Functionalized Titanium Dioxide Nanoparticle-Based Electrochemical Immunosensor for Detection of SARS-CoV-2 Antibody
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Titanium Dioxide (TiO2)
2.3. Synthesis of Chitosan-Functionalized Titanium Dioxide (TiO2-CS) Bio-Nanocomposite
2.4. Pre-Treatment of the Working Electrode
2.5. Assembly of the Immunosensing Platform for SARS-CoV-2 Antibody Detection
2.6. Morphological Analysis and Electrochemical Measurements
2.7. Sample Preparation
3. Results and Discussion
3.1. Optical Studies
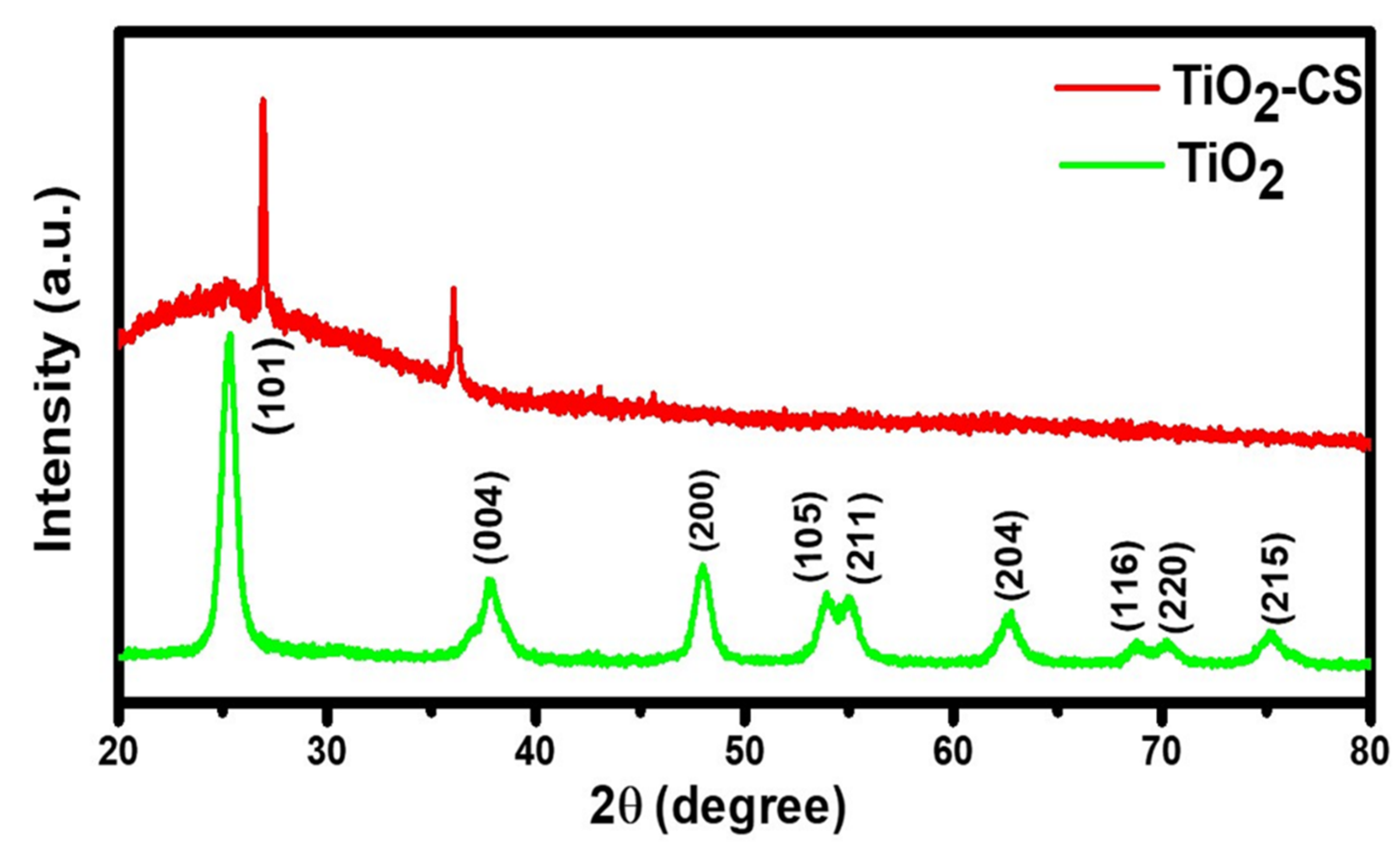
3.2. Structural Analysis
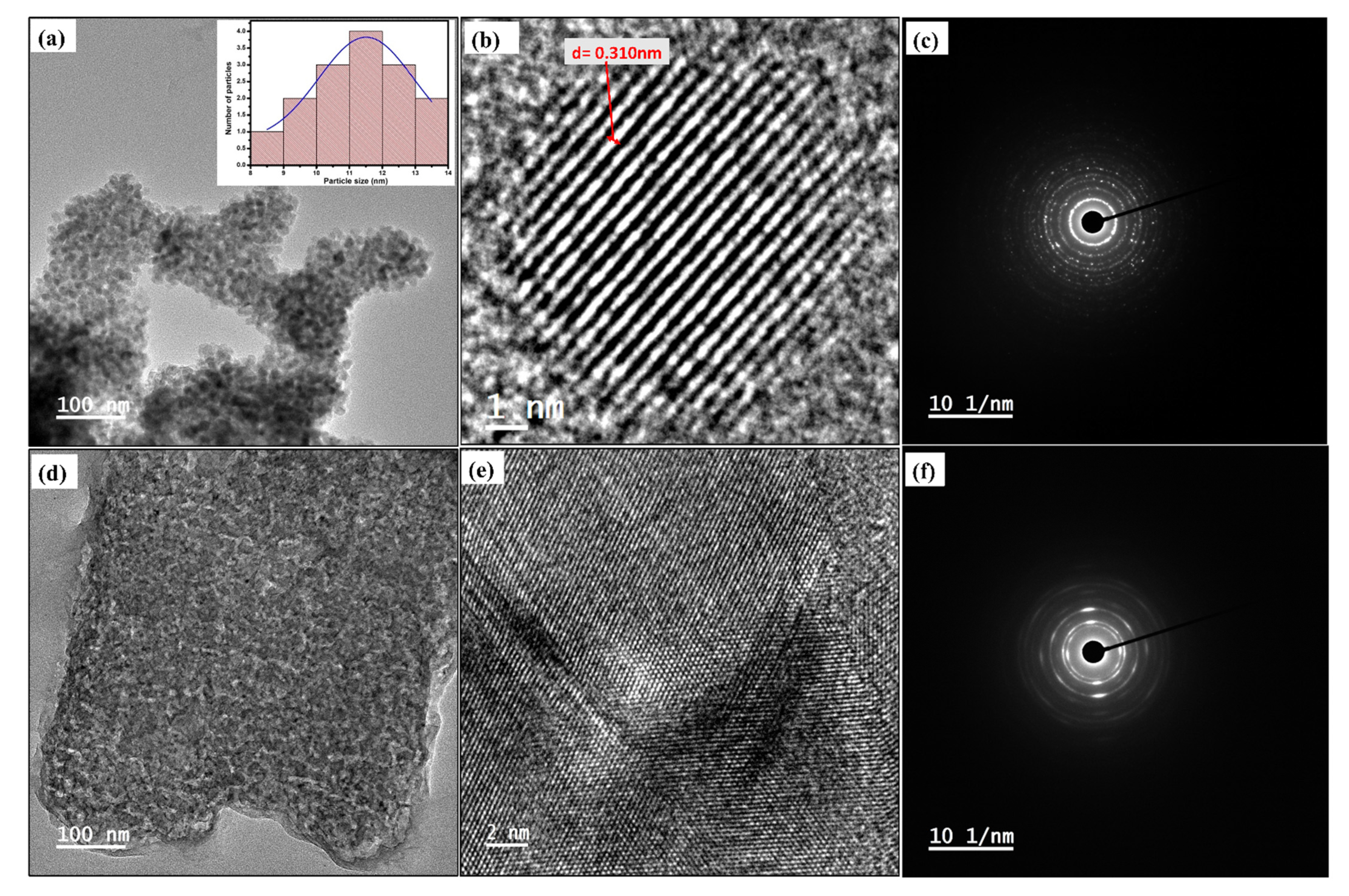
3.3. Morphological Investigation
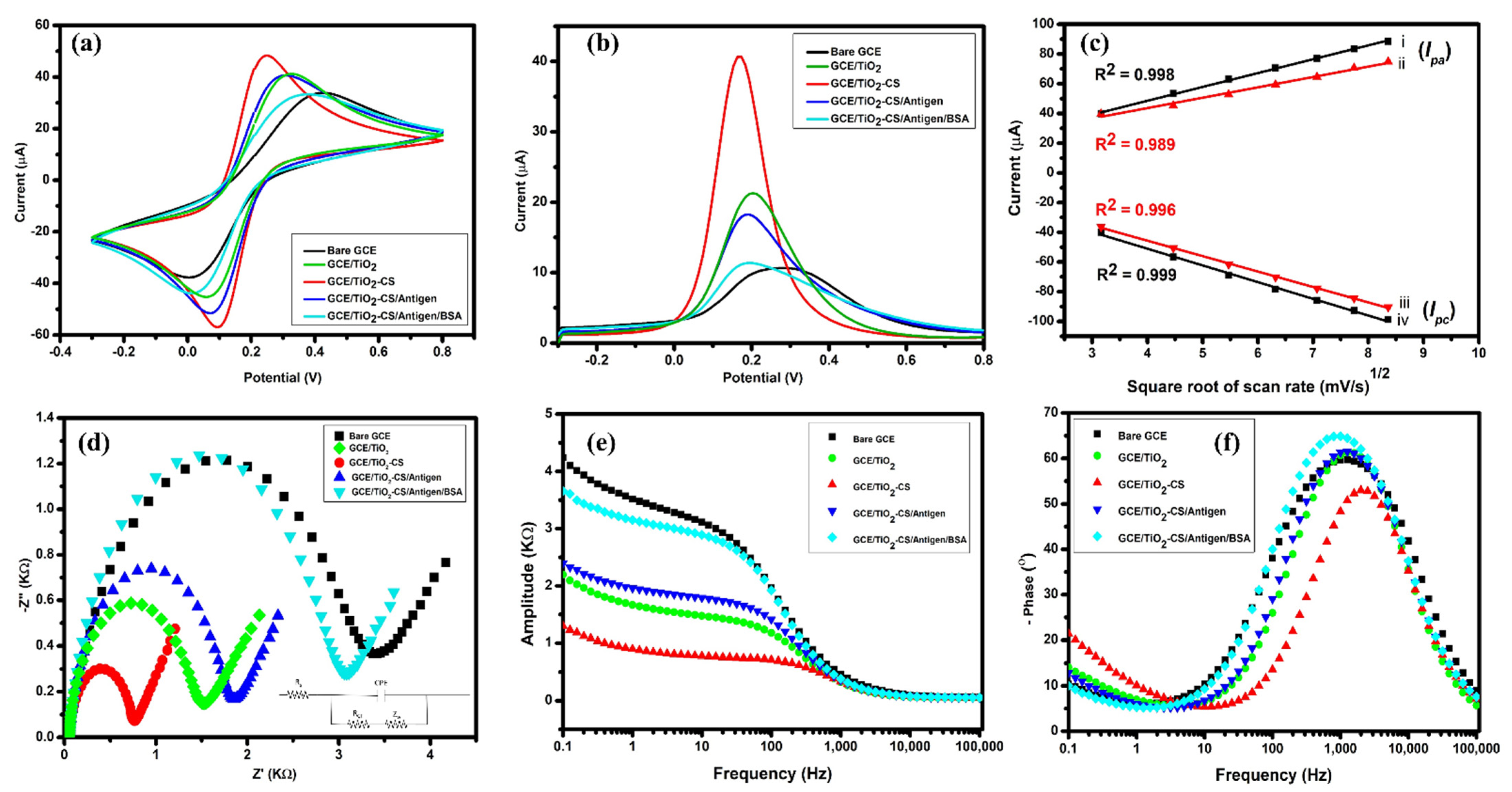
3.4. Electrochemical Analysis
3.5. Electrochemical Detection
3.6. Stability and Selectivity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parihar, A.; Ranjan, P.; Sanghi, S.K.; Srivastava, A.K.; Khan, R. Point-of-Care Biosensor-Based Diagnosis of COVID-19 Holds Promise to Combat Current and Future Pandemics. ACS Appl. Bio. Mater. 2020, 3, 7326–7343. [Google Scholar] [CrossRef]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 Diagnosis—A Review of Current Methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nagpal, S.; Kaushik, S.; Mendiratta, S. COVID-19 Diagnostic Approaches: Different Roads to the Same Destination. Virusdisease 2020, 31, 97–105. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martín-Sampedro, R.; del Real, G.; Aranda, P. Nanotechnology Responses to COVID-19. Adv. Healthc. Mater. 2020, 9, 2000979. [Google Scholar] [CrossRef]
- Bhalla, N.; Pan, Y.; Yang, Z.; Payam, A.F. Opportunities and Challenges for Biosensors and Nanoscale Analytical Tools for Pandemics: COVID-19. ACS Nano 2020, 14, 7783–7807. [Google Scholar] [CrossRef]
- Sadique, M.A.; Yadav, S.; Ranjan, P.; Verma, S.; Salammal, S.T.; Khan, M.A.; Kaushik, A.; Khan, R. High-Performance Antiviral Nano-Systems as a Shield to Inhibit Viral Infections: SARS-CoV-2 as a Model Case Study. J. Mater. Chem. B 2021, 9, 4620–4642. [Google Scholar] [CrossRef]
- Sadique, M.A.; Ranjan, P.; Yadav, S.; Khan, R. Chapter 8—Advanced High-Throughput Biosensor-Based Diagnostic Approaches for Detection of Severe Acute Respiratory Syndrome-Coronavirus-2. In Computational Approaches for Novel Therapeutic and Diagnostic Designing to Mitigate SARS-CoV2 Infection; Parihar, A., Khan, R., Kumar, A., Kaushik, A.K., Gohel, H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 147–169. ISBN 978-0-323-91172-6. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. Chapter 1—An Introduction to Nanotechnology. In An Introduction to Green Nanotechnology; Nasrollahzadeh, M., Sajadi, M., Atarod, M., Sajjadi, M., Issaabadi, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 1–27. ISBN 1573-4285. [Google Scholar]
- Ranjan, P.; Sadique, M.A.; Yadav, S.; Khan, R. Chapter 19—Approaches for Fabrication of Point-of-Care Biosensors for Viral Infection. In Advanced Biosensors for Virus Detection; Khan, R., Parihar, A., Kaushik, A., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 353–371. ISBN 978-0-12-824494-4. [Google Scholar]
- Ribeiro, B.V.; Cordeiro, T.A.R.; Freitas, G.R.O.E.; Ferreira, L.F.; Franco, D.L. Biosensors for the Detection of Respiratory Viruses: A Review. Talanta Open 2020, 2, 100007. [Google Scholar] [CrossRef]
- Malik, P.; Gupta, R.; Malik, V.; Ameta, R.K. Emerging Nanomaterials for Improved Biosensing. Meas. Sens. 2021, 16, 100050. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Kaushik, A.; Ranjan, P.; Khan, R.; Srivastava, A.K. Borophene as an Emerging 2D Flatland for Biomedical Applications: Current Challenges and Future Prospects. J. Mater. Chem. B 2022, 10, 1146–1175. [Google Scholar] [CrossRef]
- Yoo, H.; Shin, J.; Sim, J.; Cho, H.; Hong, S. Reusable Surface Plasmon Resonance Biosensor Chip for the Detection of H1N1 Influenza Virus. Biosens. Bioelectron. 2020, 168, 112561. [Google Scholar] [CrossRef]
- Woo, C.H.; Jang, S.; Shin, G.; Jung, G.Y.; Lee, J.W. Sensitive Fluorescence Detection of SARS-CoV-2 RNA in Clinical Samples via One-Pot Isothermal Ligation and Transcription. Nat. Biomed. Eng. 2020, 4, 1168–1179. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. Sers Based Lateral Flow Immunoassay for Point-of-Care Detection of Sars-Cov-2 in Clinical Samples. ACS Appl. Bio. Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef]
- Hou, Y.-H.; Wang, J.-J.; Jiang, Y.-Z.; Lv, C.; Xia, L.; Hong, S.-L.; Lin, M.; Lin, Y.; Zhang, Z.-L.; Pang, D.-W. A Colorimetric and Electrochemical Immunosensor for Point-of-Care Detection of Enterovirus 71. Biosens. Bioelectron. 2018, 99, 186–192. [Google Scholar] [CrossRef]
- Karakuş, E.; Erdemir, E.; Demirbilek, N.; Liv, L. Colorimetric and Electrochemical Detection of SARS-CoV-2 Spike Antigen with a Gold Nanoparticle-Based Biosensor. Anal. Chim. Acta 2021, 1182, 338939. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Z.; Holl, N.J.; Hassan, M.R.; Pappas, J.M.; Wei, C.; Izadi, O.H.; Wang, Y.; Dong, X.; Wang, C.; et al. MXene-Graphene Field-Effect Transistor Sensing of Influenza Virus and SARS-CoV-2. ACS Omega 2021, 6, 6643–6653. [Google Scholar] [CrossRef]
- Fathi-Hafshejani, P.; Azam, N.; Wang, L.; Kuroda, M.A.; Hamilton, M.C.; Hasim, S.; Mahjouri-Samani, M. Two-Dimensional-Material-Based Field-Effect Transistor Biosensor for Detecting COVID-19 Virus (SARS-CoV-2). ACS Nano 2021, 15, 11461–11469. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Graphene-Based Field-Effect Transistor Biosensors for the Rapid Detection and Analysis of Viruses: A Perspective in View of COVID-19. Carbon Trends 2021, 2, 100011. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, R.; Jaiswal, A. Recent Advances in Nanoparticle-Based Lateral Flow Immunoassay as a Point-of-Care Diagnostic Tool for Infectious Agents and Diseases. Analyst 2018, 143, 1970–1996. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Gu, B.; Liu, H.; Zhou, Z.; Shi, L.; Cheng, X.; Wang, S. Sensitive and Simultaneous Detection of SARS-CoV-2-Specific IgM/IgG Using Lateral Flow Immunoassay Based on Dual-Mode Quantum Dot Nanobeads. Anal. Chem. 2020, 92, 15542–15549. [Google Scholar] [CrossRef]
- Sadique, M.A.; Yadav, S.; Ranjan, P.; Khan, M.A.; Kumar, A.; Khan, R. Rapid Detection of SARS-CoV-2 Using Graphene-Based IoT Integrated Advanced Electrochemical Biosensor. Mater. Lett. 2021, 305, 130824. [Google Scholar] [CrossRef]
- Ranjan, P.; Singhal, A.; Yadav, S.; Kumar, N.; Murali, S.; Sanghi, S.K.; Khan, R. Rapid Diagnosis of SARS-CoV-2 Using Potential Point-of-Care Electrochemical Immunosensor: Toward the Future Prospects. Int. Rev. Immunol. 2021, 40, 126–142. [Google Scholar] [CrossRef]
- Farzin, M.A.; Abdoos, H. A Critical Review on Quantum Dots: From Synthesis toward Applications in Electrochemical Biosensors for Determination of Disease-Related Biomolecules. Talanta 2021, 224, 121828. [Google Scholar] [CrossRef]
- Ranjan, P.; Sadique, M.A.; Yadav, S.; Parihar, A.; Khan, R. Chapter 17—Miniaturized Analytical System for Point-of-Care Coronavirus Infection Diagnostics. In Advanced Biosensors for Virus Detection; Khan, R., Parihar, A., Kaushik, A., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 305–340. ISBN 978-0-12-824494-4. [Google Scholar]
- Ranjan, P.; Singhal, A.; Sadique, M.A.; Yadav, S.; Parihar, A.; Khan, R. Chapter 24—Scope of Biosensors, Commercial Aspects, and Miniaturized Devices for Point-of-Care Testing from Lab to Clinics Applications. In Biosensor Based Advanced Cancer Diagnostics; Khan, R., Parihar, A., Sanghi, S.K.B.T.-B.B.A.C.D., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 395–410. ISBN 978-0-12-823424-2. [Google Scholar]
- de Eguilaz, M.R.; Cumba, L.R.; Forster, R.J. Electrochemical Detection of Viruses and Antibodies: A Mini Review. Electrochem. Commun. 2020, 116, 106762. [Google Scholar] [CrossRef]
- Sayhi, M.; Ouerghi, O.; Belgacem, K.; Arbi, M.; Tepeli, Y.; Ghram, A.; Anik, Ü.; Österlund, L.; Laouini, D.; Diouani, M.F. Electrochemical Detection of Influenza Virus H9N2 Based on Both Immunomagnetic Extraction and Gold Catalysis Using an Immobilization-Free Screen Printed Carbon Microelectrode. Biosens. Bioelectron. 2018, 107, 170–177. [Google Scholar] [CrossRef]
- Layqah, L.A.; Eissa, S. An Electrochemical Immunosensor for the Corona Virus Associated with the Middle East Respiratory Syndrome Using an Array of Gold Nanoparticle-Modified Carbon Electrodes. Mikrochim. Acta 2019, 186, 224. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Wang, R.; Jiao, P.; Li, Y.; Li, Y.; Liao, M.; Yu, Y.; Wang, M. An Impedance Immunosensor Based on Low-Cost Microelectrodes and Specific Monoclonal Antibodies for Rapid Detection of Avian Influenza Virus H5N1 in Chicken Swabs. Biosens. Bioelectron. 2015, 67, 546–552. [Google Scholar] [CrossRef]
- Sadique, M.A.; Yadav, S.; Ranjan, P.; Khan, R.; Khan, F.; Kumar, A.; Biswas, D. Highly Sensitive Electrochemical Immunosensor Platforms for Dual Detection of SARS-CoV-2 Antigen and Antibody Based on Gold Nanoparticle Functionalized Graphene Oxide Nanocomposites. ACS Appl. Bio. Mater. 2022, 5, 2421–2430. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Khan, R.; Sathish, N.; Srivastava, A.K. Polydopamine Decorated MoS2 Nanosheets Based Electrochemical Immunosensor for Sensitive Detection of SARS-CoV-2 Nucleocapsid Protein in Clinical Samples. J. Mater. Chem. B 2022, 10, 8478–8489. [Google Scholar] [CrossRef]
- Khan, R. Supported TritonX-100 Polyaniline Nano-Porous Electrically Active Film onto Indium-Tin-Oxide Probe for Sensors Application. Adv. Chem. Eng. Sci. 2011, 1, 140–146. [Google Scholar] [CrossRef]
- Khan, R.; Dhayal, M. Nanocrystalline Bioactive TiO2-Chitosan Impedimetric Immunosensor for Ochratoxin-A. Electrochem. Commun. 2008, 10, 492–495. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ahour, F. An Electrochemical Biosensor Based on a Graphene Oxide Modified Pencil Graphite Electrode for Direct Detection and Discrimination of Double-Stranded DNA Sequences. Anal. Methods 2020, 12, 4541–4550. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chauhan, D.; Khan, Z.H.; Solanki, P.R. ZrO2 Nanoflowers Decorated with Graphene Quantum Dots for Electrochemical Immunosensing. ACS Appl. Nano Mater. 2020, 3, 2506–2516. [Google Scholar] [CrossRef]
- Ortiz-Casas, B.; Galdámez-Martínez, A.; Gutiérrez-Flores, J.; Baca Ibañez, A.; Panda, P.K.; Santana, G.; de la Vega, H.A.; Suar, M.; Rodelo, C.G.; Kaushik, A.; et al. Bio-Acceptable 0D and 1D ZnO Nanostructures for Cancer Diagnostics and Treatment. Mater. Today 2021, 50, 533–569. [Google Scholar] [CrossRef]
- Yu, S.; Zou, G.; Wei, Q. Ultrasensitive Electrochemical Immunosensor for Quantitative Detection of Tumor Specific Growth Factor by Using Ag@CeO2 Nanocomposite as Labels. Talanta 2016, 156–157, 11–17. [Google Scholar] [CrossRef]
- Büyüksünetçi, Y.T.; Çitil, B.E.; Tapan, U.; Anık, Ü. Development and Application of a SARS-CoV-2 Colorimetric Biosensor Based on the Peroxidase-Mimic Activity of γ-Fe2O3 Nanoparticles. Microchim. Acta 2021, 188, 335. [Google Scholar] [CrossRef]
- Li, H.; Wei, Q.; He, J.; Li, T.; Zhao, Y.; Cai, Y.; Du, B.; Qian, Z.; Yang, M. Electrochemical Immunosensors for Cancer Biomarker with Signal Amplification Based on Ferrocene Functionalized Iron Oxide Nanoparticles. Biosens. Bioelectron. 2011, 26, 3590–3595. [Google Scholar] [CrossRef]
- Luna, D.M.N.; Avelino, K.Y.P.S.; Cordeiro, M.T.; Andrade, C.A.S.; Oliveira, M.D.L. Electrochemical Immunosensor for Dengue Virus Serotypes Based on 4-Mercaptobenzoic Acid Modified Gold Nanoparticles on Self-Assembled Cysteine Monolayers. Sens. Actuators B Chem. 2015, 220, 565–572. [Google Scholar] [CrossRef]
- Guo, Y.-G.; Hu, J.-S.; Wan, L.-J. Nanostructured Materials for Electrochemical Energy Conversion and Storage Devices. Adv. Mater. 2008, 20, 2878–2887. [Google Scholar] [CrossRef]
- Lai, H.C.; Chin, S.F.; Pang, S.C.; Sum, M.S.H.; Perera, D. Carbon Nanoparticles Based Electrochemical Biosensor Strip for Detection of Japanese Encephalitis Virus. J. Nanomater. 2017, 2017, 3615707. [Google Scholar] [CrossRef]
- Li, X.; Qin, Z.; Fu, H.; Li, T.; Peng, R.; Li, Z.; Rini, J.M.; Liu, X. Enhancing the performance of paper-based electrochemical impedance spectroscopy nanobiosensors: An experimental approach. Biosens. Bioelectron. 2021, 177, 112672. [Google Scholar] [CrossRef]
- Liv, L.; Yener, M.; Çoban, G.; Can, Ş.A. Electrochemical biosensing platform based on hydrogen bonding for detection of the SARS-CoV-2 spike antibody. Anal. Bioanal. Chem. 2022, 414, 1313–1322. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. An Electrochemical Immunosensor Using SARS-CoV-2 Spike Protein-Nickel Hydroxide Nanoparticles Bio-Conjugate Modified SPCE for Ultrasensitive Detection of SARS-CoV-2 Antibodies. Microchem. J. 2021, 170, 106718. [Google Scholar] [CrossRef]
- Lorenzen, A.L.; Dos Santos, A.M.; Dos Santos, L.P.; da Silva Pinto, L.; Conceição, F.R.; Wolfart, F. PEDOT-AuNPs-based impedimetric immunosensor for the detection of SARS-CoV-2 antibodies. Electrochim. Acta 2022, 404, 139757. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Alves, J.F.; Frasco, M.F.; Piloto, A.M.; Serrano, V.; Mateus, D.; Sebastião, A.I.; Matos, A.M.; Carmo, A.; Cruz, T.; et al. An ultra-sensitive electrochemical biosensor using the Spike protein for capturing antibodies against SARS-CoV-2 in point-of-care. Mater. Today Bio. 2022, 16, 100354. [Google Scholar] [CrossRef]
- Durmus, C.; Hanoglu, S.B.; Harmanci, D.; Moulahoum, H.; Tok, K.; Ghorbanizamani, F.; Sanli, S.; Zihnioglu, F.; Evran, S.; Cicek, C.; et al. Indiscriminate SARS-CoV-2 Multivariant Detection Using Magnetic Nanoparticle-Based Electrochemical Immunosensing. Talanta 2022, 243, 123356. [Google Scholar] [CrossRef]
- Vlad-Bubulac, T.; Hamciuc, C.; Rîmbu, C.M.; Aflori, M.; Butnaru, M.; Enache, A.A.; Serbezeanu, D. Fabrication of Poly(Vinyl Alcohol)/Chitosan Composite Films Strengthened with Titanium Dioxide and Polyphosphonate Additives for Packaging Applications. Gels 2022, 8, 474. [Google Scholar] [CrossRef]
- Pehlivan, E.; Parlayıcı, Ş. Fabrication of a Novel Biopolymer-Based Nanocomposite (NanoTiO2-Chitosan-Plum Kernel Shell) and Adsorption of Cationic Dyes. J. Chem. Technol. Biotechnol. 2021, 96, 3378–3387. [Google Scholar] [CrossRef]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.; Jain, R. Polymer-Based Biomaterials: An Emerging Electrochemical Sensor BT—Handbook of Polymer and Ceramic Nanotechnology. In Handbook of Polymer and Ceramic Nanotechnology; Hussain, C.M., Thomas, S., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 1309–1327. ISBN 978-3-030-40513-7. [Google Scholar]
- Hashem, A.; Hossain, M.A.M.; Marlinda, A.R.; Mamun, M.; Simarani, K.; Johan, M.R. Nanomaterials Based Electrochemical Nucleic Acid Biosensors for Environmental Monitoring: A Review. Appl. Surf. Sci. Adv. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Prakash, M.; Ghosh, A.K. An Investigation on Optimization of Instantaneous Synthesis of TiO2 Nanoparticles and It’s Thermal Stability Analysis in PP-TiO2 Nanocomposite. Solid State Sci. 2021, 120, 106707. [Google Scholar] [CrossRef]
- Ziółkowski, R.; Jarczewska, M.; Drozd, M.; Zasada, A.A.; Malinowska, E. Studies on the Development of Electrochemical Immunosensor for Detection of Diphtheria Toxoid. J. Electrochem. Soc. 2019, 166, B472–B481. [Google Scholar] [CrossRef]
- Castañeda, C.; Tzompantzi, F.; Gómez, R.; Rojas, H. Enhanced Photocatalytic Degradation of 4-Chlorophenol and 2,4-Dichlorophenol on in Situ Phosphated Sol-Gel TiO2. J. Chem. Technol. Biotechnol. 2016, 91, 2170–2178. [Google Scholar] [CrossRef]
- Hussein, L.I.; Abdaleem, A.H.; Darwish, M.S.A.; Elsawy, M.A.; Mostafa, M.H. Chitosan/TiO2 Nanocomposites: Effect of Microwave Heating and Solution Mixing Techniques on Physical Properties. Egypt J. Chem. 2020, 63, 449–460. [Google Scholar] [CrossRef]
- Adnan, M.A.M.; Afzal, S.; Johan, M.R.; Julkapli, N.M. A Comparative Study on the Photodegradation Efficiency of TiO2-CS Hybrid Beads under Wet and Dry Conditions. Int. J. Mater. Prod. Technol. 2022, 65, 67–79. [Google Scholar] [CrossRef]
- Al-Taweel, S.S.; Saud, H.R.; Kadhum, A.A.H.; Takriff, M.S. The Influence of Titanium Dioxide Nanofiller Ratio on Morphology and Surface Properties of TiO2/Chitosan Nanocomposite. Results Phys. 2019, 13, 102296. [Google Scholar] [CrossRef]
- Bao, S.-J.; Li, C.M.; Zang, J.-F.; Cui, X.-Q.; Qiao, Y.; Guo, J. New Nanostructured TiO2 for Direct Electrochemistry and Glucose Sensor Applications. Adv. Funct. Mater. 2008, 18, 591–599. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, M.; Tai, X.; Li, H.; Han, X.; Yu, J. Analytical Transmission Electron Microscopy for Emerging Advanced Materials. Matter 2021, 4, 2309–2339. [Google Scholar] [CrossRef]
- Ranjan, P.; Sadique, M.A.; Yadav, S.; Khan, R. An Electrochemical Immunosensor Based on Gold-Graphene Oxide Nanocomposites with Ionic Liquid for Detecting the Breast Cancer CD44 Biomarker. ACS Appl. Mater. Interfaces 2022, 14, 20802–20812. [Google Scholar] [CrossRef]
- Lee, M.-H.; Liu, K.-H.; Thomas, J.L.; Chen, C.-Y.; Chen, C.-Y.; Yang, C.-H.; Lin, H.-Y. Doping of MXenes Enhances the Electrochemical Response of Peptide-Imprinted Conductive Polymers for the Recognition of C-Reactive Protein. Biosens. Bioelectron. 2022, 200, 113930. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Zarei, E.; Asghari, A. Surfactant Assisted Electrochemical Determination of Noscapine and Papaverine by TiO2 Nanoparticles/Multi-Walled Carbon Nanotubes Modified Carbon Paste Electrode. Russ. J. Electrochem. 2021, 57, 183–196. [Google Scholar] [CrossRef]
- Zarei, E.; Jamali, M.R.; Bagheri, J. Application of TiO2 Nanoparticles Modified Carbon Paste Electrode for the Determination of Vitamin B2. J. Anal. Chem. 2019, 74, 1213–1222. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.B.; Ismail, Z.H.; Subramaniam, S. Nanodiamond Conjugated SARS-CoV-2 Spike Protein: Electrochemical Impedance Immunosensing on a Gold Microelectrode. Microchimica. Acta 2022, 189, 226. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Hu, C.; Zhang, F.; Jahan, S.; Yuan, B.; Saleh, M.S.; Gao, S.-J.; Panat, R. N Protein-Based Ultrasensitive SARS-CoV-2 Antibody Detection in Seconds via 3D Nanoprinted, Microarchitected Array Electrodes. J. Med. Virol. 2022, 94, 2067–2078. [Google Scholar] [CrossRef]




| Sr. No | Nanomaterials | Electrochemical Technique | Limit of Detection | Recognition Element | Ref. |
|---|---|---|---|---|---|
| 1. | Zinc oxide nanowires (ZnO NWs) | Impedimetric | - | SARS-CoV-2 IgG antibody (CR3022) | [46] |
| 2. | Gold (Au) nanoparticles | Voltammetric | 0.03 fg mL−1 | SARS-CoV-2 spike antibody | [47] |
| 3. | Nickel hydroxide nanoparticles (Ni (OH)2 NPs) | Voltammetric | 0.3 fg mL−1 | SARS-CoV-2 specific viral antibodies | [48] |
| 4. | Poly (3,4-ethylenedioxythiophene)-Gold nanoparticles (PEDOT/AuNPs) | Impedimetric | - | SARS-CoV-2 antibodies | [49] |
| 5. | Carboxylated single-walled carbon nanotubes (SWCNTs) | Impedimetric | ~0.7 pg mL−1 | Antibodies against SARS-CoV-2 | [50] |
| 6. | TiO2-CS bio-nanocomposite | Voltammetric | 3.42 ag mL−1 | SARS-CoV-2 antibody | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadique, M.A.; Yadav, S.; Khare, V.; Khan, R.; Tripathi, G.K.; Khare, P.S. Functionalized Titanium Dioxide Nanoparticle-Based Electrochemical Immunosensor for Detection of SARS-CoV-2 Antibody. Diagnostics 2022, 12, 2612. https://doi.org/10.3390/diagnostics12112612
Sadique MA, Yadav S, Khare V, Khan R, Tripathi GK, Khare PS. Functionalized Titanium Dioxide Nanoparticle-Based Electrochemical Immunosensor for Detection of SARS-CoV-2 Antibody. Diagnostics. 2022; 12(11):2612. https://doi.org/10.3390/diagnostics12112612
Chicago/Turabian StyleSadique, Mohd Abubakar, Shalu Yadav, Vedika Khare, Raju Khan, Gagan Kant Tripathi, and Purnima Swarup Khare. 2022. "Functionalized Titanium Dioxide Nanoparticle-Based Electrochemical Immunosensor for Detection of SARS-CoV-2 Antibody" Diagnostics 12, no. 11: 2612. https://doi.org/10.3390/diagnostics12112612
APA StyleSadique, M. A., Yadav, S., Khare, V., Khan, R., Tripathi, G. K., & Khare, P. S. (2022). Functionalized Titanium Dioxide Nanoparticle-Based Electrochemical Immunosensor for Detection of SARS-CoV-2 Antibody. Diagnostics, 12(11), 2612. https://doi.org/10.3390/diagnostics12112612






