Golgi Phosphoprotein 3 Mediates Radiation-Induced Bystander Effect via ERK/EGR1/TNF-α Signal Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Irradiation
2.3. Model of RIBE In Vitro
2.4. Establishment of Stable Cell Lines
2.5. Rescue Experiment
2.6. Knockout of GOLPH3 by Using CRISPR/Cas9
2.7. Western Blotting
2.8. ELISA Assay
2.9. Micronucleus (MN) Test
2.10. Quantitative Real-Time PCR (qPCR)
2.11. Immunofluorescence
2.12. Statistical Analysis
3. Results
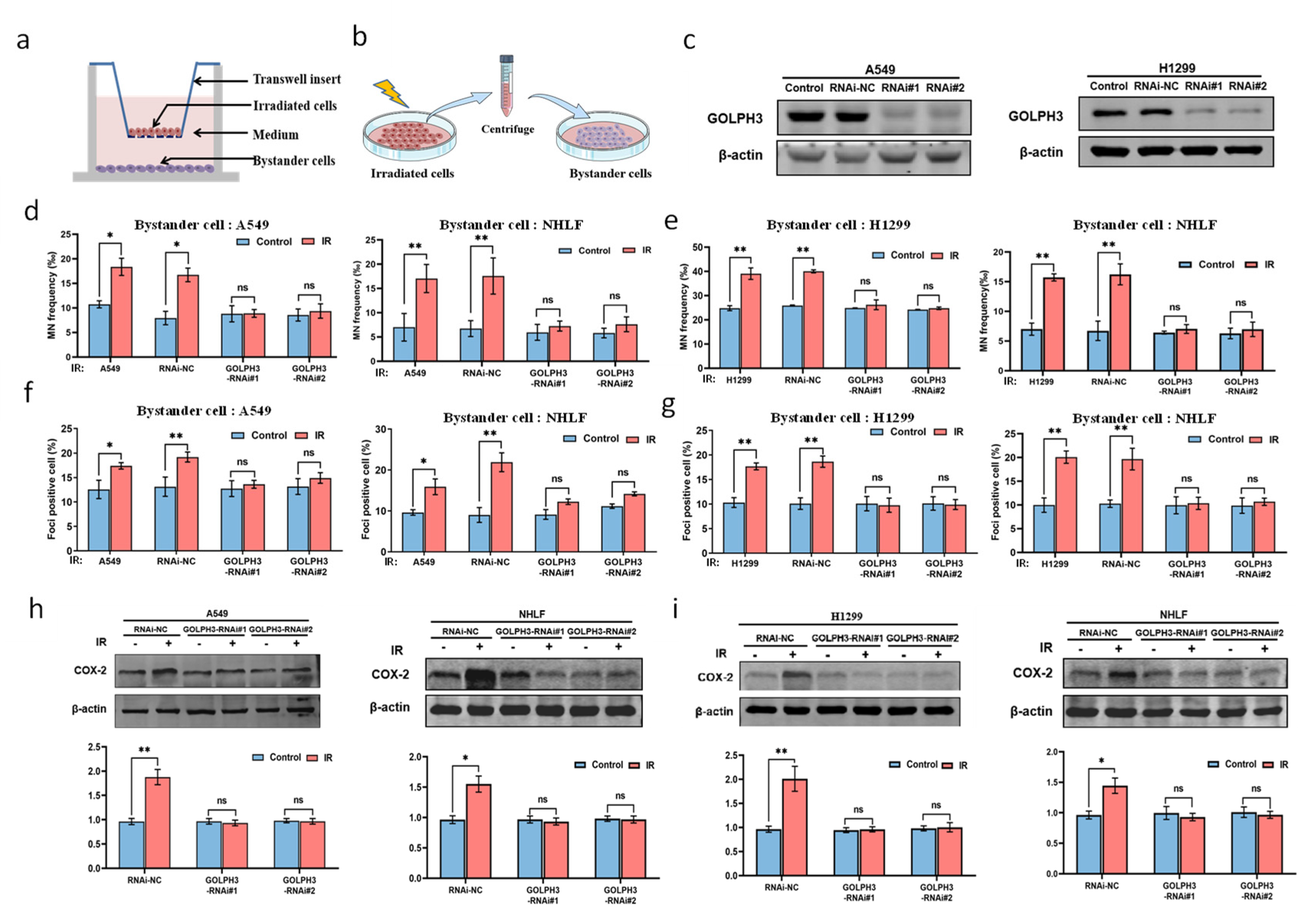
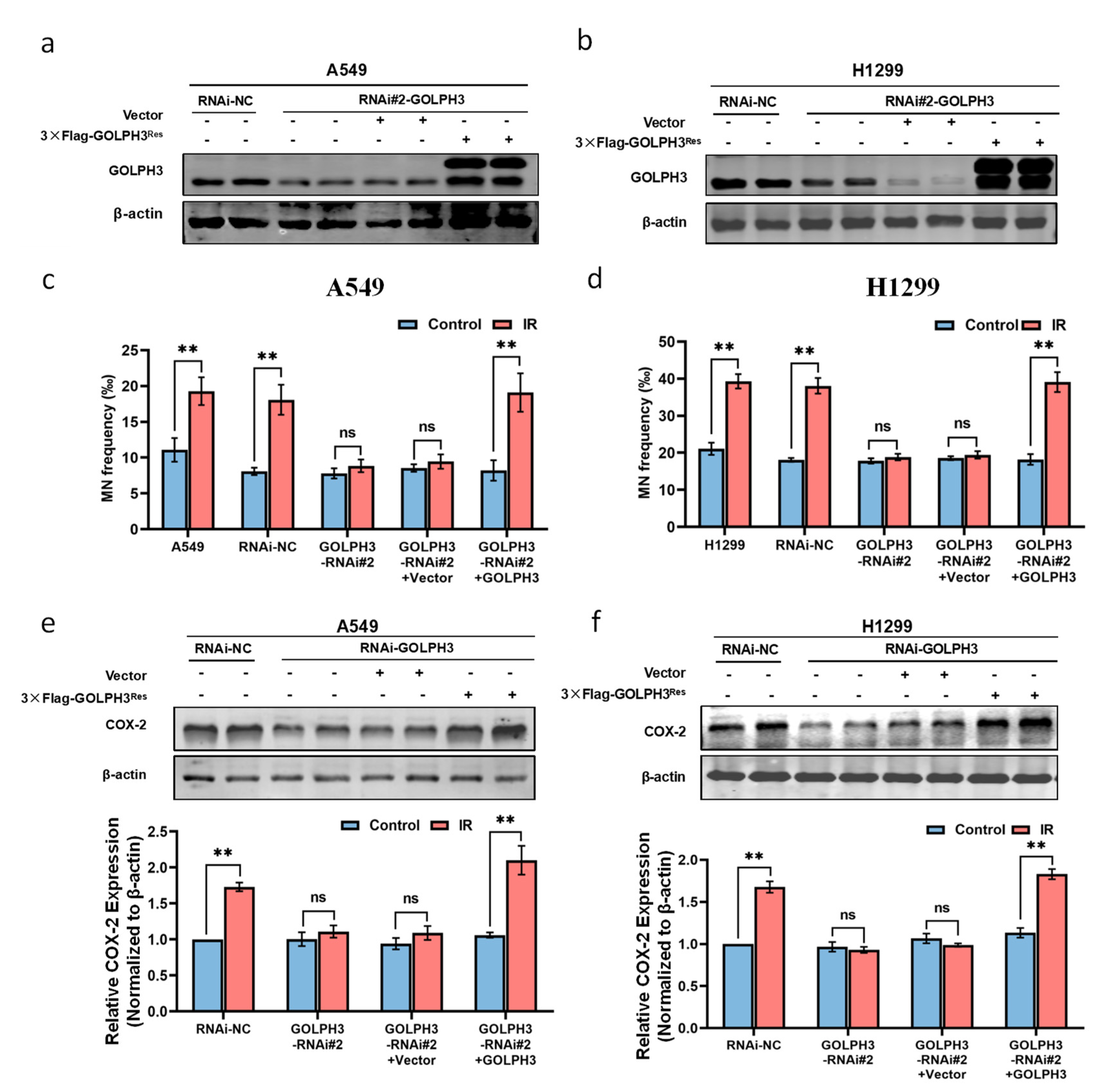
3.1. GOLPH3 Plays a Key Role in the Production of RIBE Signals Released from Irradiated Cells
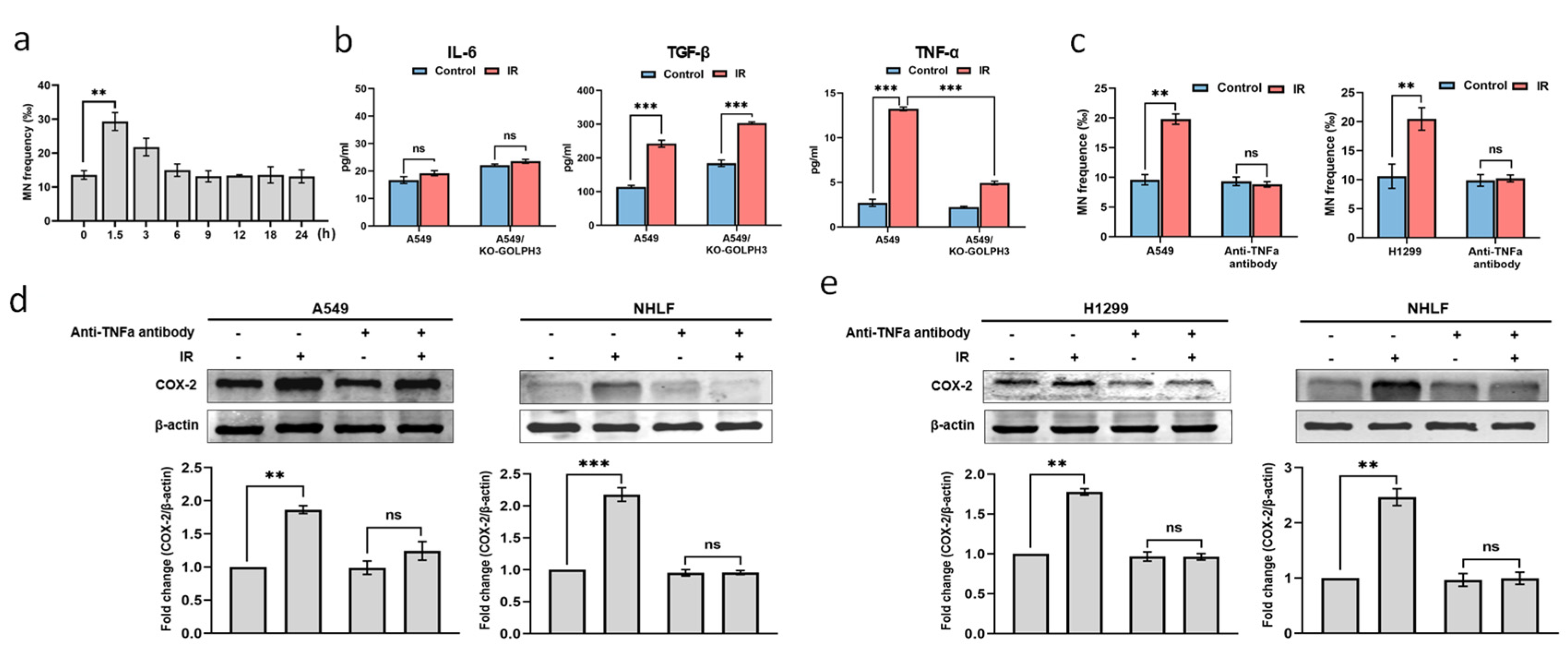
3.2. TNF-α Acts as an Intercellular Molecule in GOLPH3-Mediated RIBE
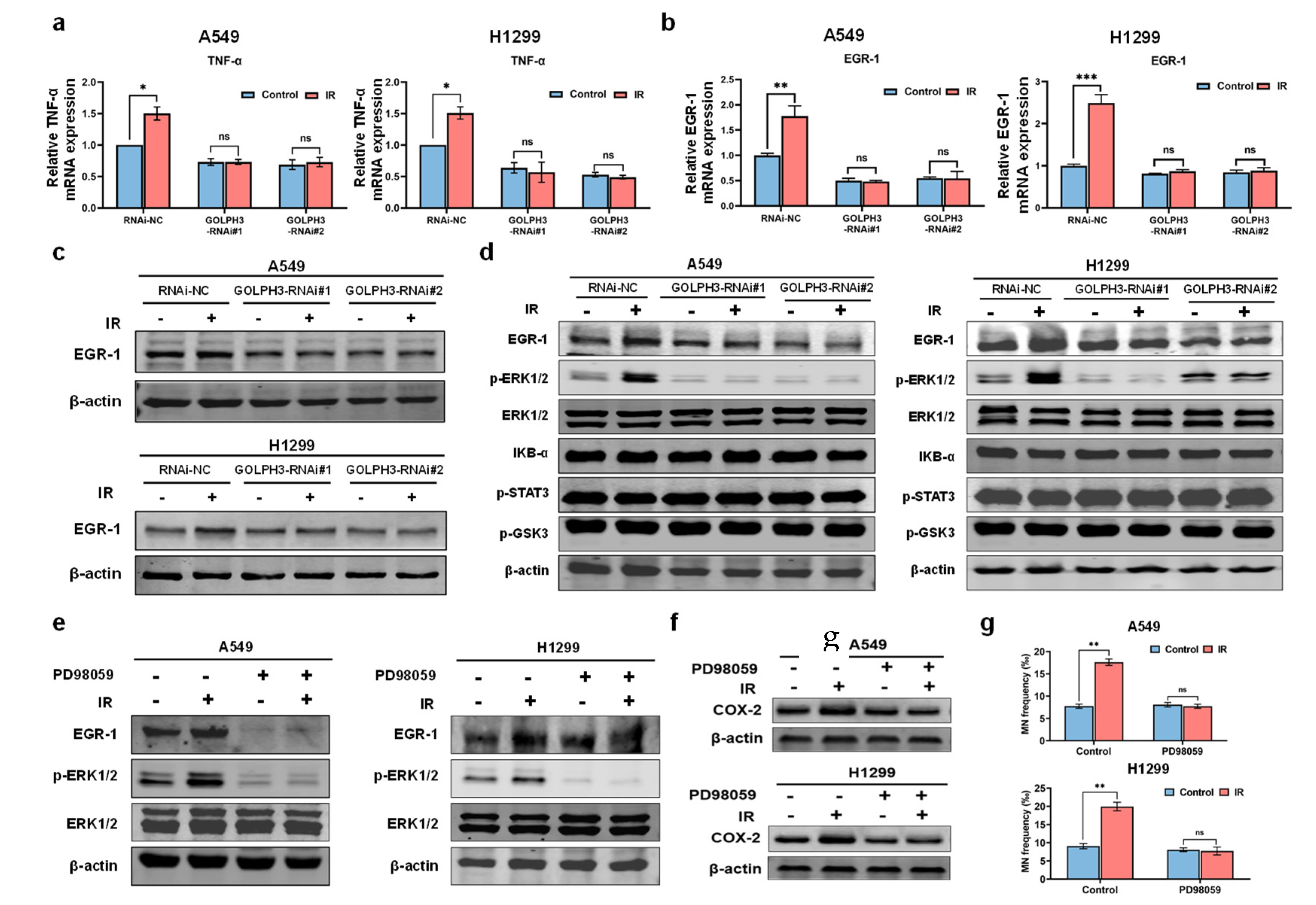
3.3. GOLPH3 Regulates the Transcription of TNF-α via ERK/EGR1 Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, W.F.; Sowa, M.B. Non-targeted bystander effects induced by ionizing radiation. Mutat. Res. 2007, 616, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Hei, T.K.; Zhou, H.; Chai, Y.; Ponnaiya, B.; Ivanov, V.N. Radiation induced non-targeted response: Mechanism and potential clinical implications. Curr. Mol. Pharmacol. 2011, 4, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, M.; Pasquali, E.; Leonardi, S.; Tanori, M.; Rebessi, S.; Di Majo, V.; Pazzaglia, S.; Toni, M.P.; Pimpinella, M.; Covelli, V.; et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc. Natl. Acad. Sci. USA 2008, 105, 12445–12450. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Hei, T.K. Radiation Induced Bystander Effect in vivo. Acta Med. Nagasaki. 2008, 53, S65–S69. [Google Scholar]
- Tamminga, J.; Koturbash, I.; Baker, M.; Kutanzi, K.; Kathiria, P.; Pogribny, I.P.; Sutherland, R.J.; Kovalchuk, O. Paternal cranial irradiation induces distant bystander DNA damage in the germline and leads to epigenetic alterations in the offspring. Cell Cycle 2008, 7, 1238–1245. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, D.; Song, Y.; Pan, Y.; Zhu, L.; Bai, Y.; Xu, Y.; Zhang, J.; Shao, C. Fractionated irradiation of right thorax induces abscopal damage on testes leading to decline in fertility. Sci. Rep. 2019, 9, 15221. [Google Scholar] [CrossRef]
- Autsavapromporn, N.; Kobayashi, A.; Liu, C.; Jaikang, C.; Tengku Ahmad, T.A.; Oikawa, M.; Konishi, T. Hypoxia and Proton microbeam: Role of Gap Junction Intercellular Communication in Inducing Bystander Responses on Human Lung Cancer Cells and Normal Cells. Radiat. Res. 2022, 197, 122–130. [Google Scholar] [CrossRef]
- Wang, H.; Yu, K.N.; Hou, J.; Liu, Q.; Han, W. Radiation-induced bystander effect: Early process and rapid assessment. Cancer Lett. 2015, 356, 137–144. [Google Scholar] [CrossRef]
- Klammer, H.; Mladenov, E.; Li, F.; Iliakis, G. Bystander effects as manifestation of intercellular communication of DNA damage and of the cellular oxidative status. Cancer Lett. 2015, 356, 58–71. [Google Scholar] [CrossRef]
- Yakovlev, V.A. Role of nitric oxide in the radiation-induced bystander effect. Redox Biol. 2015, 6, 396–400. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, X.; Li, S.; Liu, B.; Xue, D. Cathepsin B inhibitors block multiple radiation-induced side effects in C. elegans. Cell Res. 2019, 29, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, M.; Zheng, L.; Liang, Q.; Li, H.; Chen, J.T.; Guo, H.; Yoshina, S.; Chen, Y.Z.; Zhao, X.; et al. Cysteine protease cathepsin B mediates radiation-induced bystander effects. Nature 2017, 547, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tu, W.; Zhang, J.; He, M.; Ye, S.; Dong, C.; Shao, C. SirT1 knockdown potentiates radiation-induced bystander effect through promoting c-Myc activity and thus facilitating ROS accumulation. Mutat. Res. 2015, 772, 23–29. [Google Scholar] [CrossRef]
- He, M.; Zhao, M.; Shen, B.; Prise, K.M.; Shao, C. Radiation-induced intercellular signaling mediated by cytochrome-c via a p53-dependent pathway in hepatoma cells. Oncogene 2011, 30, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Mo, F.; Patel, G.; Butterworth, K.; Shao, C.; Prise, K.M. The Roles of HIF-1alpha in Radiosensitivity and Radiation-Induced Bystander Effects Under Hypoxia. Front. Cell Dev. Biol. 2021, 9, 637454. [Google Scholar] [CrossRef]
- Rizzo, R.; Parashuraman, S.; D’Angelo, G.; Luini, A. GOLPH3 and oncogenesis: What is the molecular link? Tissue Cell 2017, 49, 170–174. [Google Scholar] [CrossRef]
- Buschman, M.D.; Rahajeng, J.; Field, S.J. GOLPH3 links the Golgi, DNA damage, and cancer. Cancer Res. 2015, 75, 624–627. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, P.; Yang, M.; Shi, H.; Lu, D.; Wang, Z.; Shi, Q.; Hu, J.; Xie, S.; Zhan, W.; et al. Protein kinase D2 promotes the proliferation of glioma cells by regulating Golgi phosphoprotein 3. Cancer Lett. 2014, 355, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhuang, J.; Deng, Y.; Yang, L.; Cao, W.; Chen, W.; Lin, T.; Lv, X.; Yu, H.; Xue, Y.; et al. miR34a/GOLPH3 Axis abrogates Urothelial Bladder Cancer Chemoresistance via Reduced Cancer Stemness. Theranostics 2017, 7, 4777–4790. [Google Scholar] [CrossRef]
- Li, T.; You, H.; Zhang, J.; Mo, X.; He, W.; Chen, Y.; Tang, X.; Jiang, Z.; Tu, R.; Zeng, L.; et al. Study of GOLPH3: A potential stress-inducible protein from Golgi apparatus. Mol. Neurobiol. 2014, 49, 1449–1459. [Google Scholar] [CrossRef]
- Li, T.; You, H.; Mo, X.; He, W.; Tang, X.; Jiang, Z.; Chen, S.; Chen, Y.; Zhang, J.; Hu, Z. GOLPH3 Mediated Golgi Stress Response in Modulating N2A Cell Death upon Oxygen-Glucose Deprivation and Reoxygenation Injury. Mol. Neurobiol. 2016, 53, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ke, Z.F.; Wang, F.; Zhang, W.H.; Wang, Y.F.; Li, S.H.; Wang, L.T. GOLPH3 overexpression is closely correlated with poor prognosis in human non-small cell lung cancer and mediates its metastasis through upregulating MMP-2 and MMP-9. Cell. Physiol. Biochem. 2015, 35, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhan, W.; Bian, W.; Hua, L.; Shi, Q.; Xie, S.; Yang, D.; Li, Y.; Zhang, X.; Liu, G.; et al. GOLPH3 regulates the migration and invasion of glioma cells though RhoA. Biochem. Biophys. Res. Commun. 2013, 433, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Banerjee, P.; Pham, E.A.; Rutaganira, F.U.N.; Basu, K.; Bota-Rabassedas, N.; Guo, H.F.; Grzeskowiak, C.L.; Liu, X.; Yu, J.; et al. PI4KIIIβ is a therapeutic target in chromosome 1q-amplified lung adenocarcinoma. Sci. Transl. Med. 2020, 12, eaax3772. [Google Scholar] [CrossRef]
- Hu, P.; Wang, K.; Zhou, D.; Wang, L.; Zhao, M.; Wang, W.; Zhang, Y.; Liu, Y.; Yu, R.; Zhou, X. GOLPH3 Regulates Exosome miRNA Secretion in Glioma Cells. J. Mol. Neurosci. 2020, 70, 1257–1266. [Google Scholar] [CrossRef]
- Song, J.W.; Zhu, J.; Wu, X.X.; Tu, T.; Huang, J.Q.; Chen, G.Z.; Liang, L.Y.; Zhou, C.H.; Xu, X.; Gong, L.Y. GOLPH3/CKAP4 promotes metastasis and tumorigenicity by enhancing the secretion of exosomal WNT3A in non-small-cell lung cancer. Cell Death Dis. 2021, 12, 976. [Google Scholar] [CrossRef]
- Chen, G.; Kong, P.; Yang, M.; Hu, W.; Prise, K.M.; Yu, K.N.; Cui, S.; Qin, F.; Meng, G.; Almahi, W.A.; et al. Golgi Phosphoprotein 3 Confers Radioresistance via Stabilizing EGFR in Lung Adenocarcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 1216–1228. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, L.; Song, Y.; Zhao, X.; Chen, Q.; Pan, Y.; Zhang, J.; Bai, Y.; Zhang, H.; Shao, C. Radiation-induced abscopal reproductive effect is driven by TNF-α/p38 MAPK/Rac1 axis in Sertoli cells. Theranostics 2021, 11, 5742–5758. [Google Scholar] [CrossRef]
- Tamminga, J.; Kovalchuk, O. Role of DNA damage and epigenetic DNA methylation changes in radiation-induced genomic instability and bystander effects in germline in vivo. Curr. Mol. Pharmacol. 2011, 4, 115–125. [Google Scholar] [CrossRef]
- Siva, S.; MacManus, M.P.; Martin, R.F.; Martin, O.A. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015, 356, 82–90. [Google Scholar] [CrossRef]
- Han, W.; Wu, L.; Chen, S.; Yu, K.N. Exogenous carbon monoxide protects the bystander Chinese hamster ovary cells in mixed coculture system after alpha-particle irradiation. Carcinogenesis 2010, 31, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Nie, L.; Yu, K.N.; Wu, L.; Kong, P.; Bao, L.; Chen, G.; Yang, H.; Han, W. Low Concentration of Exogenous Carbon Monoxide Modulates Radiation-Induced Bystander Effect in Mammalian Cell Cluster Model. Int. J. Mol. Sci. 2016, 17, 2051. [Google Scholar] [CrossRef] [PubMed]
- Konopacka, M.; Rzeszowska-Wolny, J. The bystander effect-induced formation of micronucleated cells is inhibited by antioxidants, but the parallel induction of apoptosis and loss of viability are not affected. Mutat. Res. 2006, 593, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Kumar, A.; Laskar, S.; Pandey, B.N. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine 2013, 61, 54–62. [Google Scholar] [CrossRef]
- Hu, W.; Xu, S.; Yao, B.; Hong, M.; Wu, X.; Pei, H.; Chang, L.; Ding, N.; Gao, X.; Ye, C.; et al. MiR-663 inhibits radiation-induced bystander effects by targeting TGFB1 in a feedback mode. RNA Biol. 2014, 11, 1189–1198. [Google Scholar] [CrossRef]
- Shao, C.; Folkard, M.; Prise, K.M. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene 2008, 27, 434–440. [Google Scholar] [CrossRef]
- Tubin, S.; Valeriani, M.; Salerno, G.; Bracci, S.; Stoppacciaro, A.; Cardelli, P.; Osti, M.F.; De Sanctis, V.; Minniti, G.; Maurizi Enrici, R. Manipulation of radiation-induced bystander effect in prostate adenocarcinoma by dose and tumor differentiation grade: In vitro study. Int. J. Radiat. Biol. 2015, 91, 166–171. [Google Scholar] [CrossRef]
- Zhou, H.; Ivanov, V.N.; Gillespie, J.; Geard, C.R.; Amundson, S.A.; Brenner, D.J.; Yu, Z.; Lieberman, H.B.; Hei, T.K. Mechanism of radiation-induced bystander effect: Role of the cyclooxygenase-2 signaling pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 14641–14646. [Google Scholar] [CrossRef] [PubMed]
- Shareef, M.M.; Cui, N.; Burikhanov, R.; Gupta, S.; Satishkumar, S.; Shajahan, S.; Mohiuddin, M.; Rangnekar, V.M.; Ahmed, M.M. Role of tumor necrosis factor-alpha and TRAIL in high-dose radiation-induced bystander signaling in lung adenocarcinoma. Cancer Res. 2007, 67, 11811–11820. [Google Scholar] [CrossRef]
- Ansari, R.; Gaber, M.W.; Wang, B.; Pattillo, C.B.; Miyamoto, C.; Kiani, M.F. Anti-TNFA (TNF-alpha) treatment abrogates radiation-induced changes in vacular density and tissue oxygenation. Radiat. Res. 2007, 167, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qi, K.; Wang, Z.; Gu, M.; Chen, G.; Guo, F.; Wang, Z. Golgi phosphoprotein 3 regulates metastasis of prostate cancer via matrix metalloproteinase 9. Int. J. Clin. Exp. Pathol. 2015, 8, 3691–3700. [Google Scholar] [PubMed]
- Zhou, Z.P.; Wang, L.P.; Hong, Z.S.; Qiu, C.Z.; Wang, M.Z.; Chen, Z.X.; Tang, L.F.; Yu, W.S.; Wang, C.X. Silencing GOLPH3 gene expression reverses resistance to cisplatin in HT29 colon cancer cells via multiple signaling pathways. Int. J. Oncol. 2018, 53, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Narang, H.; Sarma, A.; Krishna, M. DNA damage response signaling in lung adenocarcinoma A549 cells following gamma and carbon beam irradiation. Mutat. Res. 2011, 716, 10–19. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Y.J.; Liu, C.; Yang, Y.Y.; Liu, H.; Cui, J.G.; Cheng, Y.; Gao, F.; Cai, J.M.; Li, B.L. Inhibition of TBK1 attenuates radiation-induced epithelial-mesenchymal transition of A549 human lung cancer cells via activation of GSK-3beta and repression of ZEB1. Lab. Investig. 2014, 94, 362–370. [Google Scholar] [CrossRef]
- Gao, L.; Li, F.S.; Chen, X.H.; Liu, Q.W.; Feng, J.B.; Liu, Q.J.; Su, X. Radiation induces phosphorylation of STAT3 in a dose- and time-dependent manner. Asian Pac. J. Cancer Prev. 2014, 15, 6161–6164. [Google Scholar] [CrossRef]
- Shi, L.; Kishore, R.; McMullen, M.R.; Nagy, L.E. Lipopolysaccharide stimulation of ERK1/2 increases TNF-alpha production via Egr-1. Am. J. Physiol. Cell Physiol. 2002, 282, C1205–C1211. [Google Scholar] [CrossRef]
- Katz, S.; Zsiros, V.; Doczi, N.; Kiss, A.L. Inflammation-Induced Epithelial-to-Mesenchymal Transition and GM-CSF Treatment Stimulate Mesenteric Mesothelial Cells to Transdifferentiate into Macrophages. Inflammation 2018, 41, 1825–1834. [Google Scholar] [CrossRef]
- Chen, W.; Martindale, J.L.; Holbrook, N.J.; Liu, Y. Tumor promoter arsenite activates extracellular signal-regulated kinase through a signaling pathway mediated by epidermal growth factor receptor and Shc. Mol. Cell. Biol. 1998, 18, 5178–5188. [Google Scholar] [CrossRef]
- Knebel, A.; Rahmsdorf, H.J.; Ullrich, A.; Herrlich, P. Dephosphorylation of receptor tyrosine kinases as target of regulation by radiation, oxidants or alkylating agents. EMBO J. 1996, 15, 5314–5325. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, F.; Chen, G.; Yu, K.N.; Yang, M.; Cao, W.; Kong, P.; Peng, S.; Sun, M.; Nie, L.; Han, W. Golgi Phosphoprotein 3 Mediates Radiation-Induced Bystander Effect via ERK/EGR1/TNF-α Signal Axis. Antioxidants 2022, 11, 2172. https://doi.org/10.3390/antiox11112172
Qin F, Chen G, Yu KN, Yang M, Cao W, Kong P, Peng S, Sun M, Nie L, Han W. Golgi Phosphoprotein 3 Mediates Radiation-Induced Bystander Effect via ERK/EGR1/TNF-α Signal Axis. Antioxidants. 2022; 11(11):2172. https://doi.org/10.3390/antiox11112172
Chicago/Turabian StyleQin, Feng, Guodong Chen, Kwan Ngok Yu, Miaomiao Yang, Wei Cao, Peizhong Kong, Shengjie Peng, Mingyu Sun, Lili Nie, and Wei Han. 2022. "Golgi Phosphoprotein 3 Mediates Radiation-Induced Bystander Effect via ERK/EGR1/TNF-α Signal Axis" Antioxidants 11, no. 11: 2172. https://doi.org/10.3390/antiox11112172
APA StyleQin, F., Chen, G., Yu, K. N., Yang, M., Cao, W., Kong, P., Peng, S., Sun, M., Nie, L., & Han, W. (2022). Golgi Phosphoprotein 3 Mediates Radiation-Induced Bystander Effect via ERK/EGR1/TNF-α Signal Axis. Antioxidants, 11(11), 2172. https://doi.org/10.3390/antiox11112172






_Kwan_Ngok_Yu.png)

