Assessment of the Phytotoxic Potential of Dregea volubilis (L.f.) Benth. ex Hook.f. and Identification of its Phytotoxic Substances for Weed Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Plant Material
2.2. Bioassay
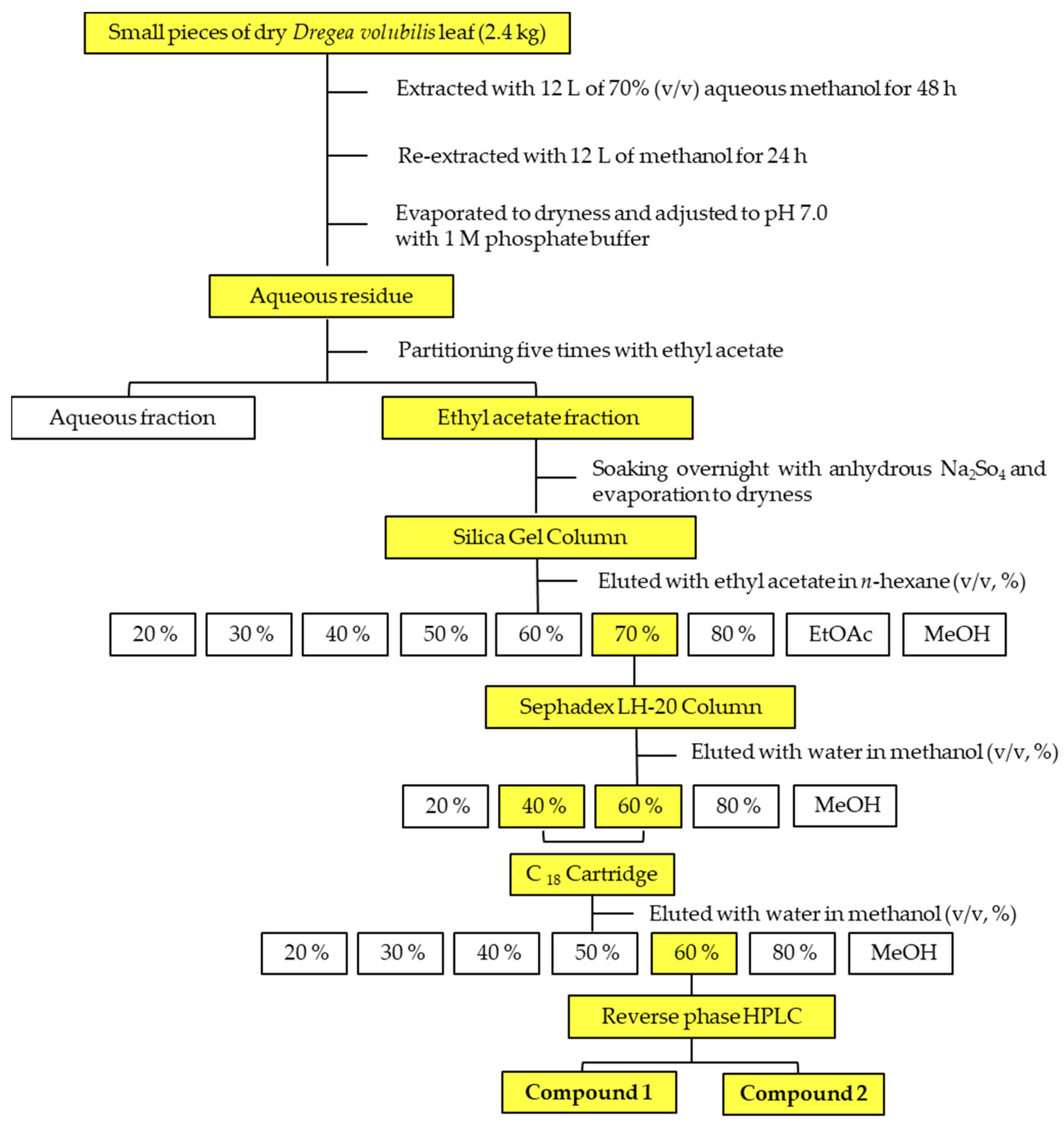
2.3. Separation of the Phytotoxic Substances in the D. volubilis Extracts
2.4. Bioassay of the Identified Compounds
2.5. Statistical Analysis
3. Results
3.1. Growth Inhibitory Effects of the D. volubilis Extracts
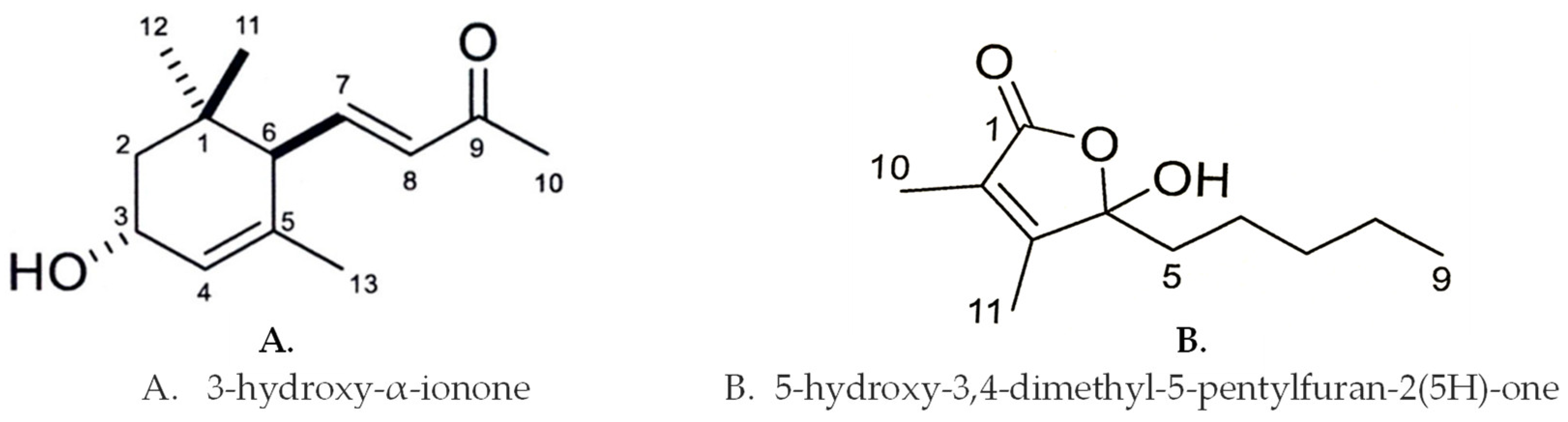
3.2. Identification of the Growth Inhibitory Substances
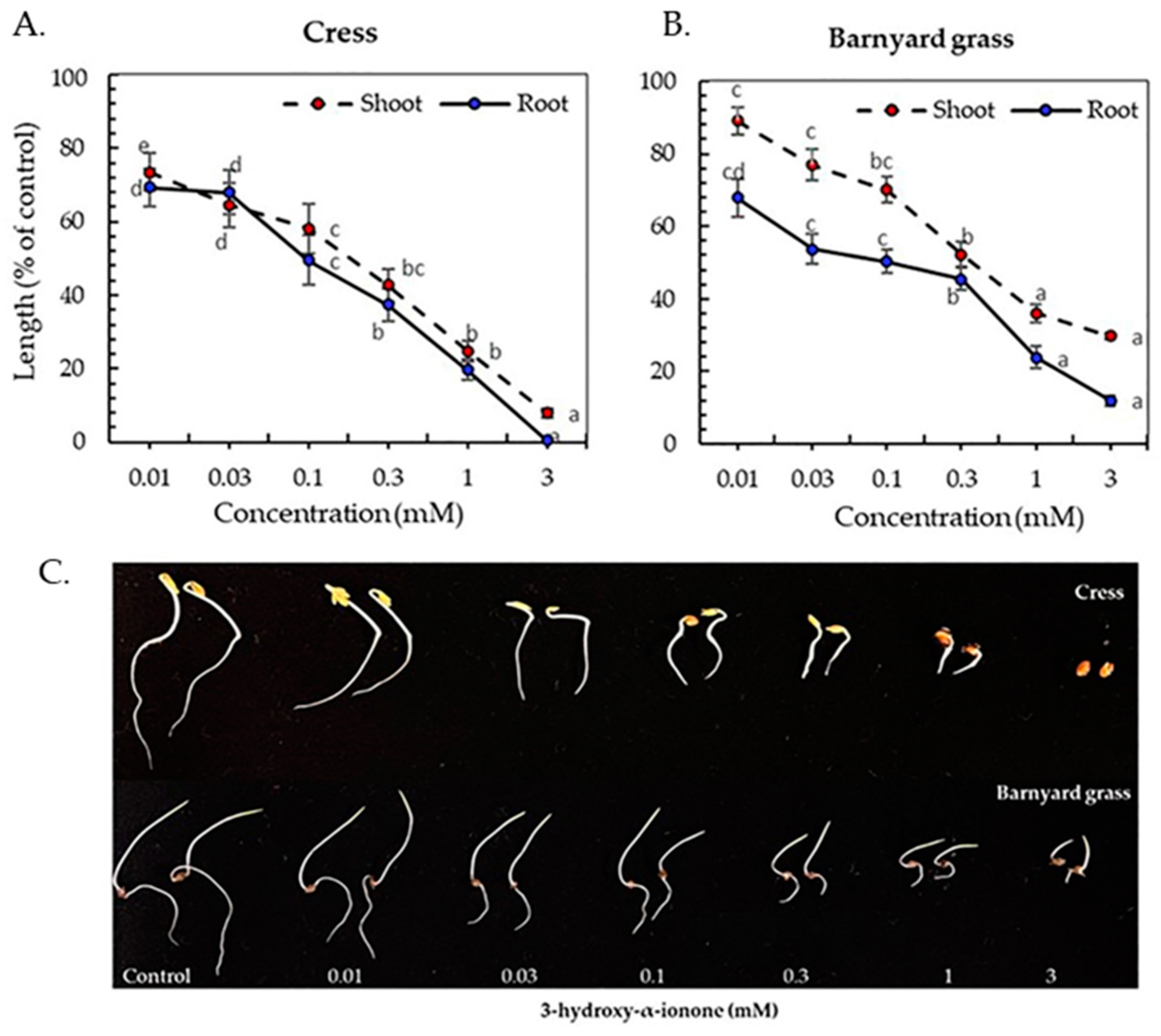
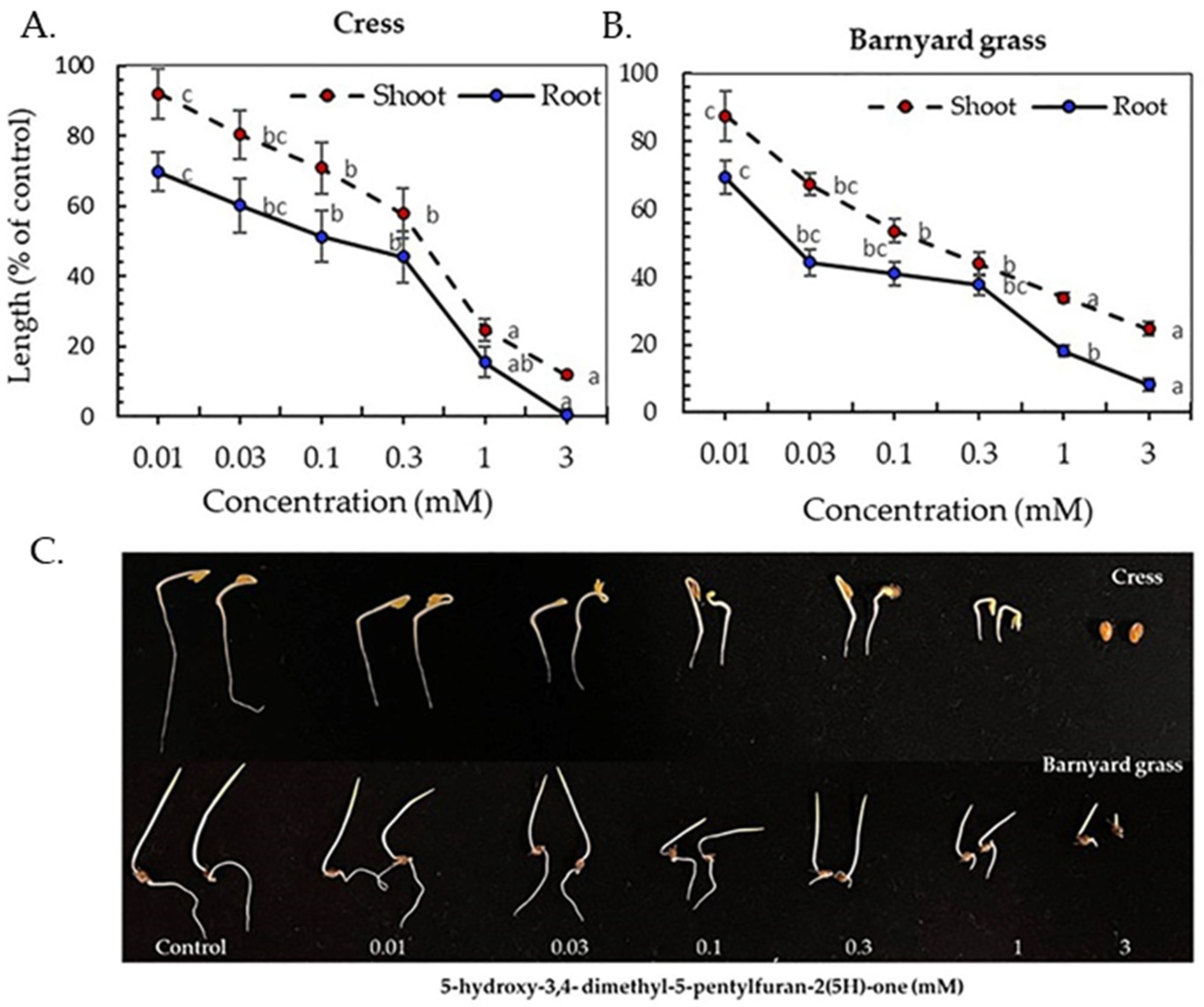
3.3. Inhibitory Activity of the Isolated Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, M.I.; Danish, S.; Sánchez-Moreiras, A.M.; Vicente, Ó.; Jabran, K.; Chaudhry, U.K.; Branca, F.; Reigosa, M.J. Unraveling sorghum allelopathy in agriculture: Concepts and implications. Plants 2021, 10, 1795. [Google Scholar] [CrossRef] [PubMed]
- Wato, T. The role of allelopathy in pest management and crop production-A review. Food Sci. Qual. Manag. 2020, 93, 13–21. [Google Scholar]
- Farooq, N.; Abbas, T.; Tanveer, A.; Jabran, K. Allelopathy for weed management. Co-Evol. Second. Metab. 2020, 505–519. [Google Scholar] [CrossRef]
- Mallik, M.A.B.; Williams, R.D. Allelopathic principles for sustainable agriculture. Allelopath. J. 2009, 24, 1–34. [Google Scholar]
- Ash, G.J. The science, art and business of successful bioherbicides. Biol. Control. 2010, 52, 230–240. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef]
- Ben Ghnaya, A.; Hamrouni, L.; Amri, I.; Ahoues, H.; Hanana, M.; Romane, A. Study of allelopathic effect of Eucalyptus erythrocorys L. crude extracts against germination and seedling growth of weeds and wheat. Nat. Prod. Res. 2016, 30, 2058–2064. [Google Scholar] [CrossRef]
- Appiah, K.; Mardani, H.; Omari, R.; Eziah, V.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Fujii, Y. Involvement of carnosic acid in the phytotoxicity of Rosmarinus officinalis leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef]
- Moreno-Robles, A.; Cala Peralta, A.; Soriano, G.; Zorrilla, J.G.; Masi, M.; Vilariño-Rodríguez, S.; Cimmino, A.; Fernández-Aparicio, M. Identification of Allelochemicals with Differential Modes of Phytotoxicity against Cuscuta campestris. Agriculture 2022, 12, 1746. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Moein, M.; Khoshghalb, H. Phytotoxic effects of several essential oils on two weed species and tomato. Biocatal. Agric. Biotechnol. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Salam, M.A.; Ohno, O.; Suenaga, K. Nimbolide B and nimbic acid B, phytotoxic substances in neem leaves with allelopathic activity. Molecules 2014, 19, 6929–6940. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.K.M.M.; Yeasmin, S.; Qasem, J.R.S.; Juraimi, A.S.; Anwar, P. Allelopathy of medicinal plants: Current status and future prospects in weed management. Agric. Sci. 2018, 9, 1569–1588. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelopathic substances of mango (Mangifera indica L.). Weed Biol. Manag. 2020, 20, 131–138. [Google Scholar] [CrossRef]
- Lun, T.L.; Kato-Noguchi, H. Assessment of the allelopathic potential of Leucas cephalotes (Roth) Spreng. extracts on the seedling growth of six test plants. Plant Omics. 2021, 14, 72–77. [Google Scholar]
- Moh, S.M.; Kato-Noguchi, H. Efficacy of Ochna integerrima (Lour.) Merr leaf extracts against seedling growth of six important plants. Aust. J. Crop Sci. 2022, 16, 555–561. [Google Scholar]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of allelopathic potential of Senna garrettiana leaves and identification of potent phytotoxic substances. Agronomy 2022, 12, 139. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic Effects of Senna garrettiana and Identification of Phytotoxic Substances for the Development of Bioherbicides. Agriculture 2022, 12, 1338. [Google Scholar] [CrossRef]
- Hossain, E.; Chakroborty, S.; Milan, A.; Chattopadhyay, P.; Mandal, S.C.; Gupta, J.K. In vitro and in vivo antitumor activity of a methanol extract of Dregea volubilis leaves with its antioxidant effect. Pharm. Biol. 2012, 50, 338–343. [Google Scholar] [CrossRef]
- Sreeramulu, N.; Suthari, S.; Ragan, A.; Raju, V.S. Ethno-botanico-medicine for common human ailments in Nalgonda and Warangal districts of Telangana, Andhra Pradesh, India. Ann Plant Sci. 2013, 2, 220–229. [Google Scholar]
- Suwitchayanon, P.; Kunasakdakul, K.; Kato-Noguchi, H. Screening the allelopathic activity of 14 medicinal plants from northern Thailand. Environ. Control Biol. 2017, 55, 143–145. [Google Scholar] [CrossRef][Green Version]
- Shankar, K.R.; Das, S.; Bujala, P. Phytochemical screening and in vitro antibacterial activity of ethanol and aqueous extracts of Dregea volubilis leaves. Biosci. Biotechnol. Res. Asia 2010, 7, 975–979. [Google Scholar]
- Purushoth, P.T.; Maheswaran, V.S.; Selvakumari, S.; Suriyapadminimoka, R.S.; Dileep, G. An antioxidant and anti- bacterial activity of Dregea volubilis leaves extract. Pharm. Lett. 2012, 4, 525–529. [Google Scholar]
- Natarajan, V.; Dhas, A.S.A.G. Effect of active fraction isolated from the leaf extract of Dregea volubilis [Linn.] Benth. on plasma glucose concentration and lipid profile in streptozotocin-induced diabetic rats. Springer Plus 2013, 2, 394. [Google Scholar] [CrossRef] [PubMed]
- Moulisha, B.; Bikash, M.N.; Partha, P.; Kumar, G.A.; Sukdeb, B.; Kanti, H.P. In vitro anti-leishmanial and anti-tumour activities of a pentacyclic triterpenoid compound isolated from the fruits of Dregea volubilis Benth Asclepiadaceae. Trop. J. Pharm. Res. 2009, 8, 2. [Google Scholar] [CrossRef]
- Sahu, N.P.; Panda, N.; Mandal, N.B.; Banerjee, S.; Koike, K.; Nikaido, T. Polyoxypregnane glycosides from the flowers of Dregea volubilis. Phytochemistry 2002, 61, 383–388. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathy of the medicinal plant Dregea volubilis (L.f.) Benth. ex Hook.f. and its phytotoxic substances with allelopathic activity. Agronomy 2022, 12, 303. [Google Scholar] [CrossRef]
- D'Abrosca, B.; Marina, D.; Antonio, F.; Pietro, M.; Palma, O.; Fabio, T. Structure elucidation and phytotoxicity of C13 nor-isoprenoids from Cestrum parqui. Phytochem. 2004, 65, 497–505. [Google Scholar] [CrossRef]
- Chen, L.; Shunsuke, I.; Diana, I.; Tomoko, I.; Ryoichi, U.; Toshifumi, H. Secretion of alleochemicals from the cultured suspension cells of Marchantia polymorpha. Chem. Lett. 1996, 3, 205–206. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Kato-Noguchi, H. Plant growth inhibitory activity of medicinal plant Hyptis suaveolens: Could allelopathy be a cause? Emir. J. Food Agric. 2013, 25, 692–701. [Google Scholar] [CrossRef]
- Bari, I.N.; Kato-Noguchi, H.; Iwasaki, A.; Suenaga, K. Allelopathic potency and an active substance from Anredera cordifolia (Tenore) Steenis. Plants 2019, 8, 134. [Google Scholar] [CrossRef]
- Boonmee, S.; Suwitchayanon, P.; Krumsri, R.; Kato-Noguchi, H. Investigation of the allelopathic potential of Nephrolepis cordifolia (L.) C. Presl against dicotyledonous and monocotyledonous plant species. Environ. Control. Biol. 2020, 58, 71–78. [Google Scholar] [CrossRef]
- Moh, S.M.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic activity of a novel compound, 5,6-dihydrogen-11α-O-acetyl-12β-O-tigloyl-17β-marsdenin, and a known steroidal glycoside from the leaves of Marsdenia tenacissima (Roxb.) Moon. Agronomy 2022, 12, 1536. [Google Scholar] [CrossRef]
- Lun, T.L.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Two allelopathic substances from Plumbago rosea stem extracts and their allelopathic effects. Agronomy 2022, 12, 2020. [Google Scholar] [CrossRef]
- El-Mergawi, R.; El-Desoki, E.R. Allelopathic activities of celery extract and its fractions against Corchorus olitorius, Echinochloa crusgalli and Portulaca oleracea weeds. Adv. Hortic. Sci. 2018, 32, 503–510. [Google Scholar]
- Rob, M.M.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Schumannione, a new butenolide derivative isolated from Schumannianthus dichotomus as a potential phytotoxic agent. Tetrahedron Lett. 2020, 61, 152–168. [Google Scholar] [CrossRef]
- Hossen, K.; Ozaki, K.; Teruya, T.; Kato-Noguchi, H. Three active phytotoxic compounds from the leaves of Albizia richardiana (Voigt.) King and Prain for the development of bioherbicides to control weeds. Cells 2021, 10, 2385. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Kato-Noguchi, H. Assessment of allelopathic activity of Tradescantia spathacea Sw. for weed control. Biol. Futur. 2021, 72, 489–495. [Google Scholar] [CrossRef]
- Pabst, A.; Barron, D.; Semont, E.; Schreier, P. A 4-hydroxy-β-ionone disaccharide glycoside from raspberry fruits. Phytochemistry 1992, 31, 3105–3107. [Google Scholar] [CrossRef]
- Das, K.R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Evaluation of phytotoxic potential and identification of phytotoxic substances in Cassia alata Linn. leaves. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 479–488. [Google Scholar] [CrossRef]
- Lutz-Wahl, S.; Fischer, P.; Schmidt-Dannert, C.; Wohlleben, W.; Hauer, B.; Schmid, R.D. Stereo- and regioselective hydroxylation of alpha-ionone by Streptomyces strains. Appl. Environ. Microbiol. 1998, 64, 3878–3881. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Seki, T. Allelopathy of the moss Rhynchostegium pallidifolium and 3-hydroxy-β-ionone. Plant Signal. Behav. 2010, 5, 702–704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xue, X.; Yao, G. A review on chemical constituents and bioactivities of Viburnum odoratissimum. Asian J. Tradit. Med. 2020, 15, 263–287. [Google Scholar]
- Yin, X.; Zhou, Y.; Zhang, S.; Zhou, Y. A new organic acid derivative from the fruits of Rosa roxburghii. Rec. Nat. Prod. 2021, 16, 264–267. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; p. 368. [Google Scholar]
- Liu, J.; Xie, M.; Li, X.; Jin, H.; Yang, X.; Yan, Z.; Su, A.; Qin, B. Main allelochemicals from the rhizosphere soil of Saussurea lappa (Decne.) Sch. Bip. and their effects on plants’ antioxidase systems. Molecules 2018, 23, 2506. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Nakamura, K.; Ohno, O.; Suenaga, K.; Okuda, N. Asparagus decline: Autotoxicity and autotoxic compounds in asparagus rhizomes. J. Plant Physiol. 2017, 213, 23–29. [Google Scholar] [CrossRef]
- Rob, M.M.; Hossen, K.; Khatun, M.R.; Iwasaki, K.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Identification and application of bioactive compounds from Garcinia xanthochymus Hook. for weed management. Appl. Sci. 2021, 11, 2264. [Google Scholar] [CrossRef]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Phytotoxic activity of Clerodendrum indicum (L.) Kuntze and its potential phytotoxic substance. Emir. J. Food Agric. 2021, 33, 884–892. [Google Scholar]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Dayan, F.E.; Romagni, J.G.; Duke, S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000, 26, 2079–2094. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, D.; Cui, H.; Zhang, D.; Sun, Y.; Jin, H.; Li, X.; Yang, X.; Guo, H.; He, X.; et al. Phytotoxicity mechanisms of two coumarin allelochemicals from Stellera chamaejasme in lettuce seedlings. Acta Physiol. Plant. 2016, 38, 248. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Muras, M.; Puig, C.G.; Pedrol, N. Complex Synergistic Interactions among Volatile and Phenolic Compounds Underlie the Effectiveness of Allelopathic Residues Added to the Soil for Weed Control. Plants 2022, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Sosa, T.; Alias, J.C.; Escudero, J.C. Identification and effects of interaction phytotoxic compounds from exudate of Cistus ladanifer leaves. J. Chem. Ecol. 2001, 27, 611–621. [Google Scholar] [CrossRef] [PubMed]

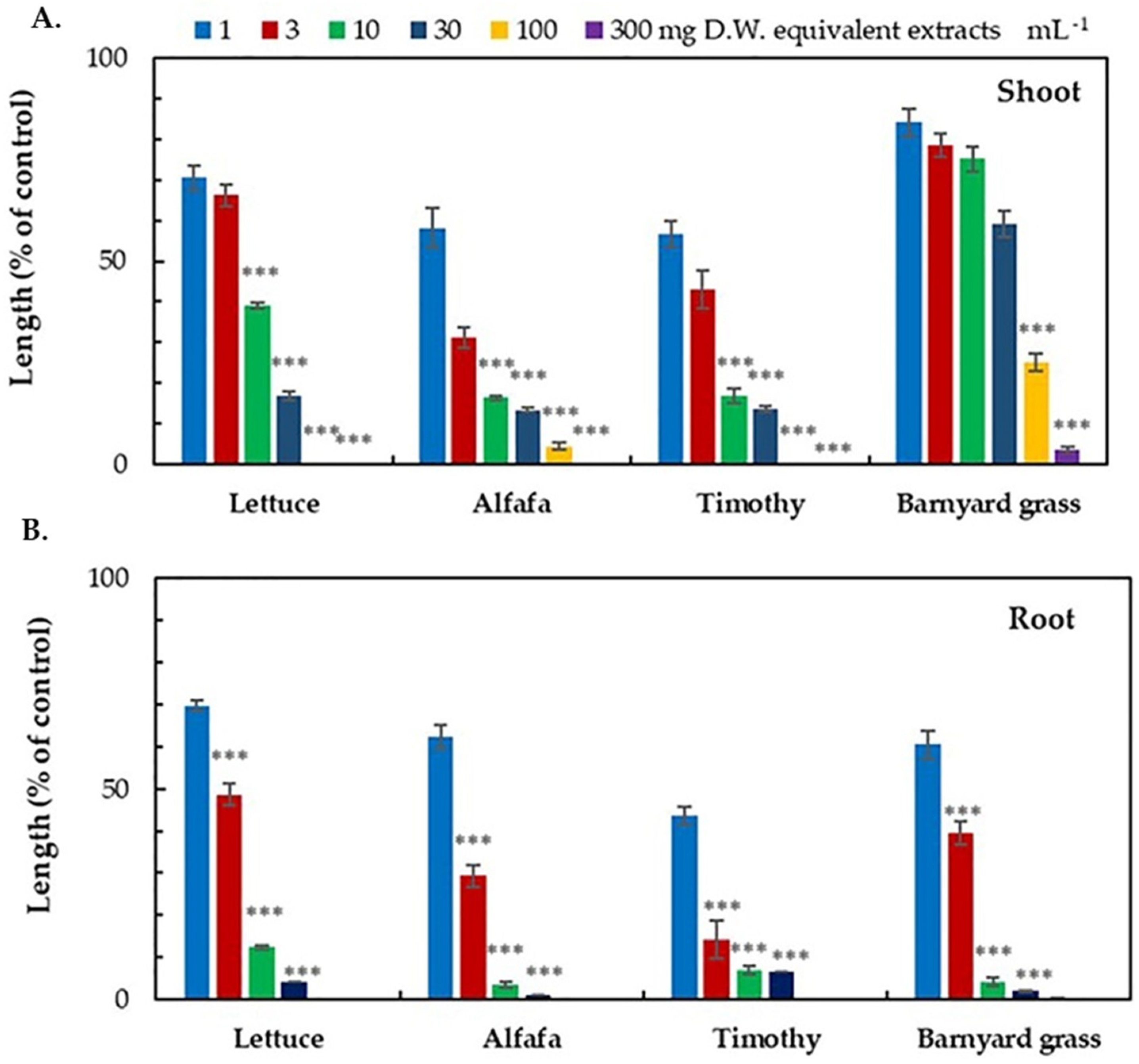
| Test Plant | I50 Values (mg DW Equivalent Extract mL−1) | ||
|---|---|---|---|
| Shoot | Root | ||
| Dicotyledonous | Alfalfa | 1.64 d | 1.49 d |
| Lettuce | 4.93 b | 2.98 c | |
| Monocotyledonous | Barnyard grass | 43.09 a | 4.97 b |
| Timothy | 2.16 c, d | 1.80 d | |
| Plant Species | I50 Value (mM) Compound 1 | I50 Value (mM) Compound 2 | ||
|---|---|---|---|---|
| Shoot | Root | Shoot | Root | |
| Cress | 0.26 b | 0.13 c, d | 0.42 a | 0.18 c |
| Barnyard grass | 0.45 a | 0.10 d | 0.19 c | 0.03 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of the Phytotoxic Potential of Dregea volubilis (L.f.) Benth. ex Hook.f. and Identification of its Phytotoxic Substances for Weed Control. Agriculture 2022, 12, 1826. https://doi.org/10.3390/agriculture12111826
Kyaw EH, Iwasaki A, Suenaga K, Kato-Noguchi H. Assessment of the Phytotoxic Potential of Dregea volubilis (L.f.) Benth. ex Hook.f. and Identification of its Phytotoxic Substances for Weed Control. Agriculture. 2022; 12(11):1826. https://doi.org/10.3390/agriculture12111826
Chicago/Turabian StyleKyaw, Ei Han, Arihiro Iwasaki, Kiyotake Suenaga, and Hisashi Kato-Noguchi. 2022. "Assessment of the Phytotoxic Potential of Dregea volubilis (L.f.) Benth. ex Hook.f. and Identification of its Phytotoxic Substances for Weed Control" Agriculture 12, no. 11: 1826. https://doi.org/10.3390/agriculture12111826
APA StyleKyaw, E. H., Iwasaki, A., Suenaga, K., & Kato-Noguchi, H. (2022). Assessment of the Phytotoxic Potential of Dregea volubilis (L.f.) Benth. ex Hook.f. and Identification of its Phytotoxic Substances for Weed Control. Agriculture, 12(11), 1826. https://doi.org/10.3390/agriculture12111826






