholar] [CrossRef]
- Mi, B.; Liu, F.; Xie, L.; Zhou, H.; Wu, F.; Dai, X. Evaluation of the uptake capacities of heavy metals in Chinese cabbage. Ecotoxicol. Environ. Saf. 2019, 171, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Ametepey, S.T.; Cobbina, S.J.; Akpabey, F.J.; Duwiejuah, A.B.; Abuntori, Z.N. Health risk assessment and heavy metal con-tamination levels in vegetables from Tamale Metropolis, Ghana. Int. J. Food Contam. 2018, 5, 5. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Merendino, N. Health Risk Assessment of Potentially Toxic Trace and Elements in Vegetables Grown under the Impact of Kajaran Mining Complex. Biol. Trace Element Res. 2019, 192, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Feseha, A.; Chaubey, A.K.; Abraha, A. Heavy metal concentration in vegetables and their potential risk for human health. Health Risk Anal. 2021, 1, 68–81. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M.; Masunaga, S. Trace metals in soil and vegetables and associated health risk assessment. Environ. Monit. Assess. 2014, 186, 8727–8739. [Google Scholar] [CrossRef]
- Singhal, P.; Jha, S.K.; Thakur, V.K.; Ravi, P.M.; Patra, A.C.; Dubey, J.S.; Tripathi, R.M. Assessment of trace element intake through some vegetables to the population of Mumbai. Vitam. Miner. 2016, 5, 1000135. [Google Scholar] [CrossRef]
- Laaouidi, Y.; Bahmed, A.; Naylo, A.; El Khalil, H.; Ouvrard, S.; Schwartz, C.; Boularbah, A. Trace Elements in Soils and Vegetables from Market Gardens of Urban Areas in Marrakech City. Biol. Trace Elem. Res. 2020, 195, 301–316. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, M.A. Concentrations and Health Risk Assessment of Trace Elements in Cereals, Fruits, and Vegetables of Bangladesh. Biol. Trace Elem. Res. 2019, 191, 243–253. [Google Scholar] [CrossRef]
- Garg, V.K.; Yadav, P.; Mor, S.; Singh, B.; Pulhani, V. Heavy Metals Bioconcentration from Soil to Vegetables and Assessment of Health Risk Caused by Their Ingestion. Biol. Trace Elem. Res. 2014, 157, 256–265. [Google Scholar] [CrossRef]
- Bati, K.; Mogobe, O.; Masamba, W.R.L. Concentrations of Some Trace Elements in Vegetables Sold at Maun Market, Botswana. J. Food Res. 2016, 6, 69–77. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ahmad, K.; Ashraf, M.; Shoaib, N.; Parveen, R.; Bibi, Z.; Mustafa, I.; Noorka, I.R.; Tahir, H.M.; Akram, N.A.; et al. Assessment of toxicological health risk of trace metals in vegetables mostly consumed in Punjab, Pakistan. Environ. Earth Sci. 2016, 75, 433. [Google Scholar] [CrossRef]
- Pipoyan, D.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. Risk assessment of population exposure to toxic trace elements via consumption of vegetables and fruits grown in some mining areas of Armenia. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 317–330. [Google Scholar] [CrossRef]
- Ramteke, S.; Sahu, B.L.; Dahariya, N.S.; Patel, K.S.; Blazhev, B.; Matini, L. Heavy Metal Contamination of Vegetables. J. Environ. Prot. 2016, 7, 996–1004. [Google Scholar] [CrossRef]
- Ngoc, N.T.M.; Van Chuyen, N.; Thao, N.T.T.; Duc, N.Q.; Trang, N.T.T.; Binh, N.T.T.; Sa, H.C.; Tran, N.B.; Van Ba, N.; Van Khai, N.; et al. Chromium, Cadmium, Lead, and Arsenic Concentrations in Water, Vegetables, and Seafood Consumed in a Coastal Area in Northern Vietnam. Environ. Health Insights 2020, 14, 1178630220921410. [Google Scholar] [CrossRef]
- Shenker, M.; Harush, D.; Ben-Ari, J.; Chefetz, B. Uptake of carbamazepine by cucumber plants—A case study related to irri-gation with reclaimed wastewater. Chemosphere 2011, 82, 905–910. [Google Scholar] [CrossRef]
- Dodgen, L.; Li, J.; Parker, D.; Gan, J. Uptake and accumulation of four PPCP/EDCs in two leafy vegetables. Environ. Pollut. 2013, 182, 150–156. [Google Scholar] [CrossRef]
- Wu, X.; Ernst, F.; Conkle, J.L.; Gan, J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.; Domínguez, C.; Pérez-Babace, L.; Cañameras, N.; Comas, J.; Bayona, J.M. Estimate of uptake and translocation of emerging organic contaminants from irrigation water concentration in lettuce grown under controlled conditions. J. Hazard. Mater. 2016, 305, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y.; Alvarez-Ruiz, R.; Alfarhan, A.H.; El-Sheikh, M.A.; Alobaid, S.M.; Barceló, D. Uptake and accumulation of emerging contaminants in soil and plant treated with wastewater under real-world environmental conditions in the Al Hayer area (Saudi Arabia). Sci. Total Environ. 2019, 652, 562–572. [Google Scholar] [CrossRef]
- Herklotz, P.A.; Gurung, P.; Heuvel, B.V.; Kinney, C.A. Uptake of human pharmaceuticals by plants grown under hydroponic conditions. Chemosphere 2010, 78, 1416–1421. [Google Scholar] [CrossRef]
- Jadia, C.D.; Fulekar, M.H. Phytoremediation of heavy metals: Recent techniques. Afr. J. Biotechnol. 2009, 8, 921–928. [Google Scholar]
- Qinsong, X.; Guoxin, S. The toxic effects of single Cd and interaction of Cd with Zn on some physiological index of [Oenanthe javanica (Blume) DC]. Nanjing Shi Da Xue Bao 2000, 23, 97–100. [Google Scholar]
- Sharma, R.K.; Agrawal, M.; Agrawal, S.B. Physiological, biochemical and growth responses of lady’s finger (Abelmoschus esculentus L.) plants as affected by Cd contaminated soil. Bull. Environ. Contam. Toxicol. 2010, 84, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Agrawal, M.; Agrawal, S.B. Physiological and biochemical responses resulting from cadmium and zinc accu-mulation in carrot plants. J. Plant Nutr. 2010, 33, 1066–1079. [Google Scholar] [CrossRef]
- Hédiji, H.; Djebali, W.; Belkadhi, A.; Cabasson, C.; Moing, A.; Rolin, D.; Brouquisse, R.; Gallusci, P.; Chaïbi, W. Impact of long-term cadmium exposure on mineral content of Solanum lycopersicum plants: Consequences on fruit production. S. Afr. J. Bot. 2015, 97, 176–181. [Google Scholar] [CrossRef]
- Bergqvist, C.; Herbert, R.; Persson, I.; Greger, M. Plants influence on arsenic availability and speciation in the rhizosphere, roots and shoots of three different vegetables. Environ. Pollut. 2014, 184, 540–546. [Google Scholar] [CrossRef]
- Nematshahi, N.; Lahouti, M.; Ganjeali, A. Accumulation of chromium and its effect on growth of (Allium cepa cv. Hybrid). Eur. J. Exp. Biol. 2012, 2, 969–974. [Google Scholar]
- Neelofer, H.; Nosheen, B.; Faiza, J. Physiological responses of Phaseolus vulgaris to different lead concentrations. Pak. J. Bot. 2010, 42, 239–246. [Google Scholar]
- Eggen, T.; Lillo, C. Antidiabetic II Drug Metformin in Plants: Uptake and Translocation to Edible Parts of Cereals, Oily Seeds, Beans, Tomato, Squash, Carrots, and Potatoes. J. Agric. Food Chem. 2012, 60, 6929–6935. [Google Scholar] [CrossRef]
- Carter, L.J.; Williams, M.; Böttcher, C.; Kookana, R.S. Uptake of Pharmaceuticals Influences Plant Development and Affects Nutrient and Hormone Homeostases. Environ. Sci. Technol. 2015, 49, 12509–12518. [Google Scholar] [CrossRef]
- Islam, M.; Hossain, M.M.; Zakaria, M.; Rahman, G.M.; Naznin, A.; Munira, S. Effect of Industrial Effluents on Germination of Summer Leafy vegetables. Int. Res. J. Earth Sci. 2015, 2015. 3, 16–23. [Google Scholar]
- Hillis, D.G.; Fletcher, J.; Solomon, K.R.; Sibley, P.K. Effects of Ten Antibiotics on Seed Germination and Root Elongation in Three Plant Species. Arch. Environ. Contam. Toxicol. 2011, 60, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.; Shenker, M.; Chefetz, B. Insights into the uptake processes of wastewater-borne pharmaceuticals by vegetables. Environ. Sci. Technol. 2014, 48, 5593–5600. [Google Scholar] [CrossRef] [PubMed]
- Yourtchi, M.S.; Bayat, H.R. Effect of cadmium toxicity on growth, cadmium accumulation and macronutrient content of durum wheat (Dena CV.). Int. J. Agric. Crop Sci. 2013, 6, 1099–1103. [Google Scholar]
- Obiora, S.C.; Chukwu, A.; Davies, T.C. Heavy metals and health risk assessment of arable soils and food crops around Pb-Zn mining localities in Enyigba, southeastern Nigeria. J. Afr. Earth Sci. 2016, 116, 182–189. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in Human Diseases: It’s More than Just a Mere Metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Abdul, K.S.M.; Jayasinghe, S.S.; Chandana, E.P.; Jayasumana, C.; De Silva, P.M.C. Arsenic and human health effects: A review. Environ. Toxicol. Pharmacol. 2015, 40, 828–846. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Gundacker, C.; Hengstschläger, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.; Qureshi, S.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Mahurpawar, M. Effects of heavy metals on human health. Int. J. Res. Granthaalayah 2015, 530, 2394–3629. [Google Scholar]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp. Cell Res. 2016, 347, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, T.; Moreira, J.C. Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef]
- Fu, Q.; Malchi, T.; Carter, L.J.; Li, H.; Gan, J.J.; Chefetz, B. Pharmaceutical and Personal Care Products: From Wastewater Treatment into Agro-Food Systems. Environ. Sci. Technol. 2019, 53, 14083–14090. [Google Scholar] [CrossRef]
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow, W.L.; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environ. Manag. 2020, 65, 131–159. [Google Scholar] [CrossRef]
- Mohanty, M.; Kumar, P.H. Effect of ionic and chelate assisted hexavalent chromium on mung bean seedlings (Vigna radiata L. wilczek. var k-851) during seedling growth. J. Stress Physiol. Biochem. 2013, 9, 232–241. [Google Scholar]
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of drinking-water by arsenic in Bangladesh: A public health emergen-cy. Bull. World Health Organ. 2000, 78, 1093–1103. [Google Scholar]
- Verslycke, T.; Mayfield, D.B.; Tabony, J.A.; Capdevielle, M.; Slezak, B. Human health risk assessment of triclosan in land-applied biosolids. Environ. Toxicol. Chem. 2016, 35, 2358–2367. [Google Scholar] [CrossRef] [PubMed]
- Malchi, T.; Maor, Y.; Chefetz, B. Comments on “Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation”. Environ. Int. 2015, 82, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, L.; Chen, L.; Li, S.; Sun, L. Bioaccumulation of antibiotics in crops under long-term manure application: Oc-currence, biomass response and human exposure. Chemosphere 2019, 219, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application Research of Biochar for the Remediation of Soil Heavy Metals Contamination: A Review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef]
- Natasha, N.; Shahid, M.; Khalid, S.; Bibi, I.; Naeem, M.A.; Niazi, N.K.; Tack, F.M.G.; Ippolito, J.A.; Rinklebe, J. Influence of biochar on trace element uptake, toxicity and detoxification in plants and associated health risks: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2803–2843. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ibrahim, M.; Zia-ur-Rehman, M.; Abbas, T.; Ok, Y.S. Mechanisms of biochar-mediated alle-viation of toxicity of trace elements in plants: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 2230–2248. [Google Scholar] [CrossRef]
- Abbas, Z.; Ali, S.; Rizwan, M.; Zaheer, I.E.; Malik, A.; Riaz, M.A.; Shahid, M.R.; Rehman, M.Z.U.; Al-Wabel, M.I. A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab. J. Geosci. 2018, 11, 448. [Google Scholar] [CrossRef]
- Hmid, A.; Al Chami, Z.; Sillen, W.; De Vocht, A.; Vangronsveld, J. Olive mill waste biochar: A promising soil amendment for metal immobilization in contaminated soils. Environ. Sci. Pollut. Res. 2015, 22, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, K.R.; Kim, H.J.; Yoon, J.H.; Yang, J.E.; Ok, Y.S.; Owens, G.; Kim, K.H. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ. Earth Sci. 2015, 74, 1249–1259. [Google Scholar] [CrossRef]
- Waqas, M.; Li, G.; Khan, S.; Shamshad, I.; Reid, B.J.; Qamar, Z.; Chao, C. Application of sewage sludge and sewage sludge biochar to reduce polycyclic aromatic hydrocarbons (PAH) and potentially toxic elements (PTE) accumulation in tomato. Environ. Sci. Pollut. Res. 2015, 22, 12114–12123. [Google Scholar] [CrossRef] [PubMed]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Uptake of Heavy Metals by Green Leafy Vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef]
- Qiu, M.; Liu, L.; Ling, Q.; Cai, Y.; Yu, S.; Wang, S.; Fu, D.; Hu, B.; Wang, X. Biochar for the removal of contaminants from soil and water: A review. Biochar 2022, 4, 19. [Google Scholar] [CrossRef]
- Coumar, M.V.; Parihar, R.S.; Dwivedi, A.K.; Saha, J.K.; Rajendiran, S.; Dotaniya, M.L.; Kundu, S. Impact of pigeon pea biochar on cadmium mobility in soil and transfer rate to leafy vegetable spinach. Environ. Monit. Assess. 2016, 188, 31. [Google Scholar] [CrossRef]
- Khan, A.Z.; Ding, X.; Khan, S.; Ayaz, T.; Fidel, R.; Khan, M.A. Biochar efficacy for reducing heavy metals uptake by Cilantro (Coriandrum sativum) and spinach (Spinaccia oleracea) to minimize human health risk. Chemosphere 2020, 244, 125543. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Lu, H.; Lonappan, L.; Brar, S.K.; He, L.; Chen, J.; Yang, S. Biochar application as a soil amendment for decreasing cadmium availability in soil and accumulation in Brassica chinensis. J. Soils Sediments 2020, 18, 2511–2519. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Zhang, R.-H.; Li, Z.-G.; Liu, X.-D.; Wang, B.-C.; Zhou, G.-L.; Huang, X.-X.; Lin, C.-F.; Wang, A.-H.; Brooks, M. Immobilization and bioavailability of heavy metals in greenhouse soils amended with rice straw-derived biochar. Ecol. Eng. 2017, 98, 183–188. [Google Scholar] [CrossRef]
- Niu, L.-Q.; Jia, P.; Li, S.-P.; Kuang, J.-L.; He, X.-X.; Zhou, W.-H.; Liao, B.; Shu, W.-S.; Li, J.-T. Slash-and-char: An ancient agricultural technique holds new promise for management of soils contaminated by Cd, Pb and Zn. Environ. Pollut. 2015, 205, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Marmiroli, M.; Pagano, L.; Pigoni, V.; Fellet, G.; Fresno, T.; Vamerali, T.; Bandiera, M.; Marmiroli, N. Biochar addition to an arsenic contaminated soil increases arsenic concentrations in the pore water but reduces uptake to tomato plants (Solanum lycopersicum L.). Sci. Total Environ. 2013, 454, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Bair, D.A.; Anderson, C.G.; Chung, Y.; Scow, K.M.; Franco, R.B.; Parikh, S.J. Impact of biochar on plant growth and uptake of ciprofloxacin, triclocarban and triclosan from biosolids. J. Environ. Sci. Health Part B 2020, 55, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Keerthanan, S.; Jayasinghe, C.; Bolan, N.; Rinklebe, J.; Vithanage, M. Retention of sulfamethoxazole by cinnamon wood biochar and its efficacy of reducing bioavailability and plant uptake in soil. Chemosphere 2022, 297, 134073. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Vithanage, M.; Lim, J.E.; Ahmed, M.B.M.; Zhang, M.; Lee, S.S.; Ok, Y.S. Invasive plant-derived biochar inhibits sulfamethazine uptake by lettuce in soil. Chemosphere 2014, 111, 500–504. [Google Scholar] [CrossRef]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Yue, Y.; Shen, C.; Ge, Y. Biochar accelerates the removal of tetracyclines and their intermediates by altering soil properties. J. Hazard. Mater. 2019, 380, 120821. [Google Scholar] [CrossRef]
- Liu, Z.; Han, Y.; Jing, M.; Chen, J. Sorption and transport of sulfonamides in soils amended with wheat straw-derived biochar: Effects of water pH, coexistence copper ion, and dissolved organic matter. J. Soils Sediments 2017, 17, 771–779. [Google Scholar] [CrossRef]
- Williams, M.; Martin, S.; Kookana, R. Sorption and plant uptake of pharmaceuticals from an artificially contaminated soil amended with biochars. Plant Soil 2015, 395, 75–86. [Google Scholar] [CrossRef]
- Qin, J.; Niu, A.; Liu, Y.; Lin, C. Arsenic in leafy vegetable plants grown on mine water-contaminated soils: Uptake, human health risk and remedial effects of biochar. J. Hazard. Mater. 2021, 402, 123488. [Google Scholar] [CrossRef]
- Almaroai, Y.A.; Eissa, M.A. Effect of biochar on yield and quality of tomato grown on a metal-contaminated soil. Sci. Hortic. 2020, 265, 109210. [Google Scholar] [CrossRef]
- Nigussie, A.; Kissi, E.; Misganaw, M.; Ambaw, G. Effect of biochar application on soil properties and nutrient uptake of lettuces (Lactuca sativa) grown in chromium polluted soils. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 369–376. [Google Scholar]
- Li, S.; Sun, X.; Li, S.; Liu, Y.; Ma, Q.; Zhou, W. Effects of amendments on the bioavailability, transformation and accumulation of heavy metals by pakchoi cabbage in a multi-element contaminated soil. RSC Adv. 2021, 11, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, K.; Ilyas, N.; Akhtar, N.; Yasmin, H.; Hefft, D.I.; El-Sheikh, M.A.; Ahmad, P. Role of biochar and compost in cadmium immobilization and on the growth of Spinacia oleracea. PLoS ONE 2022, 17, e0263289. [Google Scholar] [CrossRef] [PubMed]
- Turan, V.; Khan, S.A.; Rahman, M.U.; Iqbal, M.; Ramzani, P.M.A.; Fatima, M. Promoting the productivity and quality of brinjal aligned with heavy metals immobilization in a wastewater irrigated heavy metal polluted soil with biochar and chitosan. Ecotoxicol. Environ. Saf. 2018, 161, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Moradi, N.; Rasouli-Sadaghiani, M.; Sepehr, E. Biochar application improves lettuce (Lactuca sativa L.) growth in a lead-contaminated calcareous soil. Arab. J. Geosci. 2021, 14, 1642. [Google Scholar] [CrossRef]
- Velli, P.; Manolikaki, I.; Diamadopoulos, E. Effect of biochar produced from sewage sludge on tomato (Solanum lycopersicum L.) growth, soil chemical properties and heavy metal concentrations. J. Environ. Manag. 2021, 297, 113325. [Google Scholar] [CrossRef]
- Hagab, R.H.; Eissa, D.; Abou-Shady, A.; Abdelmottaleb, O. Effect of biochar addition on soil properties and carrot productivity grown in polluted soils. Egypt. J. Desert Res. 2016, 66, 327–350. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).