An Economic–Business Approach to Clinical Risk Management
Abstract
1. Introduction
- (a)
- Structural–technological factors represented by features of the healthcare unit, including plant design (design and maintenance); safety and logistics of the environment; equipment and tools (functioning, maintenance, renovation); and infrastructure, coverage, digitalization, and automation;
- (b)
- Organizational–managerial tools and work conditions represented by the organizational structure of the system (roles, responsibilities, work distribution); by policy and management of human resources (organization, leadership styles, incentives system, supervision and control, training and updating, workload and shifts); by the organizational communication system; by the ergonomic aspects (for example, monitoring, alarms, noises, and light); and by policies for promoting the safety of the patient (guidelines and patient diagnosis/treatment plan, error report system);
- (c)
- Human factors (both individual and team-related) identified in the characteristics of the staff (perception, attention, memory, ability to make decisions, perception of responsibility, mental and physical conditions); in the professional skills; in the interpersonal and group dynamics; as well as in the subsequent level of cooperation;
- (d)
- User characteristics, such as epidemiology and socio-cultural aspects (demographic aspects, ethnicity, socio-economic environment, education, ability to manage situations, complexity, and compresence of acute and chronic conditions) and social networking;
- (e)
- External factors including the regulation and the obligations set out by the law, the financial limits, the socio-economic–cultural context, and the influence of public opinion and of the media of the professional organizations and public protection organizations as well as of the insurance companies.
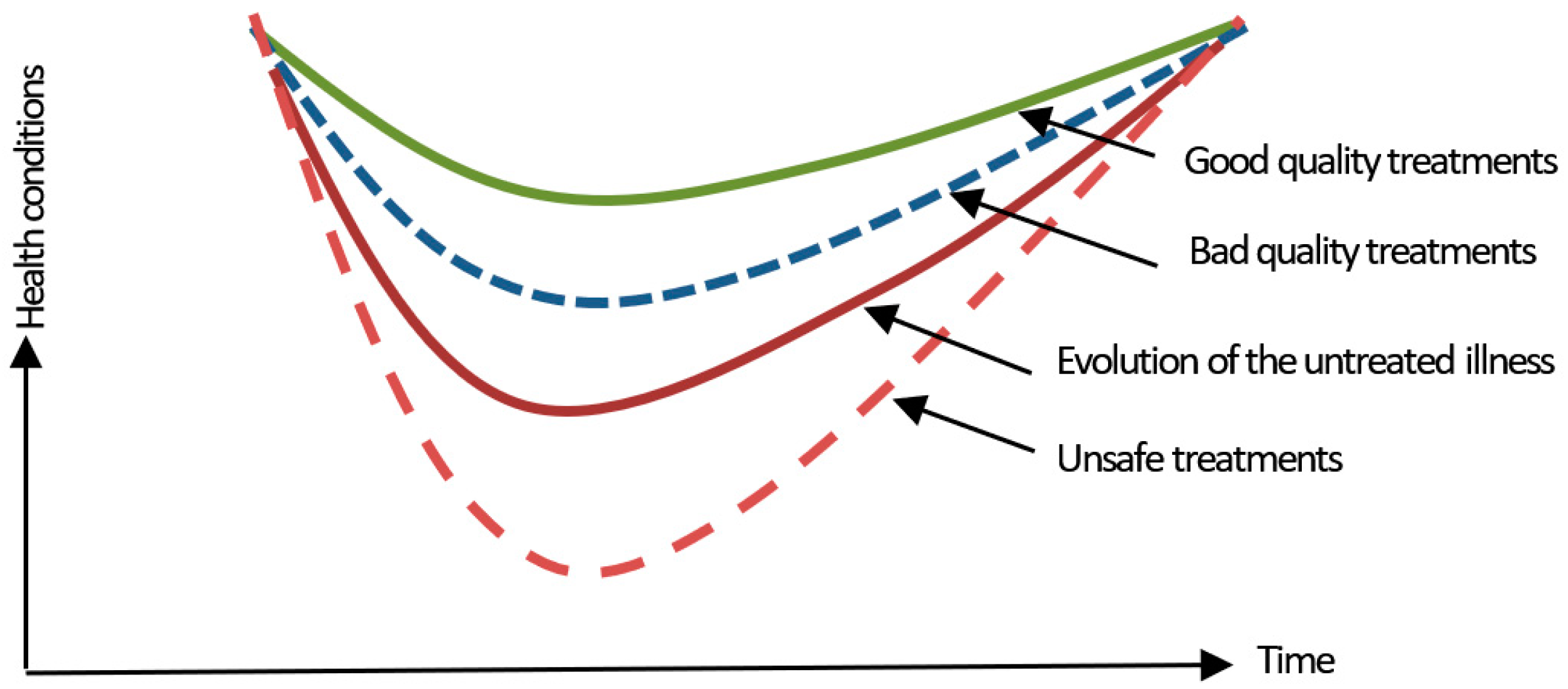
2. Clinical Risk
3. Identification, Analysis, and Risk Management Methods and Tools
- Proactive approach: The analysis begins from the review of the processes and the existing procedures, identifying critical points in the different phases. This approach can be used in the conceptual and design phases of new procedures, processes, and technologies aimed at realizing protective barriers to prevent human/active errors;
- Reactive approach: The analysis starts from an adverse event and retraces the sequence of events to identify the causative or contributory factors to the main event. Both these approaches can be used in a health organization where risk management processes are introduced (Martini and Pelati 2011).
3.1. Risk Identification Tools
- -
- open, that is, to gather any type of data related to adverse or almost-adverse events referring to the whole of the services;
- -
- predefined, that is, to gather all data related to a definite list of events (for example, sentinel events) or to a specific area (for example, drugs).
- -
- the belief in a scarce efficacy of the system and resistance to changes;
- -
- a defensive attitude;
- -
- investment in resources.
- -
- learning systems, usually voluntary systems designed to guarantee continuous improvement of the quality of the treatments. The recommendations that are elaborated after careful analysis are useful to redesign and improve healthcare processes;
- -
- accountability systems based on the principle of accountability; they are compulsory and are often limited to predefined events, for example, sentinel events. Many accountability systems use disincentivizing mechanisms such as fines and sanctions, and the efficacy of these systems depends on the ability to convince whoever is needed to report and act with consequential measures. These systems may also be considered as learning systems if the information received is analyzed with transparency, and the actions undertaken are spread to all operators.
3.2. Analysis Tools
- -
- establishment of an interdisciplinary group consisting of experts in the matter;
- -
- participation of those involved in the incident;
- -
- impartiality in highlighting potential conflicts of interest.
- -
- detection probability (score from 1 to 10);
- -
- seriousness (score from 1 to 10).
4. Safety as a Non-Dynamic Event
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barresi, Gustavo. 2014. Il Rischio Clinico Nelle Aziende Ospedaliere. Strumenti di Analisi e Profili di gestione. Milano: F. Angeli. [Google Scholar]
- Bizzarri, Giancarlo, and Massimo Farina. 2018. Strategia e Gestione del Rischio Clinico Nelle Organizzazioni Sanitarie. Milan: Approcci, Modalità, Strumenti e Risultati, F. Angeli. [Google Scholar]
- Bowen, Glenn A. 2002. Document Analysis as a Qualitative Research Method. Qualitative Research Journal 9: 27–40. [Google Scholar] [CrossRef]
- Buscemi, A. 2009. Il Risk Management in Sanità. Gestione del Rischio, Errori, Responsabilità Professionale, Aspetti Assicurativi e Risoluzione Stragiudiziale Delle Controversie. Milano: F. Angeli. [Google Scholar]
- Casati, G., ed. 2000. Il Percorso del Paziente. Milano: Egea. [Google Scholar]
- Charles, Wendy M., Natalie Elise Marler, Lauren Long, and Sean T. Manion. 2019. Blockchain Compliance by Design: Regulatory Considerations for Blockchain in Clinical Research. Frontiers in Blockchain 2: 18. [Google Scholar] [CrossRef]
- Comite, U. 2011. Healthcare Autorities: between Functional Integration and Clinical Governance. Paper presented at the Economic–Business Aspect and Managerial Determinants of Clinical Practice, Zagreb, Croatia, October 13–15; Istanbul: Ebes Publisher. [Google Scholar]
- Comite, U. 2014. Il sistema informativo aziendale a supporto del (public) reporting in sanità: un approccio manageriale, in Vincenzo Ferrari, Waldemar Tłokiński, David E. Zammit (a cura di), Responsabilità medica ed organizzazione sanitaria. Profili etico-giuridici e gestionali. Roma: Aracne. [Google Scholar]
- Comite, U., ed. 2017. Advances in Health Management. Rijeka: INTECH. [Google Scholar]
- Comite, U. 2018. Clinical assistance and management innovations in healthcare: from the patient to the integrated management. Paper presented at the Seventh International Multidisciplinary Conference on Knowledge & Human Resources Management for a Sustainable Development (ICTEA 7, 2018), University of Calabria Italy, Rende, Italy, June 29–July 1. [Google Scholar]
- Donabedian, Avedis. 2003. An Introduction to Quality Assurance in Health Care. Oxford: Oxford University Press. [Google Scholar]
- Donabedian, Avedis. 2009. Il Maestro e le Margherite. La Qualità Dell’assistenza Sanitaria Secondo Avedis Donabedian. Roma: Il Pensiero Scientifico. [Google Scholar]
- Khezr, Seyednima, Md Moniruzzaman, Abdulsalam Yassine, and Rachid Benlamri. 2019. Blockchain Technology in Healthcare: A Comprehensive Review and Directions for Future Research. Applied Science 9: 1736. [Google Scholar] [CrossRef]
- Leggeri, Riccardo, and Giuseppe Perrella. 2011. La Gestione del Rischio Clinico. La Sicurezza del Paziente e la Lotta agli Sprechi Nelle Strutture Pubbliche e Private. Milano: F. Angeli. [Google Scholar]
- Liu, Wei, Xiao-Guang Yue, and Paul B. Tchounwou. 2020. Response to the COVID-19 Epidemic: The Chinese Experience and Implications for Other Countries. International Journal of Environmental Research Public Health 17: 2304. [Google Scholar] [CrossRef] [PubMed]
- Luo, Yu-Meng, Wei Liu, Xiao-Guang Yue, and Marc. A. Rosen. 2020. Sustainable Emergency Management Based on Intelligent Information Processing. Sustainability 12: 1081. [Google Scholar] [CrossRef]
- Malinverno, Enrico. 2013. La Qualità in Sanità. Metodi e Strumenti di Clinical Governance. Bari: Carocci. [Google Scholar]
- Martini, M., and C. Pelati. 2011. La Gestione del Rischio Clinico. Milano: McGraw-Hill. [Google Scholar]
- Ministry of Health. 2018. Department of Quality, General Management of Healthcare Planning, of the Levels of Assistance and of the System Ethical Principles, Ufficio III, Sicurezza dei Pazienti e Gestione del Rischio Clinico: Manuale per la Formazione Degli Operatori Sanitari. Roma: Ministry of Health. [Google Scholar]
- Wang, Chuanyi, Zhe Cheng, Xiao-Guang Yue, and Michael McAleer. 2020. Risk Management of COVID-19 by Universities in China. Journal of Risk Financial Management 13: 36. [Google Scholar] [CrossRef]
- Wilson, Paul F., Larry D. Dell, and Gaylor F. Anderson. 1993. Root Cause Analysis: A Tool for Total Quality Management. Milwaukee: ASQ Quality Press. [Google Scholar]
- Reason, James. 2000. Human Error: Models and Management. British Medical Journal 320: 768–70. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, Diomidis H. 1995. Failure Mode and Effect Analysis: FMEA from Theory to Execution. Milwaukee: ASQ Quality Press. [Google Scholar]
- Spano, Francesco Maria, and Vania Tradori. 2015. Sistemi di Auditing e Controllo Nelle Organizzazioni Sanitarie. Roma: Rirea. [Google Scholar]
- Trinchero, Elisabetta, and Federico Lega. 2016. Governare la Sala Operatoria Nell’Ospedale del XXI secolo. Qualità, Sicurezza, Efficienza. Milano: Egea. [Google Scholar]
- Upadhyay, Nitin. 2020. Demystifying Blockchain: A Critical Analysis of Challenges, Applications and Opportunities. International Journal of Information Management 54: 102120. [Google Scholar] [CrossRef]
- Yue, Xiao-Guang, Xue-Feng Shao, Rita Y. M. Li, M. James C. Crabbe, Lili Mi, Siyan Hu, Julien S. Baker, and Gang Liang. 2020a. Risk Management Analysis for Novel Coronavirus in Wuhan, China. Journal of Risk Financial Management 13: 22. [Google Scholar] [CrossRef]
- Yue, Xiao-Guang, Xue-Feng Shao, Rita Y. M. Li, M. James C. Crabbe, Lili Mi, Siyan Hu, Julien S. Baker, Liting Liu, and Kechen Dong. 2020b. Risk Prediction and Assessment: Duration, Infections, and Death Toll of the COVID-19 and Its Impact on China’s Economy. Journal of Risk Financial Management 13: 4. [Google Scholar] [CrossRef]
- Zhang, Peng, Douglas C. Schmidt, Jules White, and Gunther Lenz. 2018. Blockchain technology use cases in healthcare. In Blockchain Technology: Platforms, Tools and Use. Edited by P. Raj and G. C. Deka. London: Elsevier Inc., pp. 1–41. [Google Scholar] [CrossRef]
| Adverse event | An unexpected, unintentional, or undesirable event related to the treatment process not associated with the clinical condition of the patient, which entails damage to the patient with or without after-effects or an extension of the time spent in hospital |
| Active fault | Failure to execute an action as planned (execution error) or choice of an incorrect plan to reach a certain objective (planning error); it occurs shortly after the adverse event |
| Latent fault | A failure to execute an action that occurred far back in time and space from the adverse event |
| Sentinel event | A particularly serious adverse event, potentially descriptive of a serious malfunction of the system, which may entail death or serious damage to the patient, and that determines a loss of trust of the citizens toward the healthcare system. Due to the seriousness, it is sufficient for any of these events to occur once for the organization to (a) operate an immediate survey to verify what eradicable or reducible factors have caused or have contributed to causing it and (b) implement adequate corrective measures |
| Damage | Any negative consequence deriving from the event |
| Accident | An event that has caused or had the potentiality to cause an adverse event |
| Near Miss | High-risk situations or events that, for fortuitous reasons or because of a prompt intervention of an operator, have not determined an accident |
| Barriers | Protection of the patient, classified as physical or technological barriers (hardware), operational barriers (software, procedures, checks, organizational system) human barriers (healthcare staff, the patient himself and/or his relatives) |
| Contributing factors | Factors that have contributed to the realization of the human error due to some characteristics of the patient, factors linked to the task, individual factors, factors linked to the work team, factors linked to the work environment, organizational factors, and factors linked to the institutional background |
| Risk | Potential condition or event, intrinsic or extrinsic to the process, that can modify the expected outcome of the process. It is measured in terms of probabilities and consequences, as the product of the probability that a specific event might occur (P) and the seriousness of the damage that results from it (G); the ability of the human factor to understand ahead of time and contain the consequences of the potentially damaging event (K factor) is also considered in the calculation of the risk |
| Themes | Representative Articles in the Literature |
|---|---|
| COVID-19 | (Yue et al. 2020b) |
| Clinical governance | (Comite 2011) |
| Risk management | (Reason 2000) |
| Analysis tools | (Wilson et al. 1993) |
| Healthcare and illness | (Donabedian 2003) |
| Safety tools and perspectives | (Khezr et al. 2019) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comite, U.; Dong, K.; Li, R.Y.M.; Crabbe, M.J.C.; Shao, X.-F.; Yue, X.-G. An Economic–Business Approach to Clinical Risk Management. J. Risk Financial Manag. 2020, 13, 135. https://doi.org/10.3390/jrfm13060135
Comite U, Dong K, Li RYM, Crabbe MJC, Shao X-F, Yue X-G. An Economic–Business Approach to Clinical Risk Management. Journal of Risk and Financial Management. 2020; 13(6):135. https://doi.org/10.3390/jrfm13060135
Chicago/Turabian StyleComite, Ubaldo, Kechen Dong, Rita Yi Man Li, M. James C. Crabbe, Xue-Feng Shao, and Xiao-Guang Yue. 2020. "An Economic–Business Approach to Clinical Risk Management" Journal of Risk and Financial Management 13, no. 6: 135. https://doi.org/10.3390/jrfm13060135
APA StyleComite, U., Dong, K., Li, R. Y. M., Crabbe, M. J. C., Shao, X.-F., & Yue, X.-G. (2020). An Economic–Business Approach to Clinical Risk Management. Journal of Risk and Financial Management, 13(6), 135. https://doi.org/10.3390/jrfm13060135









