Potential Nitrogen Contributions by Tropical Legume Summer Cover Crops in Mediterranean-Type Cropping Systems
Abstract
1. Introduction
2. Materials and Methods
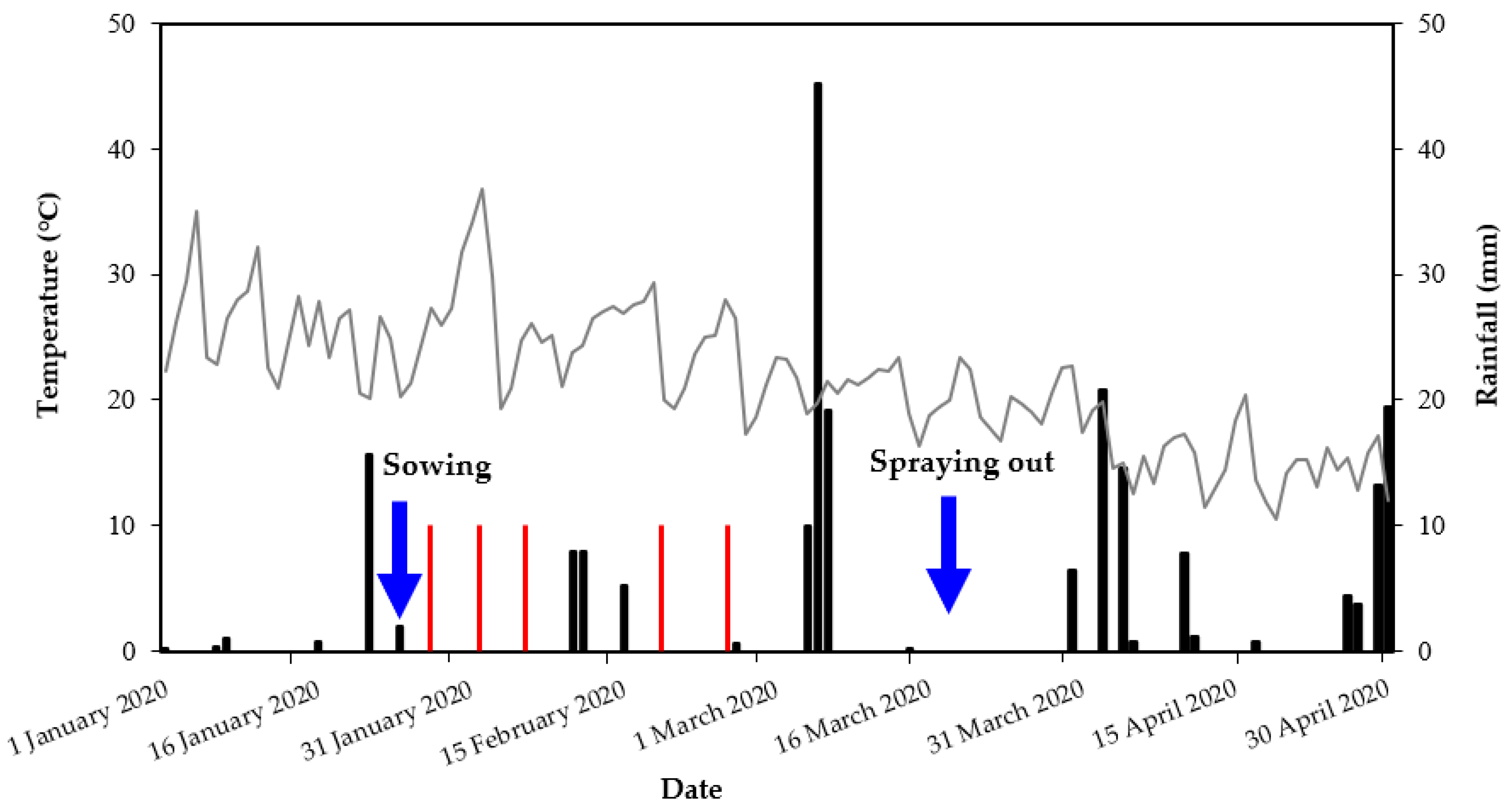
2.1. Field Trial
2.2. Cover Crop Glasshouse Trial
2.3. Statistical Analysis
3. Results
3.1. Glasshouse Experiment
3.2. Field Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Büchi, L.; Wendling, M.; Amossé, C.; Necpalova, M.; Charles, R. Importance of cover crops in alleviating negative effects of reduced soil tillage and promoting soil fertility in a winter wheat cropping system. Agric. Ecosyst. Environ. 2018, 256, 92–104. [Google Scholar] [CrossRef]
- Perrone, S.; Grossman, J.; Liebman, A.; Wells, S.; Sooksa-nguan, T.; Jordan, N. Legume cover crop contributions to ecological nutrient management in upper Midwest vegetable systems. Front. Sustain. Food Syst. 2022, 6, 712152. [Google Scholar] [CrossRef]
- Ruark, M.; Chawner, M.; Ballweg, M.; Proost, R.; Arriaga, F.; Stute, J. Does cover crop radish supply nitrogen to corn? Agron. J. 2018, 110, 1513–1522. [Google Scholar] [CrossRef]
- Dabney, S.M.; Delgado, J.A.; Reeves, D.W. Using winter cover crops to improve soil and water quality. Commun. Soil Sci. Plant Anal. 2001, 32, 1221–1250. [Google Scholar] [CrossRef]
- Hunt, J.R.; Kirkegaard, J.A. Re-evaluating the contribution of summer fallow rain to wheat yield in southern Australia. Crop Pasture Sci. 2011, 62, 915–929. [Google Scholar] [CrossRef]
- Rose, T.J.; Parvin, S.; Han, E.; Condon, J.; Flohr, B.M.; Schefe, C.; Rose, M.T.; Kirkegaard, J.A. Prospects for summer cover crops in southern Australian semi-arid cropping systems. Agric. Syst. 2022, 200, 103415. [Google Scholar] [CrossRef]
- McNee, M.E.; Rose, T.J.; Minkey, D.M.; Flower, K.C. Effects of dryland summer cover crops and a weedy fallow on soil water, disease levels, wheat growth and grain yield in a Mediterranean-type environment. Field Crop. Res. 2022, 280, 108472. [Google Scholar] [CrossRef]
- Wunsch, E.M.; Bell, L.W.; Bell, M.J. Can legumes provide greater benefits than millet as a spring cover crop in southern Queensland farming systems? Crop Pasture Sci. 2017, 68, 714, 746–759. [Google Scholar] [CrossRef]
- Angus, J.; Grace, P. Nitrogen balance in Australia and nitrogen use efficiency on Australian farms. Soil Res. 2017, 55, 435–450. [Google Scholar] [CrossRef]
- Shearer, G.; Kohl, D. N2 fixation in field settings: Estimations based on natural 15N abundance. Funct. Plant Biol. 1986, 13, 699–756. [Google Scholar] [CrossRef]
- Isbell, R.F. The Australian Soil Classification; CSIRO Publishing: Collingwood, Australia, 2002. [Google Scholar]
- McKenzie, N.J.; Isbell, R.F.; Jacquier, D.W.; Brown, K.L. Australian Soils and Landscapes: An Illustrated Compendium; CSIRO Publishing: Melbourne, Australia, 2004; Available online: http://hdl.handle.net/102.100.100/187731?index=1. (accessed on 20 March 2021).
- Rose, T.J.; Kearney, L.J.; Erler, D.V.; Rose, M.T.; Van Zwieten, L.; Raymond, C.A. Influence of growth stage and seed nitrogen on B values and potential contributions to error in estimating biological N2 fixation using the 15N natural abundance method. Plant Soil 2018, 425, 389–399. [Google Scholar] [CrossRef]
- McKenzie, N.J.; Coughlan, K.; Cresswell, H.P. Soil Physical Measurement and Interpretation for Land Evaluation; CSIRO Publishing: Melbourne, Australia, 2002; Available online: http://hdl.handle.net/102.100.100/198241?index=1 (accessed on 1 February 2021).
- Kearney, L.J.; Dutilloy, E.; Rose, T.J. Nitrogen fixation in summer-grown soybean crops and fate of fixed-N over a winter fallow in subtropical sugarcane systems. Soil Res. 2019, 57, 845–850. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models_. R Package Version 3.1-131. 2017. Available online: https://CRAN.R-project.org/package=nlme (accessed on 1 February 2021).
- Luo, D.; Ganesh, S.; Koolaard, J. Predictmeans: Calculate Predicted Means for Linear Models. R Package Version 0.99. 2014. Available online: https://CRAN.R-project.org/package=predictmeans (accessed on 5 April 2022).
- Kaveney, B.; Condon, J.; Doran, G.; Galea, F.; Rigg, J. Soil moisture impacts nitrification from nitrogen fertilisers treated with 3,4-dimethylpyrazole phosphate in acidic soils. Soil Res. 2021, 60, 86–101. [Google Scholar] [CrossRef]
- Di Bella, L.; Zahmel, M.; van Zwieten, L.; Rose, T.J. Weed suppression, biomass and nitrogen accumulation in mixed-species and single-species cover crops in a tropical sugarcane fallow. Agriculture 2021, 11, 640. [Google Scholar] [CrossRef]
- Rose, T.J.; Kearney, L.J.; Erler, D.V.; van Zwieten, L. Integration and potential nitrogen contributions of green manure inter-row legumes in coppiced tree cropping systems. Eur. J. Agron. 2019, 103, 47–53. [Google Scholar] [CrossRef]
- Herridge, D.F.; Robertson, M.J.; Cocks, B.; Peoples, M.B.; Holland, J.F.; Heuke, L. Low nodulation and nitrogen fixation of mungbean reduce biomass and grain yields. Aust. J. Exp. Agric. 2005, 45, 269–277. [Google Scholar] [CrossRef]
- Balkcom, K.; Reeves, D. Sunn-Hemp utilized as a legume cover crop for corn production. Agron. J. 2005, 97, 26–31. [Google Scholar] [CrossRef]
- Otto, R.; Pereira, G.L.; Tenelli, S.; Carvalho, J.L.N.; Lavres, J.; de Castro, S.A.Q.; Lisboa, I.P.; Sermarini, R.A. Planting legume cover crop as a strategy to replace synthetic N fertilizer applied for sugarcane production. Ind. Crop. Prod. 2020, 156, 112853. [Google Scholar] [CrossRef]
| Soil Property | Values |
|---|---|
| Soil texture | Loam |
| Soil colour | Brownish |
| Organic C (%) | 1.73 |
| Organic matter (%) | 4.0 |
| Bulk density (g cm−3) | 1.11 |
| Electrical conductivity (dS m−2) | 0.11 |
| pH (H2O) | 6.11 |
| Total C (%) | 2.3 |
| Total N (%) | 0.24 |
| Species | Cultivar | Inoculant Group | Shoot B Value (δ15N; ‰) |
|---|---|---|---|
| Balansa clover | Paradana | Group C | −0.43 ± 0.07 |
| Barrel medic | Paraggio | Group AM | −0.54 ± 0.04 |
| Mung bean | Crystal | Group I | −1.05 ± 0.08 |
| Sunn hemp | Global Sunn | Group M | −0.73 ± 0.05 |
| Lablab | Highworth | Group J | −1.26 ± 0.07 |
| Cowpea | Red Caloona | Group I | −1.85 ± 0.05 |
| Species | Shoot Biomass (g Plant−1) | Shoot N% | Shoot N Content (mg N Plant−1) | Shoot %Ndfa | Shoot N2 Fixation (mg N Plant−1) | Root N Content (mg N plant−1) | Estimated Whole Plant N2 Fixation (mg N plant−1) |
|---|---|---|---|---|---|---|---|
| Balansa clover | 0.4 ± 0.0 f | 5.2 ± 0.1 a | 22 ± 1 e | 15 ± 2 b | 3 ± 1 d | 5.8 ± 1.1 c | 4 ± 1 c |
| Barrel medic | 3.0 ± 0.1 e | 5.2 ± 0.2 a | 151 ± 7 d | 30 ± 1 ab | 47 ± 17 c | 23.2 ± 1.2 b | 54 ± 10 b |
| Mung bean | 7.6 ± 0.1 c | 4.7 ± 0.1 ab | 358 ± 12. b | 32 ± 1 a | 116 ± 5 a | 20.5 ± 3.1 b | 122 ± 5 a |
| Sunn hemp | 6.9 ± 0.2 d | 4.7 ± 0.3 ab | 324 ± 14 c | 33± 3 a | 108 ± 12 a | 22.5 ± 1.7 b | 116 ± 13 a |
| Lablab | 9.9 ± 0.4 a | 4.2 ± 0.22 b | 405 ± 4 a | 19 ± 1 ab | 75 ± 9 b | 33.3 ± 4.9 a | 81 ± 8 b |
| Cowpea | 8.7 ± 0.4 b | 4.6 ± 2.1 ab | 411 ± 22 a | 16 ± 2 b | 67 ± 6 bc | 35.8 ± 2.5 a | 73 ± 15 b |
| p-value | <0.001 | 0.011 | <0.001 | 0.006 | <0.001 | <0.001 | <0.001 |
| LSD0.05 | 0.72 | 0.54 | 39 | 10 | 30 | 8.7 | 31 |
| Treatments | Shoot Biomass (t ha−1) | Shoot N% | Shoot N Content (kg ha−1) | Shoot %Ndfa | Shoot N Fixation (kg ha−1) |
|---|---|---|---|---|---|
| Balansa clover | 0.2 ± 0.1 d | 3.1 ± 0.2 b | 8 ± 1 d | 72 ± 10 a | 6 ± 1 c |
| Barrel medic | 1.0 ± 0.5 cd | 3.1 ± 0.2 b | 32 ± 16 cd | 39 ± 6 b | 15 ± 9 c |
| Mung bean | 2.0 ± 0.2 b | 3.2 ± 0.1 b | 63 ± 6 bc | 42 ± 10 b | 26 ± 7 bc |
| Sunn hemp | 1.9 ± 0.2 b | 3.8 ± 0.3 ab | 75 ± 14 b | 73 ± 3 a | 55 ± 10 a |
| Lablab | 1.2 ± 0.3 bc | 4.1 ± 0.5 a | 49 ± 13 bc | 50 ± 4 b | 26 ± 8 bc |
| Cowpea | 3.3 ± 0 a | 3.3 ± 0.2 b | 114 ± 13 a | 40 ± 5 b | 46 ± 10 ab |
| p-value | <0.001 | 0.007 | <0.001 | <0.001 | 0.001 |
| LSD | 0.78 | 0.57 | 32 | 17 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parvin, S.; Condon, J.; Rose, T.J. Potential Nitrogen Contributions by Tropical Legume Summer Cover Crops in Mediterranean-Type Cropping Systems. Nitrogen 2022, 3, 592-599. https://doi.org/10.3390/nitrogen3040038
Parvin S, Condon J, Rose TJ. Potential Nitrogen Contributions by Tropical Legume Summer Cover Crops in Mediterranean-Type Cropping Systems. Nitrogen. 2022; 3(4):592-599. https://doi.org/10.3390/nitrogen3040038
Chicago/Turabian StyleParvin, Shahnaj, Jason Condon, and Terry J. Rose. 2022. "Potential Nitrogen Contributions by Tropical Legume Summer Cover Crops in Mediterranean-Type Cropping Systems" Nitrogen 3, no. 4: 592-599. https://doi.org/10.3390/nitrogen3040038
APA StyleParvin, S., Condon, J., & Rose, T. J. (2022). Potential Nitrogen Contributions by Tropical Legume Summer Cover Crops in Mediterranean-Type Cropping Systems. Nitrogen, 3(4), 592-599. https://doi.org/10.3390/nitrogen3040038







