Biosorption of Eriochrome Black T Using Exserohilum rostratum NMS1.5 Mycelia Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
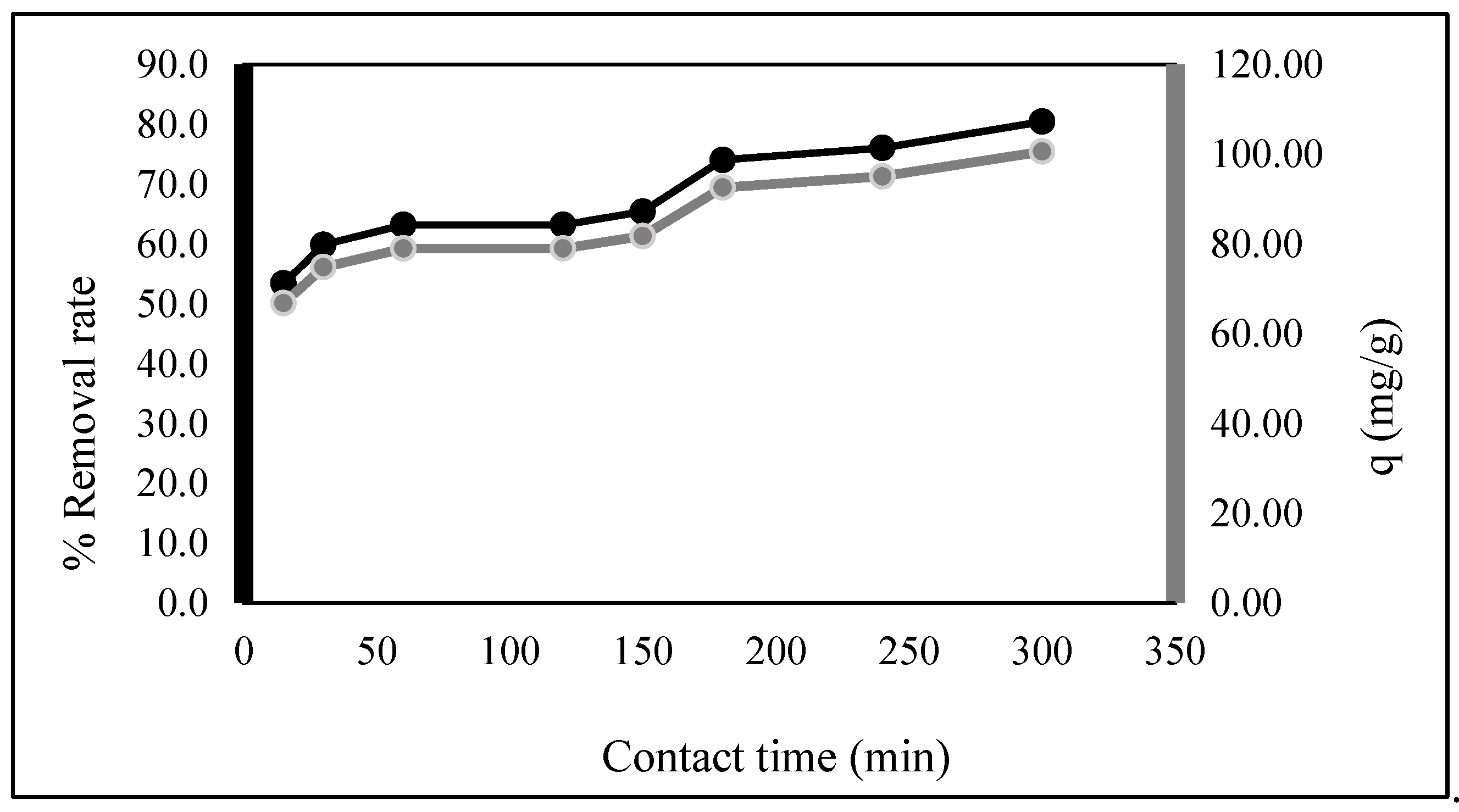
2.2. Biosorption Experiments
2.3. Characterization
3. Results and Discussion
3.1. FTIR Data before and after Adsorption
3.2. The pH Effects
3.3. Effects of Initial EBT Concentration
3.4. Isotherm Studies
3.5. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Yi, B.; Yuan, Q.; Wu, Y.; Wang, M.; Yan, S. Removal of methylene blue from aqueous solution by cattle manure-derived low temperature biochar. RSC Adv. 2018, 8, 19917–19929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Pu, S.; Hou, Y.; Zhu, R.; Zinchenko, A.; Chu, W. A highly efficient magnetic chitosan “fluid” adsorbent with a high capacity and fast adsorption kinetics for dyeing wastewater purification. J. Chem. Eng. 2018, 345, 556–565. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes-Environmental Impact and Remediation. In Environmental Protection Strategies for Sustainable Development; Strategies for Sustainability; Malik, A., Grohmann, E., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 111–162. [Google Scholar]
- Barka, N.; Abdennouri, M.; El Makhfouk, M. Removal of methylene blue and eriochrome black T from aqueous solution by biosorption on Scolymus hispanicus L.: Kinetics, equilibrium and thermodynamics. J. Taiwan Inst. Chem. Eng. 2011, 42, 320. [Google Scholar] [CrossRef]
- San, N.O.; Celebioglu, A.; Tümtaş, Y.; Uyar, T.; Tekinay, T. Reusable bacteria immobilized electrospun nanofibrous webs for decolorization of methylene blue dye in wastewater treatment. RSC Adv. 2014, 4, 32249–32255. [Google Scholar] [CrossRef] [Green Version]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Arikan, E.B.; Isik, Z.; Bouras, H.D.; Dizge, N. Investigation of immobilized filamentous fungi for treatment of real textile industry wastewater using up flow packed bed bioreactor. Bioresour. Technol. 2019, 7, 100197. [Google Scholar] [CrossRef]
- Hidayat, E.; Harada, H.; Mitoma, Y.; Yonemura, S.; Halem, H.I.A. Rapid removal of acid red 88 by zeolite/chitosan hydrogel in aqueous solution. Polymers 2022, 14, 893. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Malachite green ‘a cationic dye’ and its removal from aqueous solution by adsorption. Appl. Water Sci. 2017, 7, 3407–3445. [Google Scholar] [CrossRef] [Green Version]
- Gusmão, G.K.A.; Gurgel, A.L.V.; Melo, S.T.M.; Gil, L.F. Application of succinylated sugarcane bagasse as adsorbent to remove methylene blue and gentian violet from aqueous solutions-Kinetic and equilibrium studies. Dyes Pigm. 2012, 92, 967–974. [Google Scholar] [CrossRef] [Green Version]
- Khadijah, O.; Lee, K.K.; Abdullah, M.F.F. Isolation, screening and development of local bacterial consortia with azo dyes decolourising capability. Malays. J. Microbiol. 2009, 5, 25–32. [Google Scholar] [CrossRef]
- Boudechiche, N.; Mokaddem, H.; Sadaoui, Z.; Trari, M. Biosorption of cationic dye from aqueous solutions onto lignocellulosic biomass (Luffa cylindrica): Characterization, equilibrium, kinetic and thermodynamic studies. Int. J. Ind. Chem. 2016, 7, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Khaekhum, S.; Ekspraset, J.; Suebrasri, T.; Mongkolthanaruk, W.; Riddech, N.; Jogloy, S.; Boonlue, S. The first member of Exserohilum rostratum beneficial for promoting growth and yield of sunchoke (Helianthus tuberosus L.). Rhizosphere 2021, 19, 100379. [Google Scholar] [CrossRef]
- Sripiya, R.; Kumar, R. A novel enzymatic method for preparation and characterization of collagen film from swim bladder of fish rohu (Labeo rohita). Food Sci. Nutr. 2015, 6, 1468–1478. [Google Scholar] [CrossRef] [Green Version]
- Rezaee, J.; Salimi, F.; Karami, C. Removal of eriochrome black T dye from water solution using modified nano-boehmite by organic compounds. Desalination Water Treat 2019, 139, 342–351. [Google Scholar] [CrossRef]
- Chettri, P.; Singh, M.K.; Tripathi, A.; Pathak, A.P.; Mandal, R.K.; Tiwari, A. Eriochrome Black T sensing using silver nanoparticle-reduced graphene oxide composite via luminescent “turn-of” mechanism and its biosorption on guava (Psidium guajava) leaf powder. Graphene Technol. 2019, 4, 41–51. [Google Scholar] [CrossRef]
- Miraboutalebi, S.M.; Nikouzad, S.K.; Peydayesh, M.; Allahgholi, N.; Vafajoo, L.; McKay, G. Methylene blue adsorption via maize silk powder: Kinetic, equilibrium, thermodynamic studies and residual error analysis. Process Saf. Environ. Prot. 2017, 106, 191–202. [Google Scholar] [CrossRef]
- Leal, P.V.B.; Gregório, A.M.; Otoni, E.; da Silva, P.R.; de Krauser, M.O.; Holzbach, J.C. Study of adsorption of methylene blue dye on babassu. J. Biotec. Biodiv. 2012, 3, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, R.F.; Lima, A.C.A.; Vidal, C.B.; Melo, D.Q.; Raulino, G.S.C. Adsorption: Theoretical Aspects and Environmental Applications, 1st ed.; UFC: Fortaleza, Brazil, 2014; p. 256. [Google Scholar]
- Rangabhashiyam, S.; Lata, S.; Balasubramanian, P. Biosorption characteristics of methylene blue and malachite green from simulated wastewater onto Carica papaya wood biosorbent. Surf. Interfaces 2018, 10, 197–215. [Google Scholar] [CrossRef]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interf. Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef]
- Vadivelan, V.K.; Kumar, V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interf. Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Tang, Y.; Zeng, Y.; Hu, T.; Zhou, Q.; Peng, Y. Preparation of lignin sulfonate-based mesoporous materials for adsorbing malachite green from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 2900–2910. [Google Scholar] [CrossRef]
- Pal, S.; Ghorai, S.; Das, C.; Samrat, S.; Ghosh, A.; Panda, A.B. Carboxymethyl tamarind-g-poly (acrylamide)/silica: A high performance hybrid nanocomposite for adsorption of methylene blue dye. Ind. Eng. Chem. Res. 2012, 51, 15546–15556. [Google Scholar] [CrossRef]
- Silva, F.; Nascimento, L.; Brito, M.; da Silva, K.; Paschoal, W., Jr.; Fujiyama, R. Biosorption of methylene blue dye using natural biosorbents made from weeds. Materials 2019, 12, 2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Khan, A.A.; Chowdhury, A.; Bhakta, A.K.; Mekhalif, Z.; Hussain, S. Efficient and highly selective adsorption of cationic dyes and removal of ciprofloxacin antibiotic by surface modified nickel sulfide nanomaterials: Kinetics, isotherm and adsorption mechanism. Colloids Surf. 2020, 586, 124264. [Google Scholar] [CrossRef]





| qexp (mg/g) | Langmuir Parameters | Freundlich Parameters | |||||
|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | RL | Kf | 1/n | R2 | |
| 100.61 | 155.71 | 9.3906 | 0.877 | 0.001 | 307.38 | 0.68 | 0.988 |
| Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|
| qe (mg/g) | K1 (mg min/g) | R2 | qe (mg/g) | K2 (mg min/g) | R2 |
| 10.68 | −4.267 × 10−5 | 0.563 | 101.01 | 1.23 × 1011 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidayat, E.; Khaekhum, S.; Yonemura, S.; Mitoma, Y.; Harada, H. Biosorption of Eriochrome Black T Using Exserohilum rostratum NMS1.5 Mycelia Biomass. J 2022, 5, 427-434. https://doi.org/10.3390/j5040029
Hidayat E, Khaekhum S, Yonemura S, Mitoma Y, Harada H. Biosorption of Eriochrome Black T Using Exserohilum rostratum NMS1.5 Mycelia Biomass. J. 2022; 5(4):427-434. https://doi.org/10.3390/j5040029
Chicago/Turabian StyleHidayat, Endar, Saranya Khaekhum, Seiichiro Yonemura, Yoshiharu Mitoma, and Hiroyuki Harada. 2022. "Biosorption of Eriochrome Black T Using Exserohilum rostratum NMS1.5 Mycelia Biomass" J 5, no. 4: 427-434. https://doi.org/10.3390/j5040029






