Development of an Electrochemical Sensor Conjugated with Molecularly Imprinted Polymers for the Detection of Enrofloxacin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
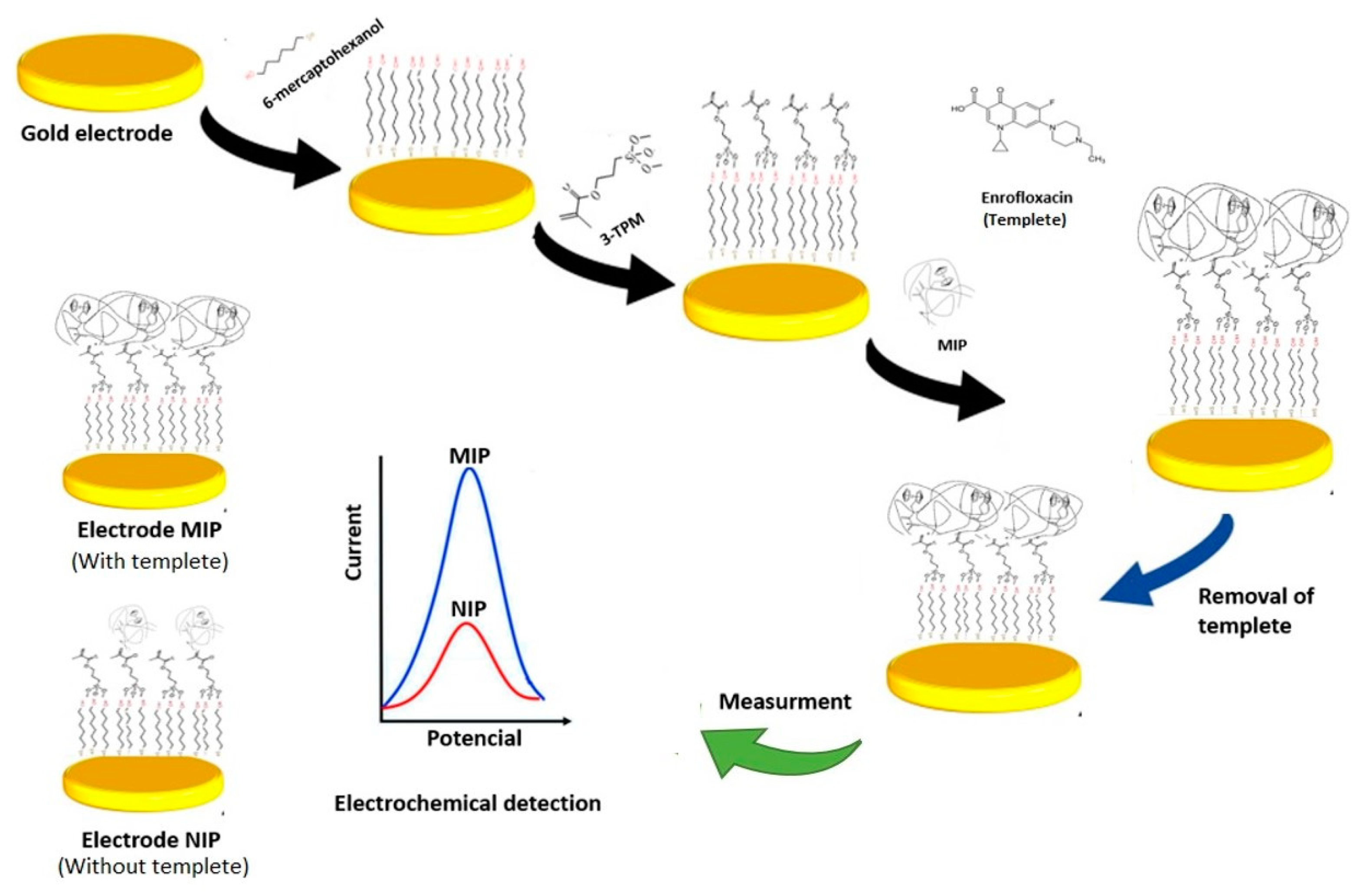
2.2. Electrode Preparation
2.3. Chemical Modification of the Screen-Printed Electrode
2.4. Synthesis of Molecularly Imprinted Polymers
2.5. Sample Preparation
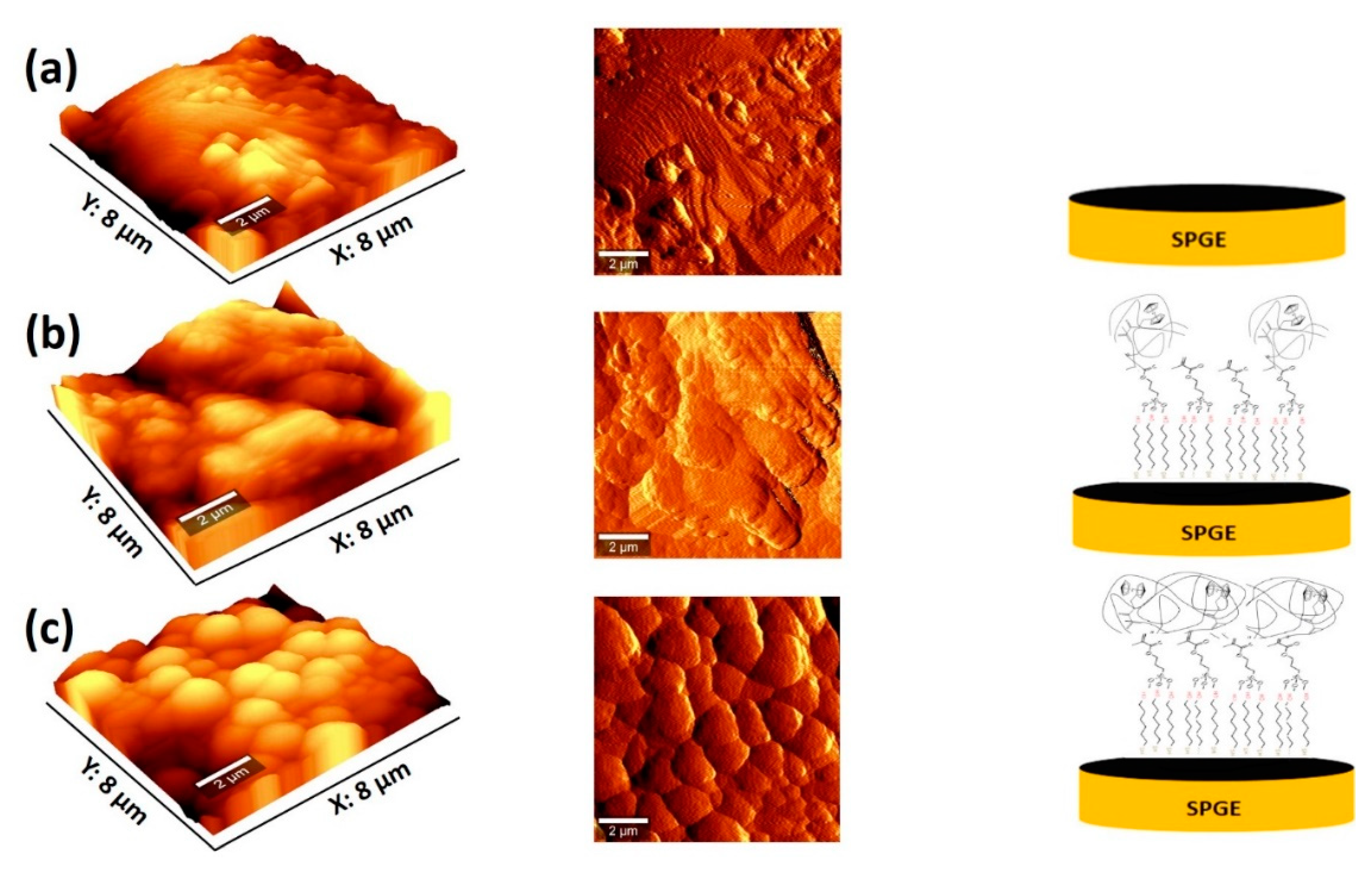
2.6. Morphological Characterization
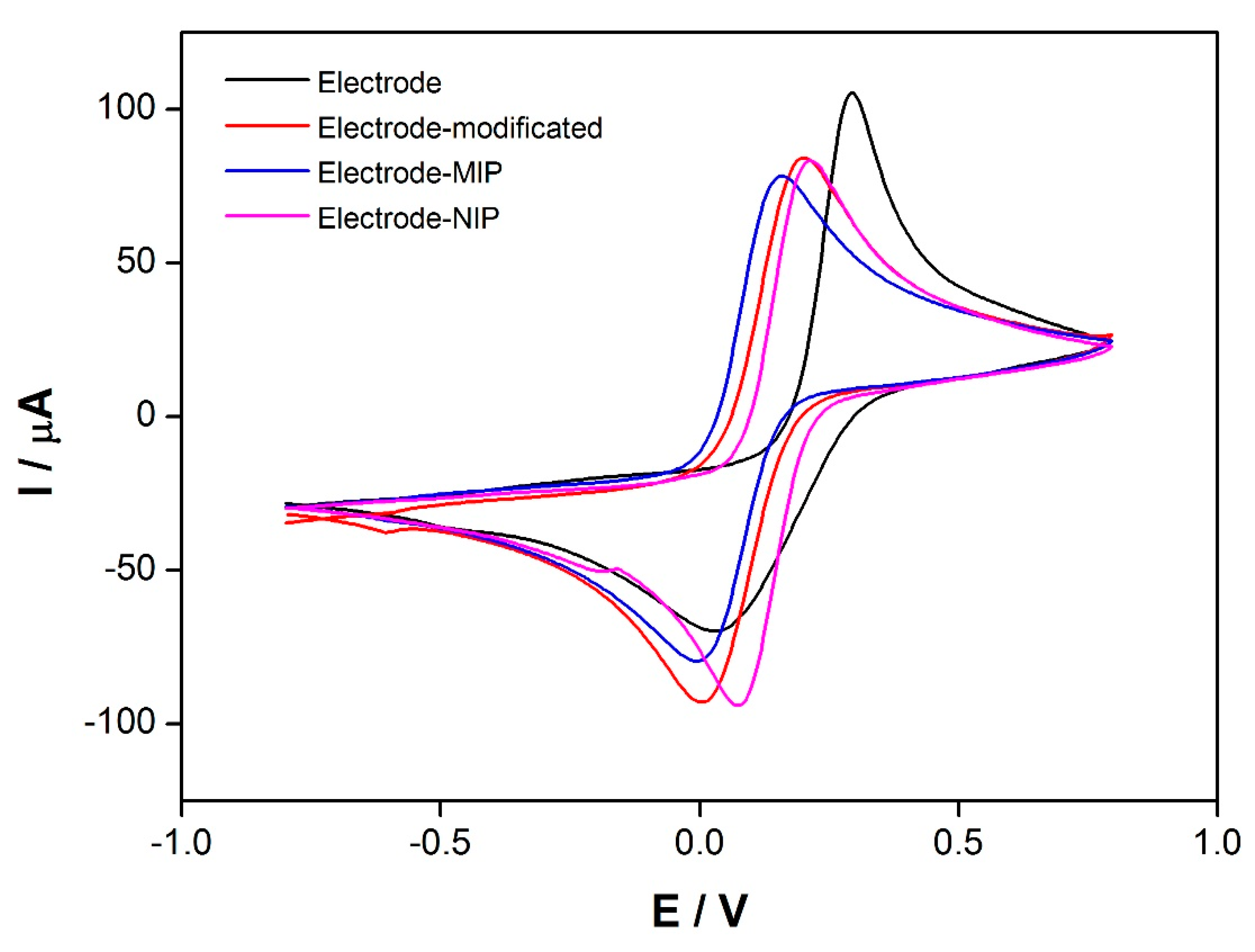
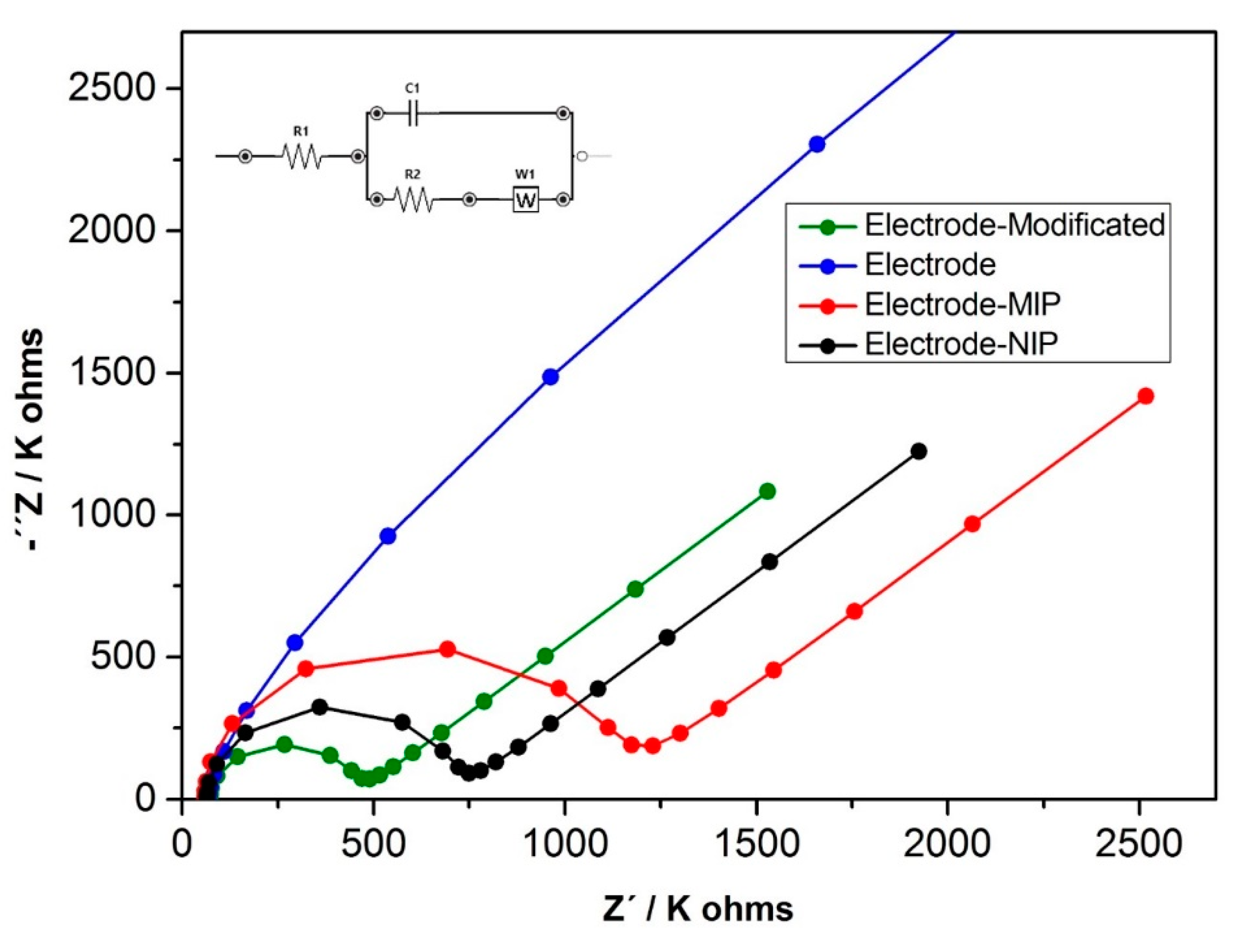
2.7. Electrochemical Characterization
2.8. Determination of the Enrofloxacin
3. Results and Discussion
3.1. Morphological Characterization
3.2. Electrochemical Characterization
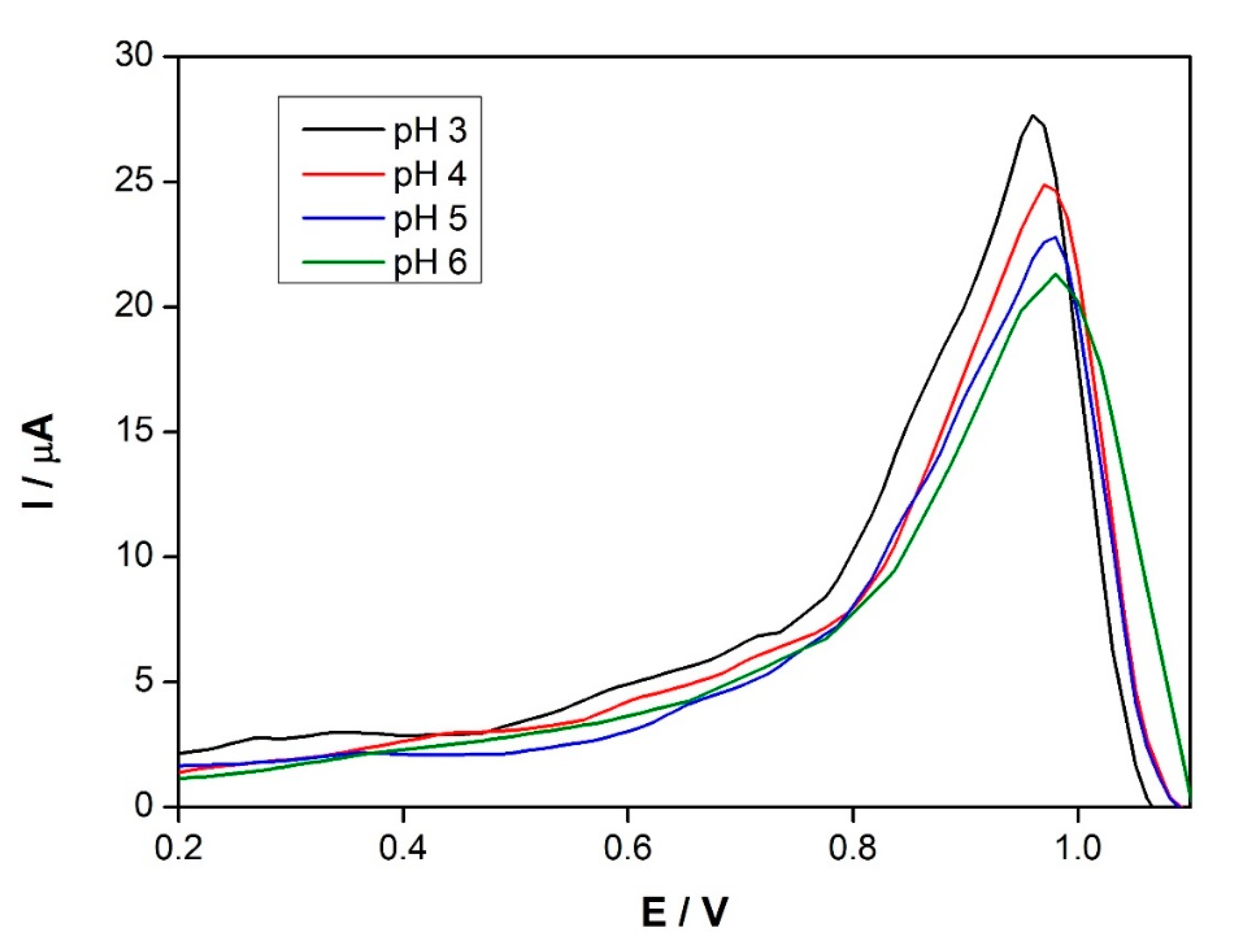
3.3. Influence of pH and Supporting Electrolyte
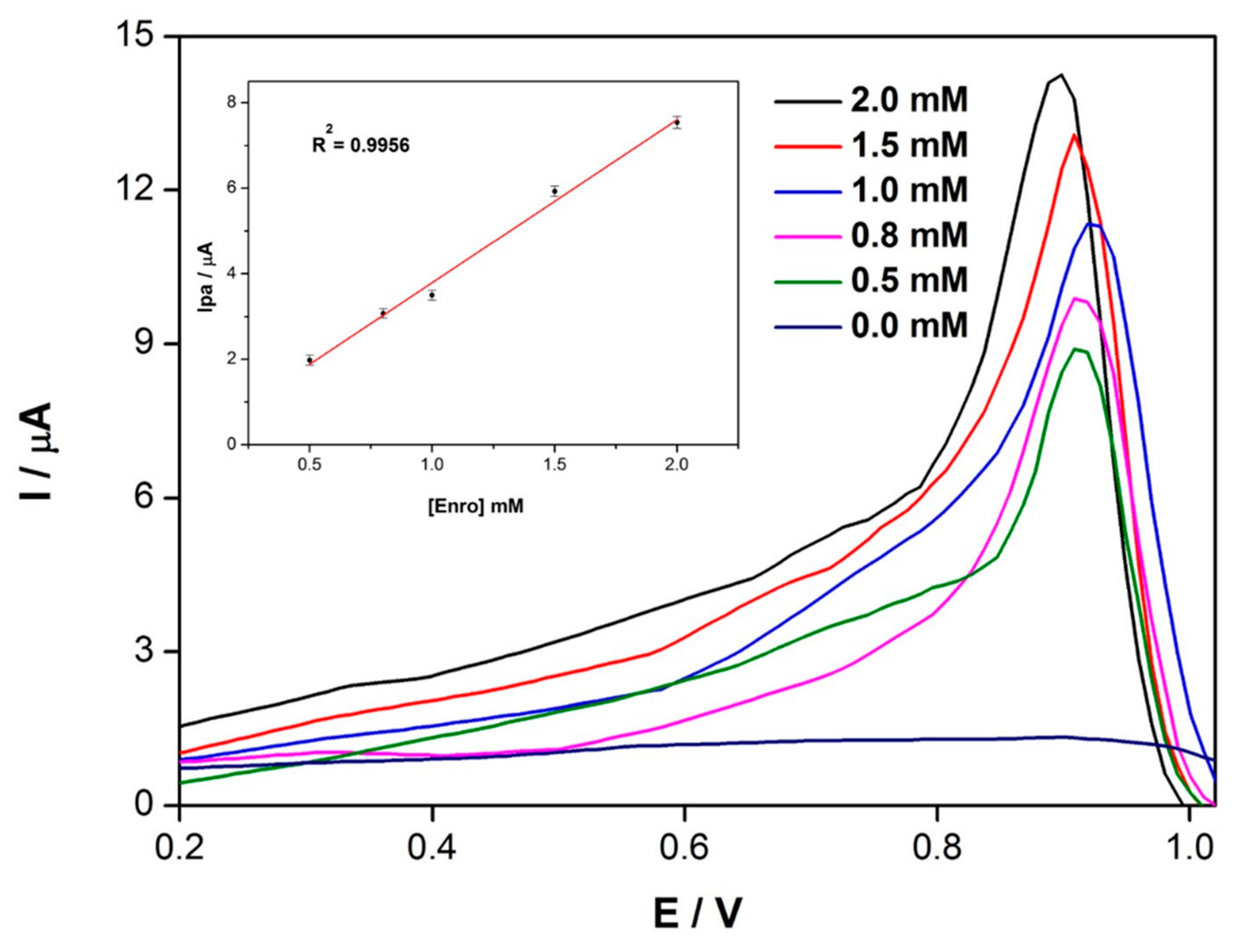
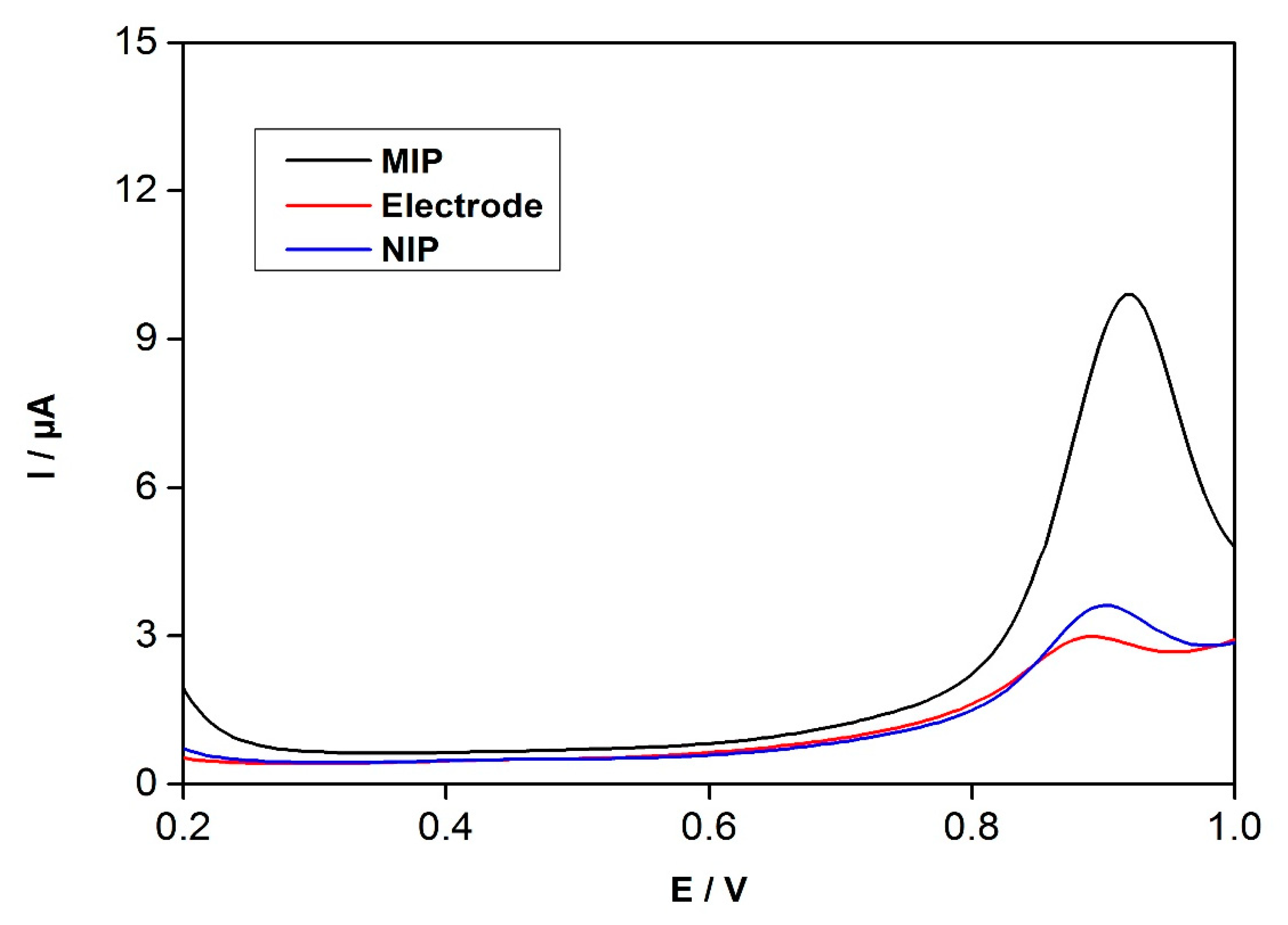
3.4. SWV Response of Electrodes to Enrofloxacin Detection
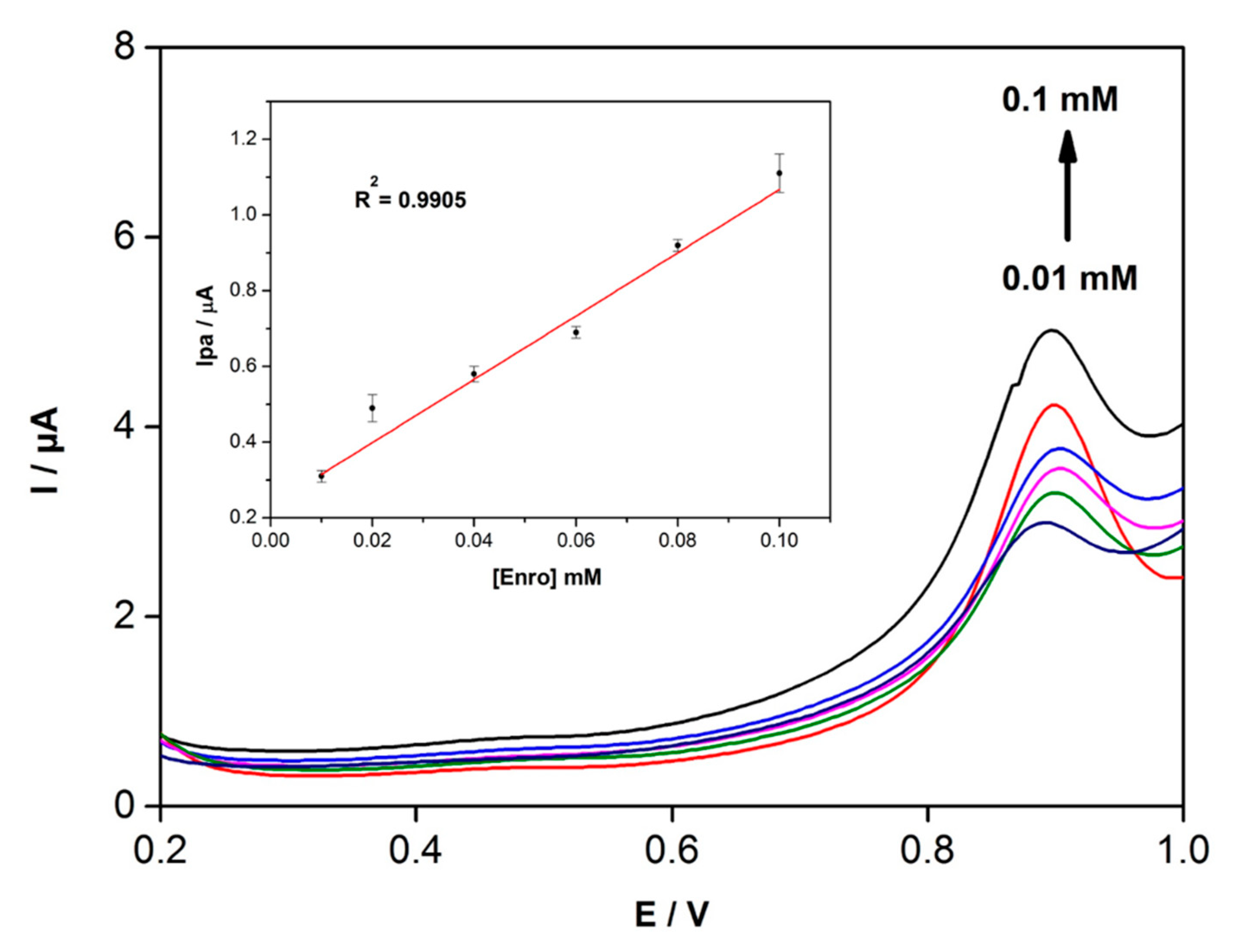
3.5. Detection of Enrofloxacin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarley, C.R.T.; Sotomayor, M.D.P.T.; Kubota, L.T. Polímeros biomiméticos em química analítica. Parte 2: Aplicações de MIP (“Molecularly Imprinted Polymers”) no desenvolvimento de sensores químicos. Química Nova 2005, 28, 1087–1101. [Google Scholar] [CrossRef]
- Abera, B.D.; Ortiz-Gómez, I.; Shkodra, B.; Romero, F.J.; Cantarella, G.; Petti, L.; Salinas-Castillo, A.; Lugli, P.; Rivadeneyra, A. Laser-Induced Graphene Electrodes Modified with a Molecularly Imprinted Polymer for Detection of Tetracycline in Milk and Meat. Sensors 2022, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Lowinsohn, D.; Bertotti, M. Sensores eletroquímicos: Considerações sobre mecanismos de funcionamento e aplicações no monitoramento de espécies químicas em ambientes microscópicos. Química Nova 2006, 29, 1318–1325. [Google Scholar] [CrossRef]
- Chu, H.; Wei, X.; Wu, M.; Yan, J.; Tu, Y. An electrochemiluminescent biosensor based on polypyrrole immobilized uricase for ultrasensitive uric acid detection. Sens. Actuators B Chem. 2012, 163, 247–252. [Google Scholar] [CrossRef]
- Pereira, A.C.; Aguiar, M.R.; Kisner, A.; Macedo, D.V.; Kubota, L.T. Amperometric biosensor for lactate based on lactate de-hydrogenase and Meldola Blue coimmobilized on multi-wall carbon-nanotube. Sens. Actuators B Chem. 2007, 124, 269–276. [Google Scholar] [CrossRef]
- Qian, T.; Yu, C.; Zhou, X.; Ma, P.; Wu, S.; Xu, L.; Shen, J. Ultrasensitive dopamine sensor based on novel molecularly imprinted polypyrrole coated carbon nanotubes. Biosens. Bioelectron. 2014, 58, 237–241. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Y.; Bai, J.; Ning, B.; Sun, S.; Hong, X.; Liu, Y.; Liu, Y.; Gao, Z. A novel electrochemical sensor based on electropolymerized molecularly imprinted polymer and gold nanomaterials amplification for estradiol detection. Sens. Actuators B Chem. 2014, 200, 69–75. [Google Scholar] [CrossRef]
- Blanco-López, M.C.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Electrochemical sensors based on molecularly imprinted polymers. TrAC Trends Anal. Chem. 2004, 23, 36–48. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Sotomayor, M.D.P.T.; Kubota, L.T. Polímeros biomiméticos em química analítica. Parte 1: Preparo e aplicações de MIP ("Molecularly Imprinted Polymers") em técnicas de extração e separação. Química Nova 2005, 28, 1076–1086. [Google Scholar] [CrossRef]
- Figueiredo, E.C.; Dias, A.C.B.; Arruda, M.A.Z. Impressão molecular: Uma estratégia promissora na elaboração de matrizes para a liberação controlada de fármacos. Rev. Bras. Cienc. Farm. 2008, 44, 361–375. [Google Scholar] [CrossRef]
- Aaryashree; Takeda, Y.; Kanai, M.; Hatano, A.; Yoshimi, Y.; Kida, M. A “Single-Use” Ceramic-Based Electrochemical Sensor Chip Using Molecularly Imprinted Carbon Paste Electrode. Sensors 2020, 20, 5847. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Poma, A.; Sacchetti, A. Advances in Molecularly Imprinted Polymers as Drug Delivery Systems. Molecules 2021, 26, 3589. [Google Scholar] [CrossRef]
- Shen, Y.; Jia, F.; Liang, A.; He, Y.; Peng, Y.; Dai, H.; Fu, Y.; Wang, J.; Li, Y. Monovalent Antigen-Induced Aggregation (MAA) Biosensors Using Immunomagnetic Beads in Both Sample Separation and Signal Generation for Label-Free Detection of Enrofloxacin. ACS Appl. Mater. Interfaces 2022, 14, 8816–8823. [Google Scholar] [CrossRef] [PubMed]
- Trouchon, T.; Lefebvre, S. A Review of Enrofloxacin for Veterinary Use. Open J. Vet. Med. 2016, 6, 40–58. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.-R.; Diao, Z.-H.; Sun, K.-F.; Hao, Q.-W.; Liu, S.-S.; Ying, G.-G. Tissue distribution, bioaccumulation characteristics and health risk of antibiotics in cultured fish from a typical aquaculture area. J. Hazard. Mater. 2018, 343, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Abu Hasan, H. Contaminants of emerging concern (CECs) in aquaculture effluent: Insight into breeding and rearing activities, alarming impacts, regulations, performance of wastewater treatment unit and future approaches. Chemosphere 2021, 290, 133319. [Google Scholar] [CrossRef] [PubMed]
- Lebelo, K.; Malebo, N.; Mochane, M.; Masinde, M. Chemical Contamination Pathways and the Food Safety Implications along the Various Stages of Food Production: A Review. Int. J. Environ. Res. Public Health 2021, 18, 5795. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Patella, B.; Daly, R.; Seymour, I.; Robinson, C.; Lovera, P.; Rohan, J.; Inguanta, R.; O’Riordan, A. A simulation and experimental study of electrochemical pH control at gold interdigitated electrode arrays. Electrochim. Acta 2021, 395, 139113. [Google Scholar] [CrossRef]
- Menon, S.; Jesny, S.; Kumar, K.G. A voltammetric sensor for acetaminophen based on electropolymerized-molecularly imprinted poly(o-aminophenol) modified gold electrode. Talanta 2018, 179, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Laschuk, N.O.; Easton, E.B.; Zenkina, O.V. Reducing the resistance for the use of electrochemical impedance spectroscopy analysis in materials chemistry. RSC Adv. 2021, 11, 27925–27936. [Google Scholar] [CrossRef]
- Hernández, P.; Aguilar-Lira, G.Y.; Islas, G.; Rodriguez, J.A. Development of a New Voltammetric Methodology for the Determination of Ciprofloxacin in Beef Samples Using a Carbon Paste Electrode Modified with Nafion and Fullerenes. Electroanalysis 2021, 33, 1539–1546. [Google Scholar] [CrossRef]
- Seguro, I.; Rebelo, P.; Pacheco, J.G.; Delerue-Matos, C. Electropolymerized, Molecularly Imprinted Polymer on a Screen-Printed Electrode—A Simple, Fast, and Disposable Voltammetric Sensor for Trazodone. Sensors 2022, 22, 2819. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos-Tabanico, A.; Jimenez-Canale, J.; Hernandez-Leon, S.G.; Burgara-Estrella, A.J.; Encinas-Encinas, J.C.; Sarabia-Sainz, J.A. Development of an Electrochemical Sensor Conjugated with Molecularly Imprinted Polymers for the Detection of Enrofloxacin. Chemosensors 2022, 10, 448. https://doi.org/10.3390/chemosensors10110448
Gallegos-Tabanico A, Jimenez-Canale J, Hernandez-Leon SG, Burgara-Estrella AJ, Encinas-Encinas JC, Sarabia-Sainz JA. Development of an Electrochemical Sensor Conjugated with Molecularly Imprinted Polymers for the Detection of Enrofloxacin. Chemosensors. 2022; 10(11):448. https://doi.org/10.3390/chemosensors10110448
Chicago/Turabian StyleGallegos-Tabanico, Amed, Jorge Jimenez-Canale, Sergio G. Hernandez-Leon, Alexel J. Burgara-Estrella, Jose Carmelo Encinas-Encinas, and Jose A. Sarabia-Sainz. 2022. "Development of an Electrochemical Sensor Conjugated with Molecularly Imprinted Polymers for the Detection of Enrofloxacin" Chemosensors 10, no. 11: 448. https://doi.org/10.3390/chemosensors10110448
APA StyleGallegos-Tabanico, A., Jimenez-Canale, J., Hernandez-Leon, S. G., Burgara-Estrella, A. J., Encinas-Encinas, J. C., & Sarabia-Sainz, J. A. (2022). Development of an Electrochemical Sensor Conjugated with Molecularly Imprinted Polymers for the Detection of Enrofloxacin. Chemosensors, 10(11), 448. https://doi.org/10.3390/chemosensors10110448




