Challenges and Solutions for Integrating and Financing Personalized Medicine in Healthcare Systems: A Systematic Literature Review
Abstract
1. Introduction
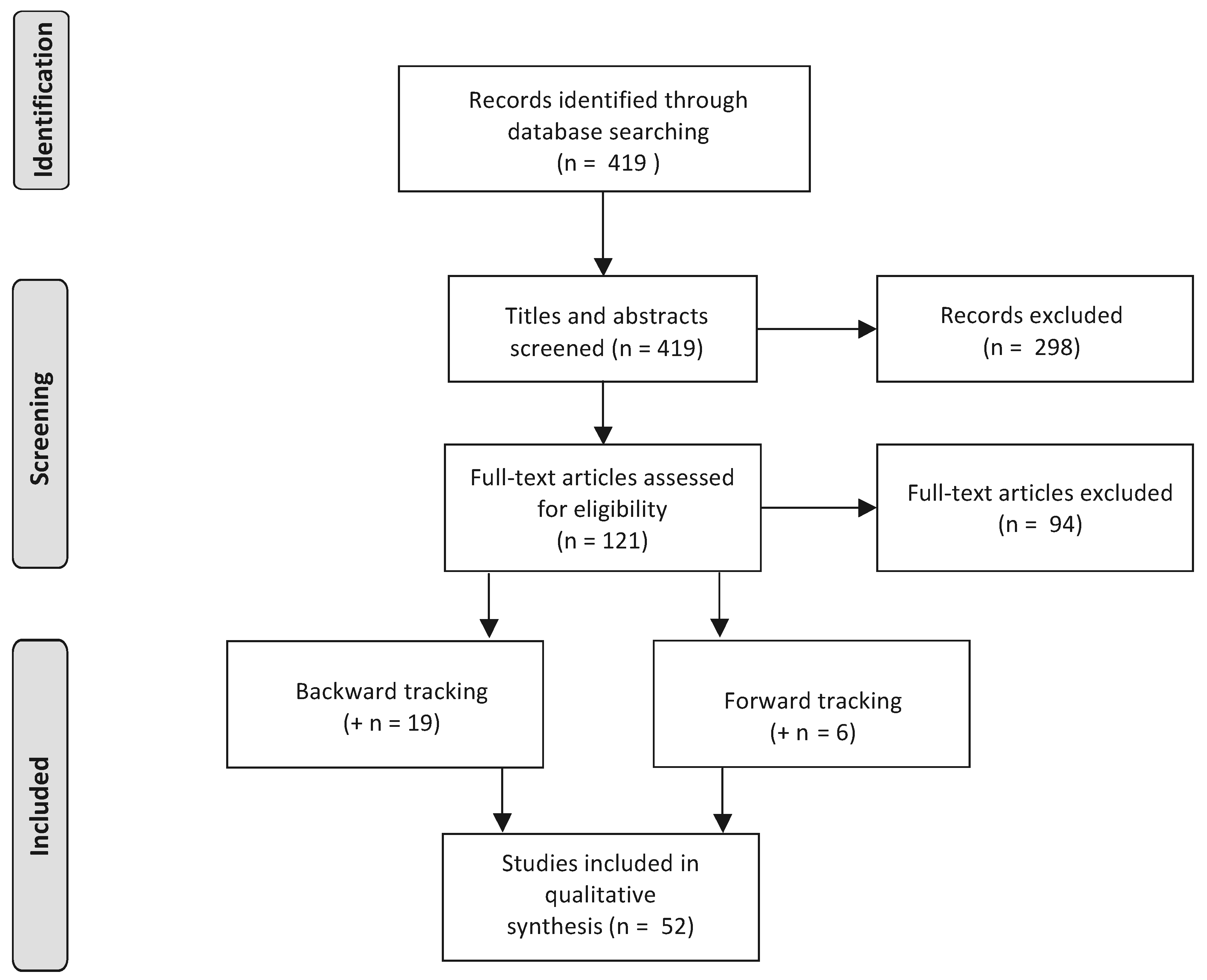
2. Methodology
2.1. Review Strategy
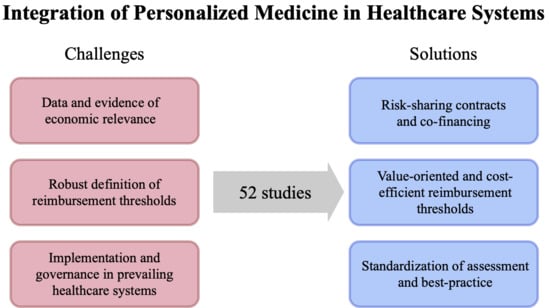
- Economic relevance of PM. Insurers as rational economic agents need proof of the cost-effectiveness of PM technologies before granting coverage. We include all literature that provides evidence, economic indicators related to PM, and tools and methods to foster the building of evidence. This includes but is not restricted to the two major approaches, i.e., contracts between manufacturers and payers to generate the evidence and standardization of data provision by assessment bodies.
- Governance challenges. In this stream we include literature that tackles the heterogeneity for inter- and intra-countries PM coverage. This includes, for example, papers that highlight the emerging discrepancies from the existence of a myriad of contracts, the standardization of reimbursement thresholds, and the need for enhanced communication between stakeholders.
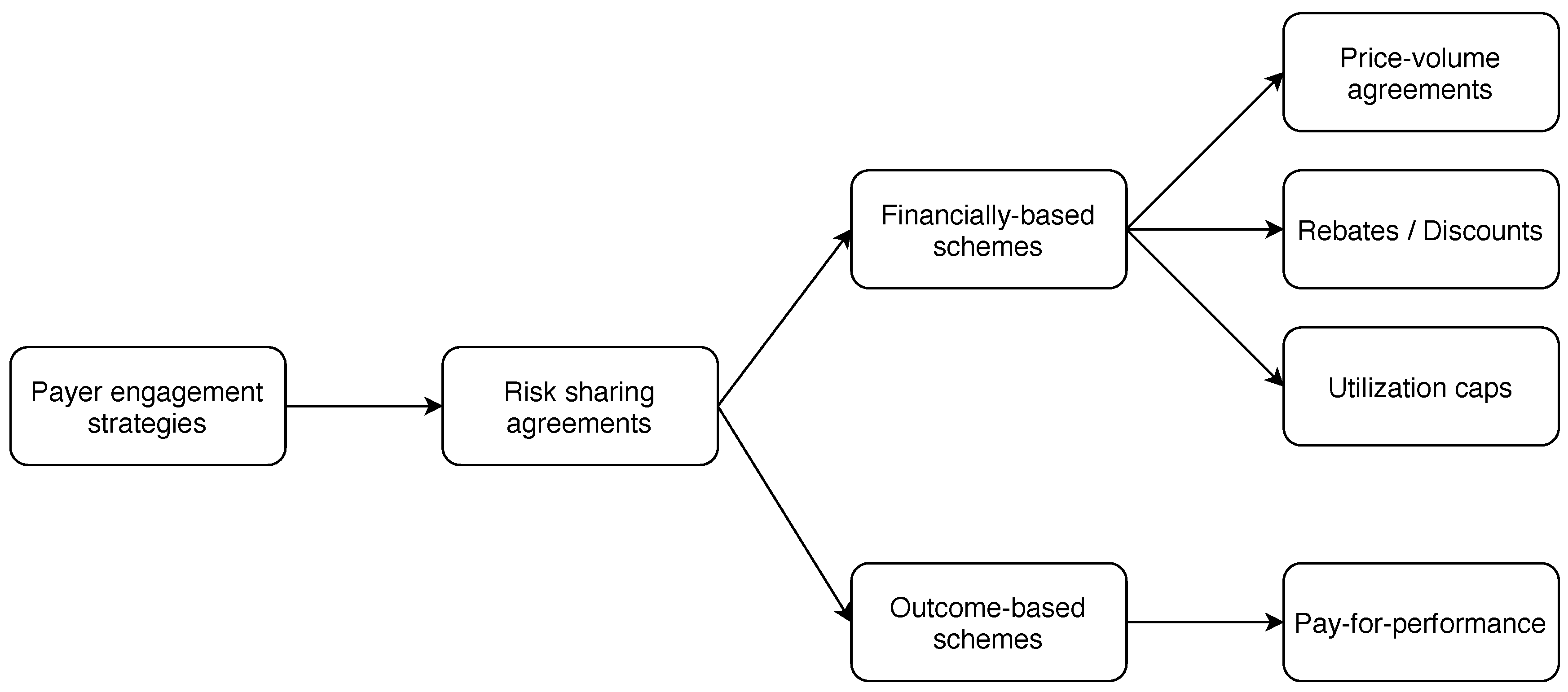
- Healthcare system implementation. Here, we are interested in all actionable ideas to overcome the hurdles linked to, for example, technology and pricing, that impede a successful implementation in current healthcare systems. Insights to overcome these obstacles include, for example, risk-sharing or evidence development contracts between manufacturers and payers.
2.2. Synopsis of the Results
3. Economic Relevance: Lack of Evidence and Efficiency Metrics
3.1. Description of the Challenges
3.2. Discussion of Potential Solutions
4. Governance: Heterogeneity in Coverage
4.1. Description of the Challenges
4.2. Discussion of Potential Solutions
5. Implementation in the Healthcare System: Characteristics of Pm
5.1. Description of the Challenges
5.2. Discussion of Potential Solutions
6. Concluding Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CDx | Companion diagnostics |
| CED | Contract with evidence development |
| HTA | Health technology assessment |
| MCDA | Multiple criteria decision analysis |
| MEA | Managed entry agreement |
| NGS | Next generation sequencing |
| NHS | National Health Service |
| NICE | National Institute for Health and Care Excellence |
| PAS | Patient access scheme |
| PGx | Pharmacogenomics / Pharmacogenetics |
| PM | Personalized medicine |
| PRISMA | Preferred reporting items for systematic reviews and meta-analysis |
| PVA | Price-volume agreement |
| QALY | Quality-adjusted life year |
| RSA | Risk-sharing agreement |
| WTP | Willingness-to-pay |
Appendix A
| Reference | Region | Methodology | Key Contents and Main Results | ER | GC | HS |
|---|---|---|---|---|---|---|
| Akhmetov and Bubnov (2017) | US | Survey/interviews () | – Manufacturers may benefit from accessing claims data – Collaboration and trust are key, data exchange improves evidence paucity – Early dialogue between producers and payers enables better integration | ✔ | ✔ | ✔ |
| Amendola et al. (2019) | US | Review of coverage () | – Guidelines not meaningfully identify patients who may benefit from testing – Germline cancer test often deemed experimental or not medically necessary – Difference in denial because of difference in evidence assessment | ✔ | ✔ | |
| Basu (2015) | Conceptual article | – HealthCoins to address the “free-rider” problem among insurers – HealthCoins to enable smooth investments across insurers – HealthCoins are produced with investments and bought out by next insurer | ✔ | |||
| Boon et al. (2015) | NL | Discussion | – None of 46 orphan drugs on conditional reimbursement were de-listed – De-listing solely on cost-effectiveness faces social pressure – Four year re-evaluation for quality evidence production is too short | ✔ | ✔ | |
| Carrera and IJzerman (2016) | Discussion | – PM drugs are particularly costly due to narrower customer base – Stakeholders have different WTP thresholds per QALY – MCDA incorporates in HTA the multidimensional value of PM | ✔ | |||
| Chalkidou and Rawlins (2011) | Discussion & case studies | – Discussion of interrelated impact between CED and pharmacogenetics – CED contracts offer an alternative solution for public reimbursement – Healthcare system has to be adjusted for optimal RSA implementation | ✔ | |||
| Cohen et al. (2013) | US | Review of reimbursement ( PGx) | – Lack of comprehensive reimbursement of CDx and high costs of PGx – Often low evidence of clinical usefulness – Payers report that tests cost for everyone but help only a few | ✔ | ||
| Cohen and Felix (2014) | US | Review (10 drug- diagnostics) and survey () | – Variable and relatively high patient co-insurance – Drug reimbursement is not necessarily coupled with diagnostic coverage – Need to increase the body of evidence; CED to increase data | ✔ | ||
| Degtiar (2017) | 42 countries | Literature review ( articles) | – Private payers deem tests investigational and cover them less – Value-based assessment for reimbursement to incorporate other criteria – Need for evidence guidelines from payers | ✔ | ✔ | ✔ |
| Deverka et al. (2007) | US | Survey/interviews () | – Lack of clinical utility as a barrier for molecular medicine coverage – Public–private partnership for effectiveness research data generation – Establishment of accurate regulation to avoid uncertainty | ✔ | ||
| Deverka and Dreyfus (2014) | Review of NGS coverage | – Lack of clinical utility information and standards – Move reimbursement from a cost-based to a value-based approach – Payers concerned with reimbursement of confirmation of incidental findings | ✔ | |||
| Deverka (2009) | Commentary | – Payers have evidence requirements more rigorous than regulators – Coverage assessment needs positive net benefit as compared to usual care – Opportunity for informed decision-making by linking payer information | ✔ | ✔ | ✔ | |
| Faulkner et al. (2012) | US | Review | – Research prioritization and early standardized value assessment – Best practice for clinical evidence development/health economic assessment – New incentive and reimbursement approaches for PM | ✔ | ||
| Faulkner et al. (2016) | EU & US | Review of pricing and reimbursement | – Earlier cross-stakeholder engagement and regulatory tools – Flexible and adaptive payer approaches to pricing and reimbursement – Iterative evidence generation and specific funding | ✔ | ✔ | |
| Ferrario and Kanavos (2015) | BE, UK, NL, SE | Review of MEA () | – Conceptual framework for MEA agreements and tests – Different types of agreement and medicine-indications across countries – Variation from governance and risk-perception | ✔ | ✔ | ✔ |
| Fugel et al. (2012) | EU & US | Review of pricing and reimbursement | – Lack of a consistent process for value assessment of more complex diagnostics – More flexible pricing and reimbursement systems are needed – Further development of framework for access of diagnostic-based therapies | ✔ | ✔ | |
| Garfield (2011) | 10 EU countries | Review of reimbursement | – European reimbursement systems are not appropriately aligned – Change health technology assessment methodologies – Need for integrated reimbursement pathways and drug coding systems | ✔ | ✔ | |
| Garrison and Austin (2006) | Commentary | – Limitations of genetic prediction and lack of economic incentives slows PM – Clinical successes often on a case-by-case basis – Develop strong intellectual property and value-based, flexible pricing systems | ✔ | |||
| Garrison and Towse (2017) | Concepts for pricing and reimbursement | – Take an economic perspective and a broader concept of value – Valuations beyond QALY including changing preferences over life – Inflexible or cost-based reimbursement systems as barriers for PM | ✔ | |||
| Garrison et al. (2013) | Review of RSA () | – Performance-based risk-sharing arrangements to reduce uncertainty – Practical recommendations for state-of-the-art methods – Data regulation and long-run societal perspective needed | ✔ | ✔ | ||
| Graf et al. (2013) | US | Review of coverage ( policies) | – Half of insurers do not cover specific genetic-related services – One-third of the insurers addressed genetic testing – Challenges in ensuring consistency and homogeneity among insurers | ✔ | ||
| Hall and McCabe (2013) | Commentary | – Cost-effectiveness standards are more poorly defined for PM – Regulation of diagnostic tests less rigorous – Harmonize methods and increase modeling transparency | ✔ | |||
| Hresko and Haga (2012) | US | Review of coverage ( policies) | – Lack of evidence of clinical utility as a barrier for coverage – Variable coverage determinations and factors considered – Inclusion of PGx information in drug package inserts seems relevant | ✔ | ✔ | ✔ |
| Kanavos and Angelis (2013) | Concept for value assessment | – Multiple criteria decision analysis: HTA for broader value inclusion – Values: illness burden, innovation, therapeutic and socioeconomic impact – Score: weights are assigned according to an institution’s priorities | ✔ | |||
| Knowles et al. (2017) | Literature review ( articles) | – Science of PM requires broadening beyond genetics – Lack of clinical uptake due to structural and human factors – Recommendations on financial and regulatory barriers to be addressed | ✔ | ✔ | ||
| Leopold et al. (2013) | 27 EU countries | Survey () | – In the EU four broad models for PM funding (case study trastuzumab) – Most EU countries: combined hospital and 3rd party payer strategy – No combined funding for diagnostic test and medical treatment | ✔ | ✔ | |
| Lu et al. (2018) | US | Review of coverage ( payers) | – Important variation among guidelines, especially in private payers – A second HTA agency assessment could reduce coverage variation – Increased dialogue and sharing prior information to reduce coverage variation | ✔ | ✔ | ✔ |
| Lu et al. (2015) | Asia-Pacific countries | Literature review | – Most PAS focus on pharmaceuticals, few on medical technologies – Majority involve pricing arrangements, evidence generation rarely used – Australia has strong experience with PAS | ✔ | ✔ | ✔ |
| Lu et al. (2018) | Commentary | – Clinical utility unanswered for many genomic technologies – Propose building blocks for rapid generation of evidence – Proven analytical and clinical validity needed, collaborative models for action | ✔ | ✔ | ||
| Mattke et al. (2017) | Discussion | – Policy options to remedy the “free-rider" problem with high-cost cures – Incentives for patients, coordination among payers, government intervention – Collaborations for equitable mechanisms for cost-benefits distribution | ✔ | |||
| McCabe et al. (2008) | UK | Commentary | – NICE is the only entity to assesses effectiveness and cost-effectiveness – Cost-effectiveness threshold of NICE is £20,000 per QALY – Threshold should be regularly re-evaluated to match budget and innovation | ✔ | ||
| Meckley and Neumann (2010) | US | Case studies of diagnostics and treatments () | – Strength of evidence is the strongest predictor for drug reimbursement – Regulatory oversight and cost-effectiveness not associated to reimbursement – Absence of coverage triggers direct-to-consumer marketing | ✔ | ✔ | |
| Merlin et al. (2013) | Australia | Review of reimbursement | – Safety, effectiveness, and cost-effectiveness for reimbursement decisions – Linkage of different types of evidence and likely clinical benefits of drugs – Framework allows merging different data sources to increase the database | ✔ | ||
| Messner et al. (2016) | US | Policy Delphi panel () | – Proprietary variant databases are a key challenge for NGS coverage – Payer policies and perceived inconsistency in standards as a barrier – FDA regulation not strongly perceived as a barrier | ✔ | ✔ | ✔ |
| Miller et al. (2011) | EU | Market study | – Insufficient clarity on reimbursement and regulatory pathways for PM tests – Value-based public sector pricing required in Europe – EU market suffers from decentralization | ✔ | ✔ | |
| Pauly (2019) | Review of coverage | – Study on patterns of insurance coverage for PM and efficiency – Heterogeneity in marginal benefits call for partial coverage – Case studies: tests providing more benefits than costs should be fully covered | ✔ | |||
| Payne and Annemans (2013) | EU | Literature review | – Successful market access driven by generation of robust evidence – Take account of the different stakeholders’ perspectives – Suggestion of possible approaches and necessary timescales | ✔ | ✔ | |
| Phillips et al. (2017) | US | Review of coverage ( policies) | – Multigene tests do not fit standard coverage framework – High degree of variability in coverage assessment for multigene tests – Payers deny coverage because of lack of evidence and actionability | ✔ | ||
| Ramsey et al. (2006) | Commentary | – Currently, reimbursement is based on the price rather than clinical value – Reimbursement to move to an evidence- and value-based paradigm – Standardize presentation and filling information gap benefits all | ✔ | |||
| Schwarzer et al. (2015) | 11 countries | Review of thresholds | – Explicit cost-effectiveness thresholds only in two countries (UK and TH) – Implicit values in other countries and different decision-making rules – No PM-specific threshold found | ✔ | ||
| Simeonidis et al. (2019) | Literature review ( articles) | – Outcome of interventions mostly measured in QALYs – Total cost estimated upon direct medical cost data – Need for cost-utility analyses within national healthcare systems | ✔ | ✔ | ||
| Sullivan et al. (2011) | High-income countries | Review of cancer care delivery | – Clinicians require analytic and clinical validity before testing – Coverage with evidence development is an opportunity to generate data – Alternative business models to be developed and encouraged | ✔ | ||
| Terkola et al. (2017) | Commentary | – Lack of real-world data regarding costs and health outcomes – No study confronting clinical trial and real-world data – International coordination between regulators to establish standards | ✔ | |||
| Thomas et al. (2010) | US | Industry perspective | – Need for reimbursement that fosters evidence development – Reimbursement systems should develop clearer standards – Regulatory process has to integrate CDx in the appraisal of the drug | ✔ | ✔ | ✔ |
| Towse and Garrison (2013) | Commentary | – Collaboration between stakeholders needed to increase evidence creation – CED for realistic expectations for evidence standards – Public investment along with manufacturers and payers to generate data | ✔ | |||
| Trosman et al. (2010) | US | Interviews () | – Heterogeneity in clinical evidence perception among payers – Clinical effectiveness is a paramount factor in coverage decision for all payers – Approach to consider both clinical evidence and health care system factors | ✔ | ✔ | |
| Trosman et al. (2011) | US | Literature review and interviews () | – Payers use HTA more extensively for PM than for other technologies – Limited relevance if HTA unavailable and insufficient nonclinical factors – HTA organizations to improve their relevance to payers and clinicians | ✔ | ||
| Trosman et al. (2015) | US | Interviews ( experts / payers) | – Next-generation tumor sequencing deemed experimental/investigational – Efforts for evidence generation and incorporation into policies necessary – Misalignment between evidentiary methods and payers’ needs | ✔ | ||
| Trosman et al. (2017) | US | Interviews ( payers) | – Adjustment needed for PM to fit the coverage framework – All interviewees find that lack of evidence is a coverage barrier – Manufacturers need to include payers’ evidentiary requirements | ✔ | ||
| Trosman et al. (2018) | US | Review of coverage | – Three approaches to adapt coverage framework for tumor sequencing – RSA with manufacturer for performance data; CED for evidence generation – Technology-specific coverage based on number of genes | ✔ | ||
| Vegter et al. (2008) | Literature review ( articles) | – Level of consistency among economic analyses generally poor – Extensive sensitivity analyses and incorporate evidence-based data – Checklist for performing pharmacoeconomic analysis | ✔ | |||
| Vozikis et al. (2016) | EU | Review of pricing and reimbursement | – Overview of basic principles guiding governance of genomic testing services – Need for one single HTA agency for selection of priority areas – Merge all the current reimbursement processes under one committee | ✔ | ✔ |
References
- Akhmetov, Ildar, and Rostyslav V. Bubnov. 2017. Innovative payer engagement strategies: Will the convergence lead to better value creation in personalized medicine? EPMA Journal 8: 5–15. [Google Scholar] [CrossRef][Green Version]
- Amendola, Laura M., M. Ragan Hart, Robin L. Bennett, Martha Horike-Pyne, Michael Dorschner, Brian Shirts, and Gail P. Jarvik. 2019. Insurance coverage does not predict outcomes of genetic testing: The search for meaning in payer decisions for germline cancer tests. Journal of Genetic Counseling 28: 1208–13. [Google Scholar] [CrossRef]
- Antonanzas, Fernando, Reyes Juárez-Castelló, Carmeloand Lorente, and Roberto Rodríguez-Ibeas. 2019. The use of risk-sharing contracts in healthcare: Theoretical and empirical assessments. PharmacoEconomics 37: 1469–83. [Google Scholar] [CrossRef]
- Basu, Anirban. 2015. Financing cures in the United States. Expert Review of Pharmacoeconomics & Outcomes Research 15: 1–4. [Google Scholar] [CrossRef][Green Version]
- Boon, Wouter, Luis Martins, and Marc Koopmanschap. 2015. Governance of conditional reimbursement practices in the Netherlands. Health Policy 119: 180–85. [Google Scholar] [CrossRef] [PubMed]
- Carrera, Pricivel, and Maarten J. IJzerman. 2016. Are current ICER thresholds outdated? Valuing medicines in the era of personalized healthcare. Expert Review of Pharmacoeconomics & Outcomes Research 16: 435–37. [Google Scholar] [CrossRef]
- Chalkidou, Kalipso, and Sir Michael Rawlins. 2011. Pharmacogenetics and cost-effectiveness analysis: A two-way street. Drug Discovery Today 16: 873–77. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J., A. Wilson, and K. Manzolillo. 2013. Clinical and economic challenges facing pharmacogenomics. Pharmacogenomics Journal 13: 378–88. [Google Scholar] [CrossRef]
- Cohen, P. Joshua, and E. Abigail Felix. 2014. Personalized medicine’s bottleneck: Diagnostic test evidence and reimbursement. Journal of Personalized Medicine 4: 163–75. [Google Scholar] [CrossRef] [PubMed]
- Degtiar, Irina. 2017. A review of international coverage and pricing strategies for personalized medicine and orphan drugs. Health Policy 121: 1240–48. [Google Scholar] [CrossRef]
- Deverka, Patricia A., T. Doksum, and R. J. Carlson. 2007. Integrating Molecular Medicine into the US Health-care System: Opportunities, Barriers, and Policy Challenges. Clinical Pharmacology & Therapeutics 82: 427–34. [Google Scholar] [CrossRef]
- Deverka, Patricia A., and Jennifer C. Dreyfus. 2014. Clinical integration of next generation sequencing: Coverage and reimbursement challenges. The Journal of Law, Medicine & Ethics 42: 22–41. [Google Scholar] [CrossRef]
- Deverka, Patricia A. 2009. Pharmacogenomics, evidence, and the role of payers. Public Health Genomics 12: 149–57. [Google Scholar] [CrossRef] [PubMed]
- Dunn, Jessilyn, Ryan Runge, and Michael Snyder. 2018. Wearables and the medical revolution. Personalized Medicine 15: 429–48. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, Eric, Leven Annemans, Lou Garrison, Mark Helfand, Anke-Peggy Holtorf, John Hornberger, Dyfrig Hughes, Tracy Li, Daniel Malone, Katherine Payne, and et al. 2012. Challenges in the development and reimbursement of personalized medicine-payer and manufacturer perspectives and implications for health economics and outcomes research: A report of the ispor personalized medicine special interest group. Value in Health 15: 1162–71. [Google Scholar] [CrossRef]
- Faulkner, S. D., M. Lee, D. Qin, L. Morrell, E. Xoxi, A. Sammarco, S. Cammarata, P. Russo, L. Pani, and R. Barker. 2016. Pricing and reimbursement experiences and insights in the european union and the united states: Lessons learned to approach adaptive payer pathways. Clinical Pharmacology & Therapeutics 100: 730–42. [Google Scholar] [CrossRef]
- Ferrario, Alessandra, and Panos Kanavos. 2015. Dealing with uncertainty and high prices of new medicines: A comparative analysis of the use of managed entry agreements in Belgium, England, the Netherlands and Sweden. Social Science & Medicine 124: 39–47. [Google Scholar] [CrossRef]
- Fugel, Hans-Joerg, Mark Nuijten, and Maarten Postma. 2012. Stratified medicine and reimbursement issues. Frontiers in Pharmacology 3: 181. [Google Scholar] [CrossRef]
- Garfield, Susan. 2011. Advancing Access to Personalized Medicine: A Comparative Assessment of European Reimbursement Systems. Technical Report. Washington, DC: Personalized Medicine Coalition. [Google Scholar]
- Garrison, Louis P., and M.J. Finley Austin. 2006. Linking pharmacogenetics-based diagnostics and drugs for personalized medicine. Health Affairs 25: 1281–90. [Google Scholar] [CrossRef]
- Garrison, Louis P., and Adrian Towse. 2017. Value-based pricing and reimbursement in personalised healthcare: Introduction to the basic health economics. Journal of Personalized Medicine 7: 10. [Google Scholar] [CrossRef]
- Garrison, Louis P., Adrian Towse, Andrew Briggs, Gerard de Pouvourville, Jens Grueger, Penny E. Mohr, J.L. (Hans) Severens, Paolo Siviero, and Miguel Sleeper. 2013. Performance-based risk-sharing arrangements—Good practices for design, implementation, and evaluation: Report of the ISPOR good practices for performance-based risk-sharing arrangements task force. Value in Health 16: 703–19. [Google Scholar] [CrossRef] [PubMed]
- Graf, Michael D, Denise F Needham, Nicole Teed, and Trisha Brown. 2013. Genetic testing insurance coverage trends: A review of publicly available policies from the largest US payers. Personalized Medicine 10: 235–43. [Google Scholar] [CrossRef] [PubMed]
- Hall, Peter S., and Christopher McCabe. 2013. What evidence is there for the reimbursement of personalised medicine? Pharmacoeconomics 31: 181–83. [Google Scholar] [CrossRef] [PubMed]
- Hammond, Ray. 2020. The World In 2040—Future Health, Care & Well-Being. Available online: https://www.allianz-partners.com/en_US/press-and-media/reports/future-health-care-wellbeing.html (accessed on 15 October 2020).
- Henry, JaWanna, Yuriy Pylypchuk, Talisha Searcy, and Vaishali Patel. 2016. Adoption of electronic health record systems among u.s. non-federal acute care hospitals: 2008–2015. ONC Data Brief 35, Office of the National Coordinator for Health Information Technology. Available online: https://dashboard.healthit.gov/evaluations/data-briefs/non-federal-acute-care-hospital-ehr-adoption-2008-2015.php (accessed on 15 October 2020).
- Hresko, Andrew, and B. Susanne Haga. 2012. Insurance coverage policies for personalized medicine. Journal of Personalized Medicine 2: 201–16. [Google Scholar] [CrossRef] [PubMed]
- Kanavos, Panos, and Aris Angelis. 2013. Multiple criteria decision analysis for valuebased assessment of new medical technologies: A conceptual framework. Technical Report. London School of Economics and Political Science. Available online: http://eprints.lse.ac.uk/51211 (accessed on 15 October 2020).
- Knowles, Lori, Westerly Luth, and Tania Bubela. 2017. Paving the road to personalized medicine: Recommendations on regulatory, intellectual property and reimbursement challenges. Journal of Law and the Biosciences 4: 453–506. [Google Scholar] [CrossRef] [PubMed]
- Leopold, C., S. Vogler, C. Habl, A. K. Mantel-Teeuwisse, and J. Espin. 2013. Personalised medicine as a challenge for public pricing and reimbursement authorities—A survey among 27 European countries on the example of trastuzumab. Health Policy 113: 313–22. [Google Scholar] [CrossRef]
- Lu, Christine Y., Stephanie Loomer, Rachel Ceccarelli, Kathleen M. Mazor, James Sabin, Ellen Wright Clayton, Geoffrey S. Ginsburg, and Ann Chen Wu. 2018. Insurance coverage policies for pharmacogenomic and multi-gene testing for cancer. Journal of Personalized Medicine 8: 19. [Google Scholar] [CrossRef]
- Lu, Christine Y., Caitlin Lupton, Shana Rakowsky, Zaheer-Ud-Din Babar, Dennis Ross-Degnan, and Anita K. Wagner. 2015. Patient access schemes in Asia-pacific markets: Current experience and future potential. Journal of Pharmaceutical Policy and Practice 8: 6. [Google Scholar] [CrossRef]
- Lu, Christine Y., Marc S. Williams, Geoffrey S. Ginsburg, Sengwee Toh, Jeff S. Brown, and Muin J. Khoury. 2018. A proposed approach to accelerate evidence generation for genomic-based technologies in the context of a learning health system. Genetics in Medicine 20: 390–96. [Google Scholar] [CrossRef]
- Mattke, Soeren, Hangsheng Liu, Emily Hoch, and Andrew W Mulcahy. 2017. Avoiding the tragedy of the commons in health care: Policy options for covering high-cost cures. Rand Health Quarterly 6: 1. [Google Scholar]
- McCabe, Christopher, Karl Claxton, and Anthony J. Culyer. 2008. The NICE cost-effectiveness threshold. PharmacoEconomics 26: 733–44. [Google Scholar] [CrossRef] [PubMed]
- Meckley, Lisa M., and Peter J. Neumann. 2010. Personalized medicine: Factors influencing reimbursement. Health Policy 94: 91–100. [Google Scholar] [CrossRef]
- Merlin, Tracy, Claude Farah, Camille Schubert, Andrew Mitchell, Janet E. Hiller, and Philip Ryan. 2013. Assessing personalized medicines in Australia: A national framework for reviewing codependent technologies. Medical Decision Making 33: 333–42. [Google Scholar] [CrossRef] [PubMed]
- Messner, Donna A., Jennifer Al Naber, Pei Koay, Robert Cook-Deegan, Mary Majumder, Gail Javitt, Patricia Deverka, Rachel Dvoskin, Juli Bollinger, Margaret Curnutte, and et al. 2016. Barriers to clinical adoption of next generation sequencing: Perspectives of a policy Delphi panel. Applied and Translational Genomics 10: 19–24. [Google Scholar] [CrossRef][Green Version]
- Miller, Iain, Joanna Ashton-Chess, Herman Spolders, Vincent Fert, Joseph Ferrara, Werner Kroll, Jon Askaa, Patrick Larcier, Patrick F. Terry, Anne Bruinvels, and et al. 2011. Market access challenges in the EU for high medical value diagnostic tests. Personalized Medicine 8: 137–48. [Google Scholar] [CrossRef] [PubMed]
- Moher, David, Larissa Shamseer, Mike Clarke, Davina Ghersi, Alessandro Liberati, Mark Petticrew, Paul Shekelle, Lesley A. Stewart, and PRISMA-P Group. 2015. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 4: 1. [Google Scholar] [CrossRef]
- National Human Genome Research Institute. 2020. DNA Sequencing Costs: Data. Available online: https://www.genome.gov/about-genomics/fact-sheets/DNA-Sequencing-Costs-Data (accessed on 15 October 2020).
- Orofino, Javier, Javier Soto, Miguel A. Casado, and Itziar Oyagüez. 2010. Global spending on orphan drugs in France, Germany, the UK, Italy and Spain during 2007. Applied Health Economics and Health Policy 8: 301–15. [Google Scholar] [CrossRef]
- O’Sullivan, Brian P., David M. Orenstein, and Carlos E. Milla. 2013. Pricing for orphan drugs: Will the market bear what society cannot? JAMA 310: 1343–44. [Google Scholar] [CrossRef]
- Pauly, Mark V. 2019. Cost sharing in insurance coverage for precision medicine. In Economic Dimensions of Personalized and Precision Medicine. Chicago: University of Chicago Press, Chapter 10. pp. 159–84. [Google Scholar] [CrossRef]
- Payne, Katherine, and Lieven Annemans. 2013. Reflections on market access for personalized medicine: Recommendations for Europe. Value in Health 16: S32–S38. [Google Scholar] [CrossRef][Green Version]
- Phillips, Kathryn A., Patricia A. Deverka, Julia R. Trosman, Michael P. Douglas, James D. Chambers, Christine B. Weldon, and Andrew P. Dervan. 2017. Payer coverage policies for multigene tests. Nature Biotechnology 35: 614–17. [Google Scholar] [CrossRef]
- Pokorska-Bocci, Anna, Alison Stewart, Gurdeep S Sagoo, Alison Hall, Mark Kroese, and Hilary Burton. 2014. ’Personalized medicine’: What’s in a name? Personalized Medicine 11: 197–210. [Google Scholar] [CrossRef]
- Ramsey, Scott D., David L. Veenstra, Louis P. Garrison, Rick Carlson, Paul Billings, Josh Carlson, and Sean D. Sullivan. 2006. Toward evidence-based assessment for coverage and reimbursement of laboratory-based diagnostic and genetic tests. The American Journal of Managed Care 12: 197–202. [Google Scholar] [PubMed]
- Schey, Carina, Tsveta Milanova, and Adam Hutchings. 2011. Estimating the budget impact of orphan medicines in Europe: 2010–2020. Orphanet Journal of Rare Diseases 6: 62. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, Ruth, Ursula Rochau, Kim Saverno, Beate Jahn, Bernhard Bornschein, Nikolai Muehlberger, Magdalena Flatscher-Thoeni, Petra Schnell-Inderst, Gaby Sroczynski, Martina Lackner, and et al. 2015. Systematic overview of cost-effectiveness thresholds in ten countries across four continents. Journal of Comparative Effectiveness Research 4: 485–504. [Google Scholar] [CrossRef] [PubMed]
- Simeonidis, Stavros, Stefania Koutsilieri, Athanassios Vozikis, David N. Cooper, Christina Mitropoulou, and George P. Patrinos. 2019. Application of economic evaluation to assess feasibility for reimbursement of genomic testing as part of personalized medicine interventions. Frontiers in Pharmacology 10: 830. [Google Scholar] [CrossRef] [PubMed]
- Spear, Brian B, Margo Heath-Chiozzi, and Jeffrey Huff. 2001. Clinical application of pharmacogenetics. Trends in Molecular Medicine 7: 201–4. [Google Scholar] [CrossRef]
- Sullivan, Richard, Jeffrey Peppercorn, Karol Sikora, John Zalcberg, Neal J Meropol, Eitan Amir, David Khayat, Peter Boyle, Philippe Autier, Ian F Tannock, and et al. 2011. Delivering affordable cancer care in high-income countries. The Lancet Oncology 12: 933–80. [Google Scholar] [CrossRef]
- Sultana, Janet, Paola Cutroneo, and Gianluca Trifirò. 2013. Clinical and economic burden of adverse drug reactions. Journal of Pharmacology & Pharmacotherapeutics 4: S73–S77. [Google Scholar] [CrossRef]
- Terkola, Robert, Fernando Antoñanzas, and Maarten Postma. 2017. Economic evaluation of personalized medicine: A call for real-world data. The European Journal of Health Economics 18: 1065–67. [Google Scholar] [CrossRef]
- Thomas, Adrian, Audrey Phillips, Robert Donnelly, and Catherine Tak Piech. 2010. Comparative effectiveness, personalized medicine and innovation. PharmacoEconomics 28: 923–30. [Google Scholar] [CrossRef]
- Towse, Adrian, and Louis P. Garrison. 2013. Economic incentives for evidence generation: Promoting an efficient path to personalized medicine. Value in Health 16: S39–S43. [Google Scholar] [CrossRef]
- Trosman, Julia R., Stephanie L. Van Bebber, and Kathryn A. Phillips. 2010. Coverage policy development for personalized medicine: Private payer perspectives on developing policy for the 21-gene assay. Journal of Oncology Practice 6: 238–42. [Google Scholar] [CrossRef]
- Trosman, Julia R., Stephanie L. Van Bebber, and Kathryn A. Phillips. 2011. Health technology assessment and private payers’ coverage of personalized medicine. Journal of Oncology Practice 7: 18s–24s. [Google Scholar] [CrossRef] [PubMed]
- Trosman, Julia R., Christine B. Weldon, Michael P. Douglas, Allison W. Kurian, R. Kate Kelley, Patricia A. Deverka, and Kathryn A. Phillips. 2017. Payer coverage for hereditary cancer panels: Barriers, opportunities, and implications for the precision medicine initiative. Journal of the National Comprehensive Cancer Network 15: 219–28. [Google Scholar] [CrossRef] [PubMed]
- Trosman, Julia R., Christine B. Weldon, William J. Gradishar, Al B. Benson, Massimo Cristofanilli, Allison W. Kurian, James M. Ford, Alan Balch, John Watkins, and Kathryn A. Phillips. 2018. From the past to the present: Insurer coverage frameworks for next-generation tumor sequencing. Value in Health 21: 1062–68. [Google Scholar] [CrossRef] [PubMed]
- Trosman, Julia R., Christine B. Weldon, R. Kate Kelley, and Kathryn A. Phillips. 2015. Challenges of coverage policy development for next-generation tumor sequencing panels: Experts and payers weigh in. Journal of the National Comprehensive Cancer Network 13: 311–18. [Google Scholar] [CrossRef]
- Vegter, Stefan, Cornelis Boersma, Mark Rozenbaum, Bob Wilffert, GerJan Navis, and Maarten J. Postma. 2008. Pharmacoeconomic evaluations of pharmacogenetic and genomic screening programmes. PharmacoEconomics 26: 569–87. [Google Scholar] [CrossRef]
- Vozikis, Athanassios, David N. Cooper, Christina Mitropoulouc, Manousos E. Kambouris, Angela Brand, Vita Dolzan, Paolo Fortina, Federico Innocenti, Ming Ta Michael Lee, Lada Leyens, and et al. 2016. Test pricing and reimbursement in genomic medicine: Towards a general strategy. Public Health Genomics 19: 352–63. [Google Scholar] [CrossRef]
| 1. | The Web of Science Core Collection is a curated bibliographic database containing peer-reviewed scholarly journals, books and conference proceedings published worldwide in the sciences, social sciences, and arts and humanities disciplines. It is available at http://isiknowledge.com/wos. |
| 2. | The full query used is as follows: (ALL = “personalized health" OR ALL= “personalized health" OR ALL= “personalized healthcare" OR ALL= “personalised healthcare" OR ALL= “personalized health care" OR ALL= “personalised health care" OR ALL= “precision medicine" OR ALL= “individualized medicine" OR ALL= “individualised medicine" OR ALL= “personalized medicine" OR ALL= “personalised medicine" OR ALL= “stratified medicine" OR ALL= “genetic medicine" OR ALL= “genomic medicine") AND (ALL = “health insur*" OR ALL= “payer*" OR ALL =“reimbursement"). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalouguina, V.; Wagner, J. Challenges and Solutions for Integrating and Financing Personalized Medicine in Healthcare Systems: A Systematic Literature Review. J. Risk Financial Manag. 2020, 13, 283. https://doi.org/10.3390/jrfm13110283
Kalouguina V, Wagner J. Challenges and Solutions for Integrating and Financing Personalized Medicine in Healthcare Systems: A Systematic Literature Review. Journal of Risk and Financial Management. 2020; 13(11):283. https://doi.org/10.3390/jrfm13110283
Chicago/Turabian StyleKalouguina, Veronika, and Joël Wagner. 2020. "Challenges and Solutions for Integrating and Financing Personalized Medicine in Healthcare Systems: A Systematic Literature Review" Journal of Risk and Financial Management 13, no. 11: 283. https://doi.org/10.3390/jrfm13110283
APA StyleKalouguina, V., & Wagner, J. (2020). Challenges and Solutions for Integrating and Financing Personalized Medicine in Healthcare Systems: A Systematic Literature Review. Journal of Risk and Financial Management, 13(11), 283. https://doi.org/10.3390/jrfm13110283