Thioterpenoids as Potential Antithrombotic Drugs: Molecular Docking, Antiaggregant, Anticoagulant and Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
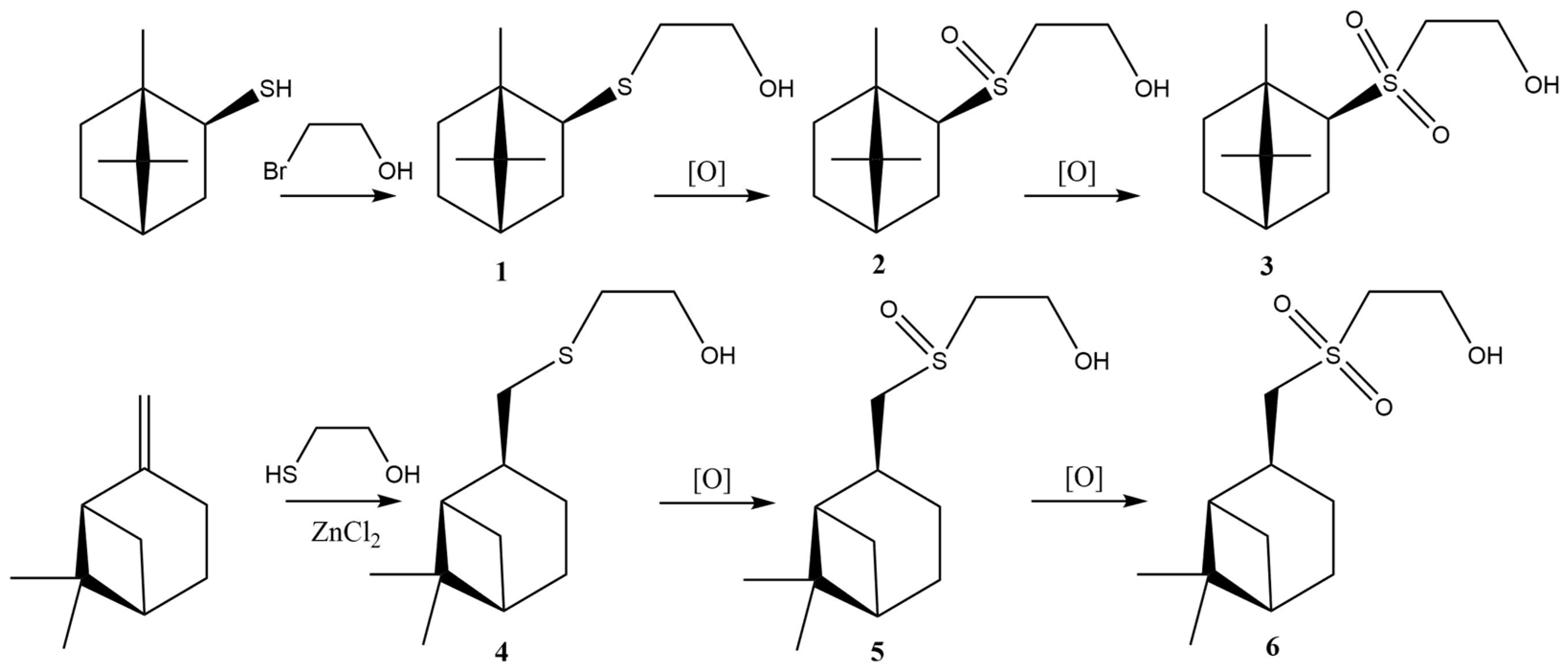
2.1. Synthesis
2.1.1. Sulfide 1
2.1.2. Sulfide 4
2.1.3. General Procedure for Sulfoxides 2 and 5 Synthesis
2.1.4. General Procedure for Sulfones 3 and 6 Synthesis
2.2. Molecular Docking
2.3. Biochemical Assays
2.3.1. TBARS Assay
2.3.2. Mouse RBC Test for Erythrotoxicity, Antioxidant and Membrane-Protective Activities
2.3.3. Antiaggregant and Anticoagulant Activities
3. Results
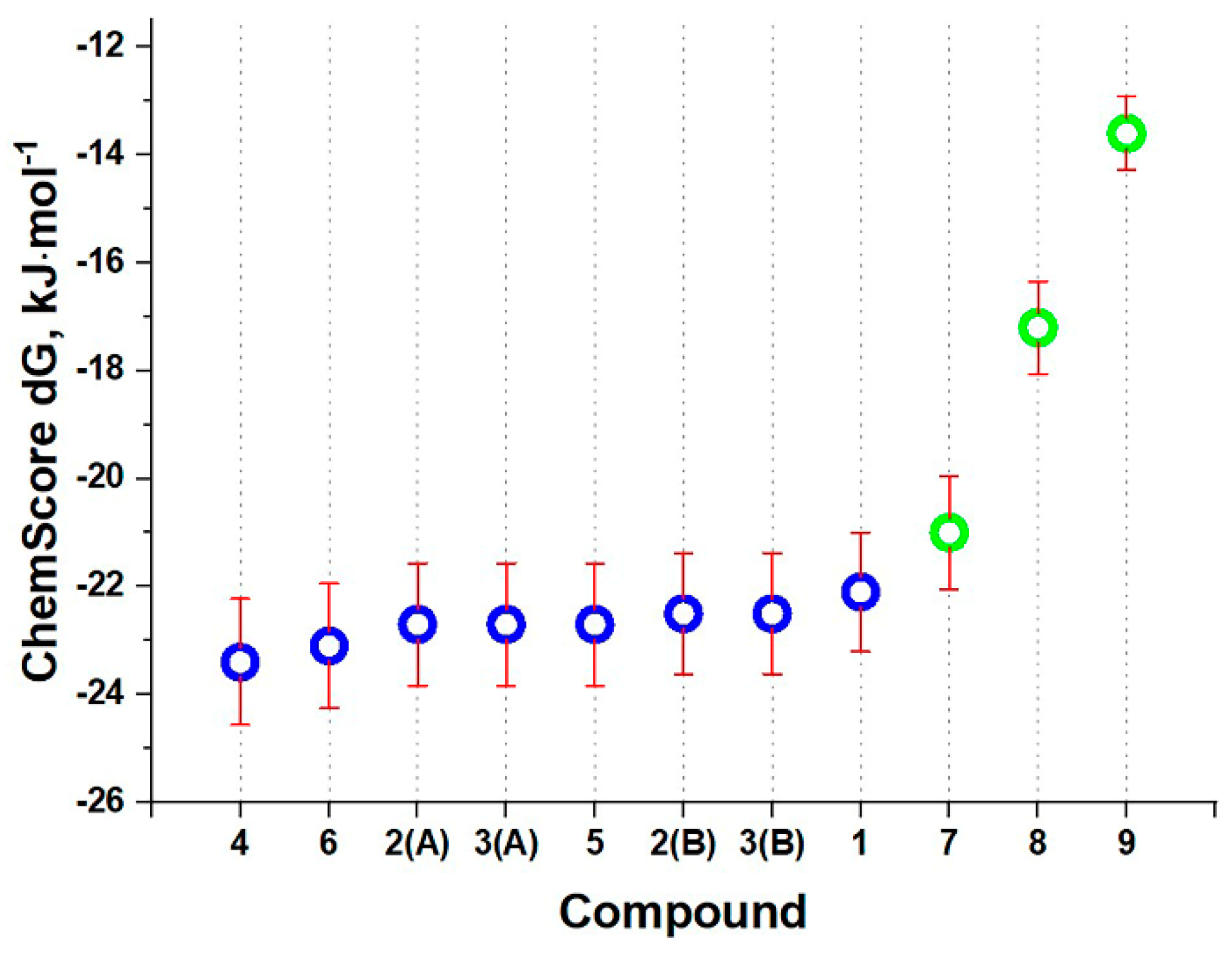
3.1. Molecular Docking Study
3.2. Antioxidant Activity
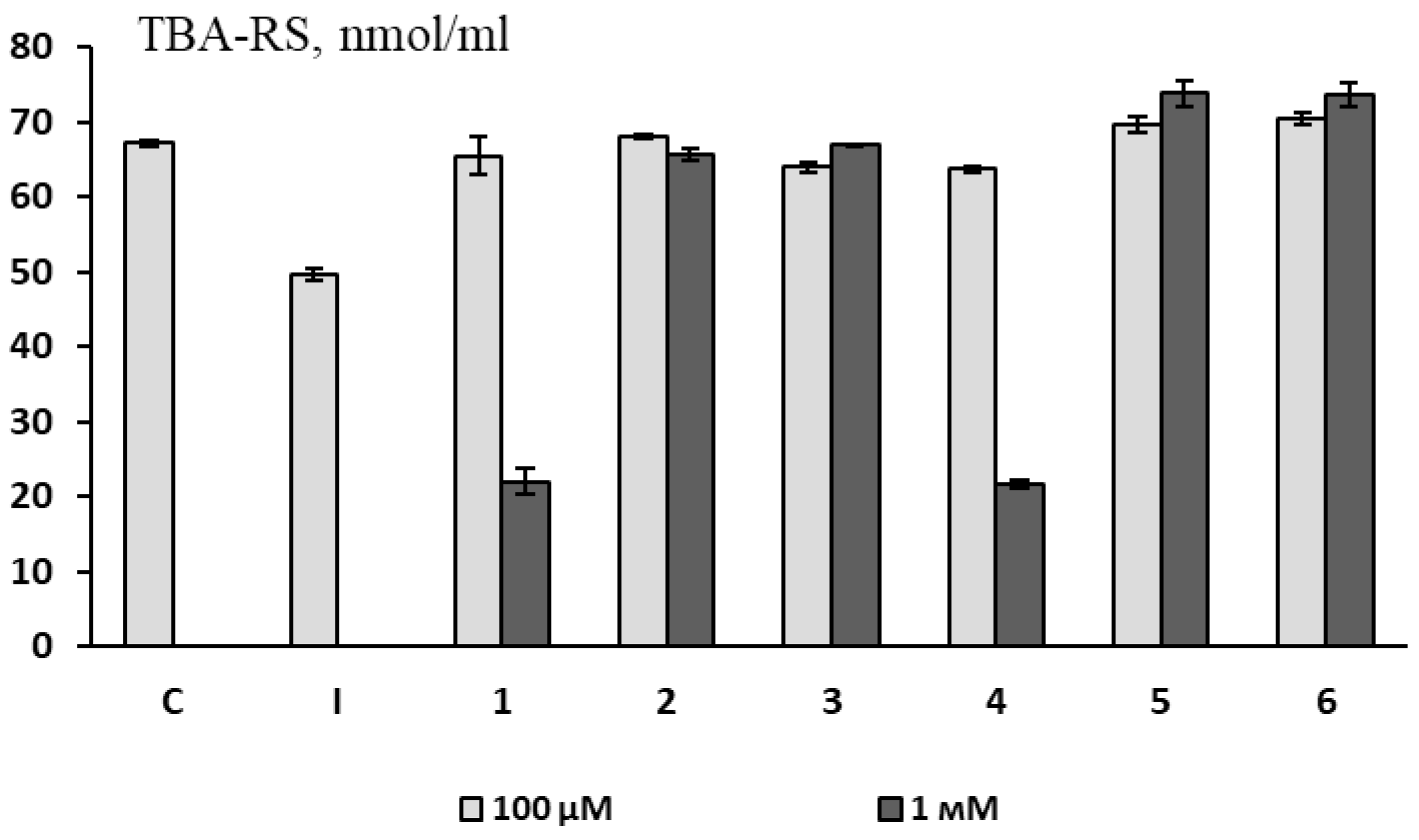
3.2.1. Non-Cellular Model (Antioxidant Activity in a Substrate Containing Animal Brain Lipids)
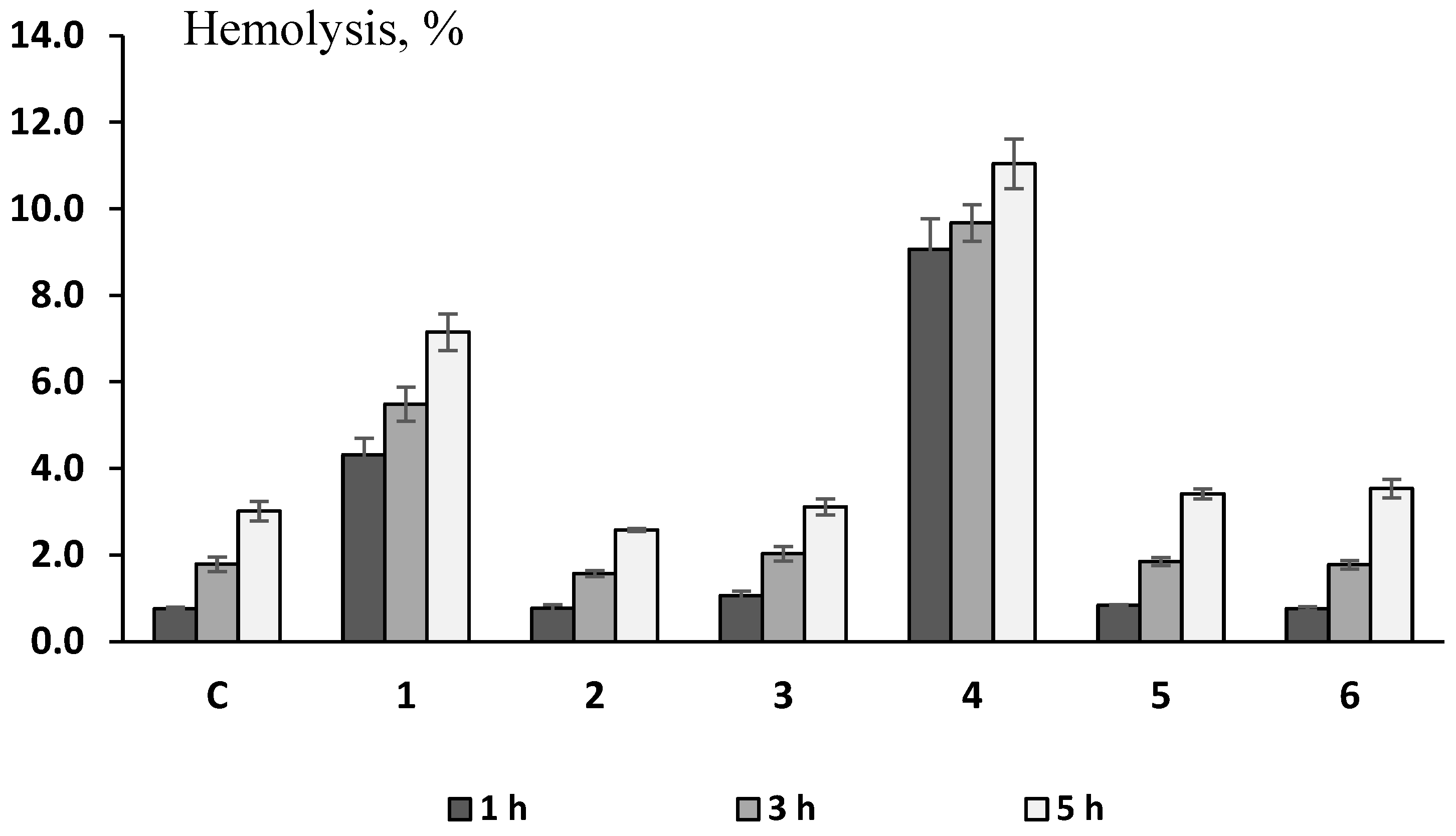
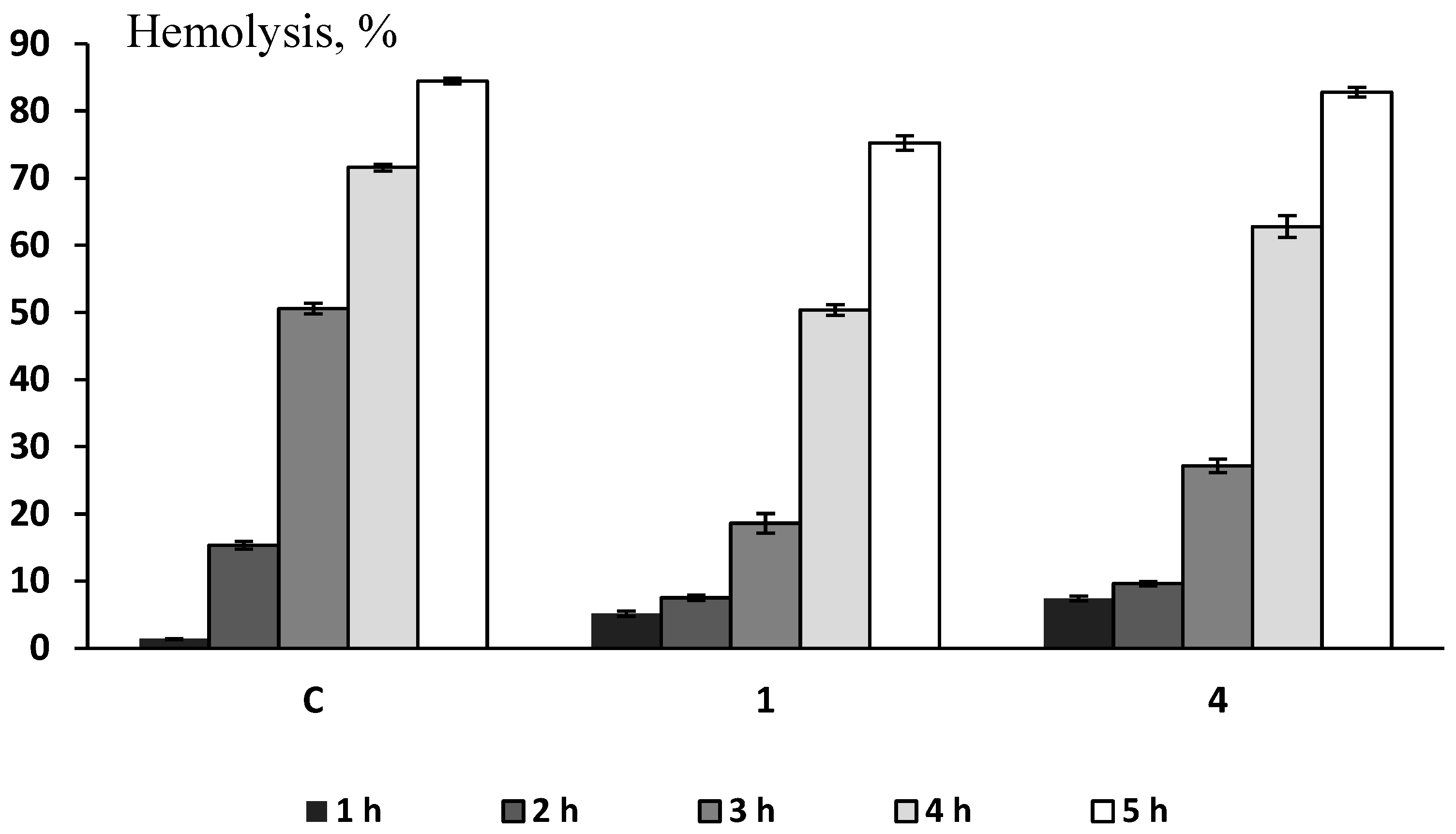
3.2.2. Mouse RBC Test for Erythrotoxicity, Antioxidant and Membrane-Protective Activities
3.3. Antiaggregant and Anticoagulant Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and Their Derivatives—Recent Development in Biological and Medical Applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Moreira, F.; Fraga, B.; Sousa, D.; Bonjardim, L.; Quintans-Júnior, L. Cardiovascular effects of monoterpenes: A review. Rev. Bras. Farmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Ishmuratov, G.Y.; Yakovleva, M.P.; Tukhvatshin, V.S.; Talipov, R.F.; Nikitina, L.E.; Artemova, N.P.; Startseva, V.A.; Tolstikov, A.G. Sulfur-Containing Derivatives of Mono- and Bicyclic Natural Monoterpenoids. Chem. Nat. Compd. 2014, 50, 22–47. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Pavelyev, R.S.; Gilfanov, I.R.; Kiselev, S.V.; Azizova, Z.R.; Ksenofontov, A.A.; Bocharov, P.S.; Antina, E.V.; Klochkov, V.V.; Timerova, A.F.; et al. Unraveling the Mechanism of Platelet Aggregation Suppression by Monoterpenoids. Bioengineering 2022, 9, 24. [Google Scholar] [CrossRef]
- Wickens, A.P. Ageing and the Free Radical Theory. Respir. Physiol. 2001, 128, 379–391. [Google Scholar] [CrossRef]
- Lodochnikova, O.A.; Islamov, D.R.; Gerasimova, D.P.; Zakharychev, D.V.; Saifina, A.F.; Pestova, S.V.; Izmest’ev, E.S.; Rubtsova, S.A.; Pavelyev, R.S.; Rakhmatullin, I.Z. Isobornanyl Sulfoxides and Isobornanyl Sulfone: Physicochemical Characteristics and the Features of Crystal Structure. J. Mol. Struct. 2021, 1239, 130491. [Google Scholar] [CrossRef]
- Gavrilov, V.V.; Startseva, V.A.; Nikitina, L.E.; Lodochnikova, O.A.; Gnezdilov, O.I.; Lisovskaya, S.A.; Glushko, N.I.; Klimovitskii, E.N. Synthesis and Antifungal Activity of Sulfides, Sulfoxides, and Sulfones Based on (1S)-(-)-β-Pinene. Pharm. Chem. J. 2010, 44, 126–129. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Kiselev, S.V.; Startseva, V.A.; Bodrov, A.V.; Azizova, Z.R.; Shipina, O.T.; Fedyunina, I.V.; Boichuk, S.V.; Lodochnikova, O.A.; Klochkov, V.V.; et al. Sulfur-Containing Monoterpenoids as Potential Antithrombotic Drugs: Research in the Molecular Mechanism of Coagulation Activity Using Pinanyl Sulfoxide as an Example. Front. Pharmacol. 2018, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Lodochnikova, O.A.; Startseva, V.A.; Nikitina, L.E.; Bodrov, A.V.; Klimovitskii, A.E.; Klimovitskii, E.N.; Litvinov, I.A. When Two Symmetrically Independent Molecules Must Be Different: “Crystallization-Induced Diastereomerization” of Chiral Pinanyl Sulfone. CrystEngComm 2014, 16, 4314–4321. [Google Scholar] [CrossRef]
- Solis, F.J.; Wets, R.J.-B. Minimization by Random Search Techniques. Math. Oper. Res. 1981, 6, 19–30. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748.19. [Google Scholar] [CrossRef] [Green Version]
- Acker, C.I.; Brandão, R.; Rosário, A.R.; Nogueira, C.W. Antioxidant Effect of Alkynylselenoalcohol Compounds on Liver and Brain of Rats in Vitro. Environ. Toxicol. Pharmacol. 2009, 28, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, S.T.; Prestes, A.S.; Ogunmoyole, T.; Salman, S.M.; Schwab, R.S.; Brender, C.R.; Dornelles, L.; Rocha, J.B.T.; Soares, F.A.A. Evaluation of in Vitro Antioxidant Effect of New Mono and Diselenides. Toxicol. Vitr. 2013, 27, 1433–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J. Preliminary Evaluation for Comparative Antioxidant Activity in the Water and Ethanol Extracts of Dried Citrus Fruit (Citrus unshiu) Peel Using Chemical and Biochemical in Vitro Assays. Food Nutr. Sci. 2013, 4, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Chawla, R.; Arora, R.; Kumar, R.; Sharma, A.; Prasad, J.; Singh, S.; Sagar, R.; Chaudhary, P.; Shukla, S.; Kaur, G. Antioxidant Activity of Fractionated Extracts of Rhizomes of High-Altitude Podophyllum Hexandrum: Role in Radiation Protection. Mol. Cell. Biochem. 2005, 273, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Asakawa, T.; Matsushita, S. Coloring Conditions of Thiobarbituric Acid Test for Detecting Lipid Hydroperoxides. Lipids 1980, 15, 137–140. [Google Scholar] [CrossRef]
- Takebayashi, J.; Chen, J.; Tai, A. A Method for Evaluation of Antioxidant Activity Based on Inhibition of Free Radical-Induced Erythrocyte Hemolysis. In Advanced Protocols in Oxidative Stress II; Springer: Berlin/Heidelberg, Germany, 2010; pp. 287–296. [Google Scholar]
- Van den Berg, J.J.M.; den Kamp, J.A.F.O.; Lubin, B.H.; Roelofsen, B.; Kuypers, F.A. Kinetics and Site Specificity of Hydroperoxide-Induced Oxidative Damage in Red Blood Cells. Free. Radic. Biol. Med. 1992, 12, 487–498. [Google Scholar] [CrossRef]
- Classics Born, G.V.R. Aggregation of Blood Platelets by Adenosine Diphosphate and Its Reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef]
- Mironov, A.N. Handbook for Preclinical Drug Trials [in Russian], In Guidelines for Conducting Preclinical Studies of Drugs. Part 1; GRIF-K: Moscow, Russia, 2012; 944p, ISBN 978-5-8125-17667-0. [Google Scholar]
- Hollopeter, G.; Jantzen, H.M.; Vincent, D.; Li, G.; England, L.; Ramakrishnan, V.; Yang, R.B.; Nurden, P.; Nurden, A.; Julius, D. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 2001, 409, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, M. The Platelet P2 Receptors in Inflammation. Hamost 2015, 35, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Luis, J.C.; Johnson, C.B. Seasonal Variations of Rosmarinic and Carnosic Acids in Rosemary Extracts. Analysis of Their in Vitro Antiradical Activity. Span. J. Agric. Res. 2005, 3, 106. [Google Scholar] [CrossRef] [Green Version]
- Sinha, M.; Manna, P.; Sil, P.C. Protective Effect of Arjunolic Acid against Arsenic-induced Oxidative Stress in Mouse Brain. J. Biochem. Mol. Toxicol. 2008, 22, 15–26. [Google Scholar] [CrossRef]
- Maleknia, S.D.; Adams, M.A. Reactions of Oxygen-Containing Terpenes with Peptides and Proteins. In Proceedings of the 4th International Peptide Symposium, Australian Peptide Association, Cairns, Australia, 21–25 October 2007; pp. 334–335. [Google Scholar]
- Nikitina, L.E.; Lisovskaya, S.A.; Startseva, V.A.; Frolova, L.L.; Kutchin, A.V.; Shevchenko, O.G.; Ostolopovskaya, O.V.; Pavelyev, R.S.; Khelkhal, M.A.; Gilfanov, I.R.; et al. Biological Activity of Bicyclic Monoterpene Alcohols. BionanoSci 2021, 11, 970–976. [Google Scholar] [CrossRef]
- Sudarikov, D.V.; Krymskaya, Y.V.; Melekhin, A.K.; Shevchenko, O.G.; Rubtsova, S.A. Synthesis and antioxidant activity of monoterpene nitrobenzylidenesulfenimines. Chem. Pap. 2021, 75, 2957–2963. [Google Scholar] [CrossRef]
- Sudarikov, D.V.; Gyrdymova, Y.V.; Borisov, A.V.; Lukiyanova, J.M.; Rumyantcev, R.V.; Shevchenko, O.G.; Baidamshina, D.R.; Zakarova, N.D.; Kayumov, A.R.; Sinegubova, E.O.; et al. Synthesis and Biological Activity of Unsymmetrical Monoterpenylhetaryl Disulfides. Molecules 2022, 27, 5101. [Google Scholar] [CrossRef]
- Shevchenko, O.G.; Plyusnina, S.N. Antioxidant effectiveness in the presence of uranyl ions at low concentrations in a model cell system. Biol. Membr. 2016, 33, 435–444. [Google Scholar] [CrossRef]



| Experiment 1 (H2O2-Induced Hemolysis) | |||
|---|---|---|---|
| Control | 2.14 ± 0.05 | 1.03 ± 0.07 | 0.87 ± 0.03 |
| 1 | 1.59 ± 0.06 ** | 0.99 ± 0.06 | 0.72 ± 0.03 * |
| 2 | 1.87 ± 0.05 ** | 1.34 ± 0.11 | 0.86 ± 0.04 |
| 3 | 1.85 ± 0.03 ** | 1.65 ± 0.10 | 0.83 ± 0.05 |
| Experiment 2 (H2O2-induced hemolysis) | |||
| Control | 2.34 ± 0.04 | 1.14 ± 0.06 | 0.88 ± 0.04 |
| 1 | 2.08 ± 0.04 ** | 0.98 ± 0.06 * | 0.66 ± 0.02 ** |
| 4 | 1.82 ± 0.03 ** | 0.94 ± 0.10 * | 0.59 ± 0.03 ** |
| 5 | 2.07 ± 0.06 * | 1.77 ± 0.12 | 0.77 ± 0.05 |
| 6 | 2.11 ± 0.04 * | 1.51 ± 0.07 | 0.65 ± 0.04 * |
| Experiment 3 (AAPH-induced hemolysis) | |||
| Control | 2.17 ± 0.04 | 2.85 ± 0.13 | 1.67 ± 0.10 |
| 1 | 1.69 ± 0.04 ** | 1.90 ± 0.19 ** | 1.00 ± 0.05 ** |
| 4 | 2.03 ± 0.06 | 3.38 ± 0.28 | 1.59 ± 0.10 |
| Compound | Latent Period, % to the Monitoring | Maximum Amplitude, % to the Monitoring | Aggregation Rate, % to the Monitoring | Time to Reach MA, % to the Control | APTT, % to the Monitoring |
|---|---|---|---|---|---|
| Collagen—Induced Aggregation | ADP—Induced Aggregation | ||||
| 1 | +5.4 (3.7–6.2) *,# | −26.4 (21.7–29.3) **,## | −15.6 (13.2–18.7) *,# | +10.1 (8.2–12.5) * | + 9.2 (7.9–10.4) * |
| 4 | +13.4 (11.2–15.3) *,## | −14.5 (11.2–16.7)* | −8.5 (6.1–11.7)* | +18.7 (16.4–21.5) *,# | +5.6 (3.9–6.1) * |
| Acetylsalicylic acid | −2.1 (1.1–2.6) | −13.7 (10.8–16.4) * | −10.5 (7.6–12.3) * | +10.5 (8.7–13.4) * | - |
| Sodium heparin | - | - | - | - | 20.3 (19.7–21.4) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ksenofontov, A.A.; Bocharov, P.S.; Antina, E.V.; Shevchenko, O.G.; Samorodov, A.V.; Gilfanov, I.R.; Pavelyev, R.S.; Ostolopovskaya, O.V.; Startseva, V.A.; Fedyunina, I.V.; et al. Thioterpenoids as Potential Antithrombotic Drugs: Molecular Docking, Antiaggregant, Anticoagulant and Antioxidant Activities. Biomolecules 2022, 12, 1599. https://doi.org/10.3390/biom12111599
Ksenofontov AA, Bocharov PS, Antina EV, Shevchenko OG, Samorodov AV, Gilfanov IR, Pavelyev RS, Ostolopovskaya OV, Startseva VA, Fedyunina IV, et al. Thioterpenoids as Potential Antithrombotic Drugs: Molecular Docking, Antiaggregant, Anticoagulant and Antioxidant Activities. Biomolecules. 2022; 12(11):1599. https://doi.org/10.3390/biom12111599
Chicago/Turabian StyleKsenofontov, Alexander A., Pavel S. Bocharov, Elena V. Antina, Oksana G. Shevchenko, Aleksandr V. Samorodov, Ilmir R. Gilfanov, Roman S. Pavelyev, Olga V. Ostolopovskaya, Valeriya A. Startseva, Inna V. Fedyunina, and et al. 2022. "Thioterpenoids as Potential Antithrombotic Drugs: Molecular Docking, Antiaggregant, Anticoagulant and Antioxidant Activities" Biomolecules 12, no. 11: 1599. https://doi.org/10.3390/biom12111599






