Fermented and Aged Ginseng Sprouts (Panax ginseng) and Their Main Component, Compound K, Alleviate Asthma Parameters in a Mouse Model of Allergic Asthma through Suppression of Inflammation, Apoptosis, ER Stress, and Ferroptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented and Aged Ginseng Sprouts Extract
2.2. Analysis of Ginsenoside Compounds
2.3. Preparation of 50% EtOH Extract Concentrates for Animal Experiments
2.4. Animal Care
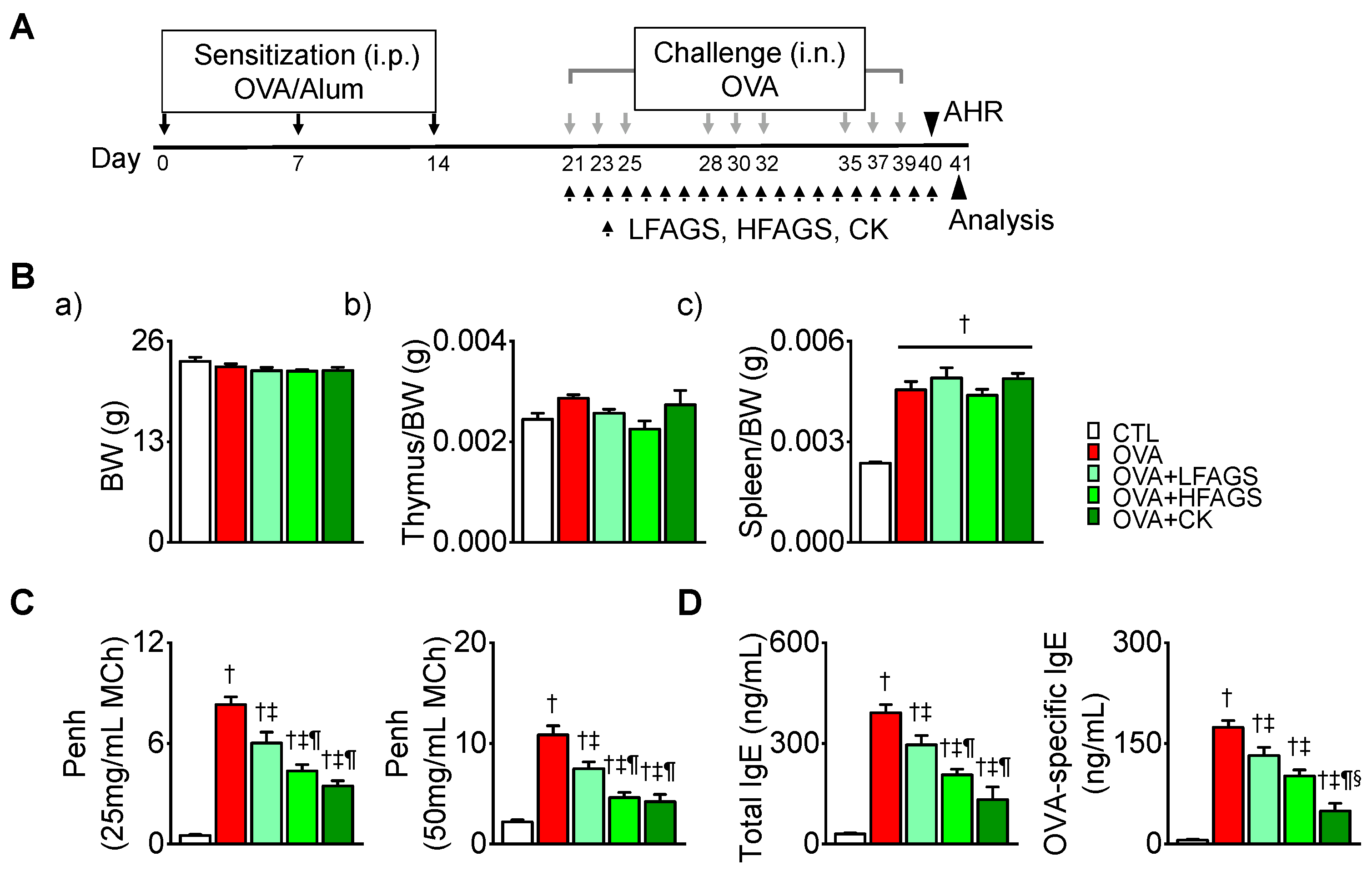
2.5. Induction of Allergic Asthma and Treatment
2.6. MCh Test
2.7. Collection of Bronchoalveolar Lavage Fluid (BALF) and Differential Cell Count
2.8. Measurement of Total and OVA-Specific Immunoglobulin E (IgE) in Plasma
2.9. Immunoassay for T helper Type 2 (Th2) Cytokines, Histamine, and Tryptase in BALF
2.10. Lung Histology
2.11. Prussian Blue Staining for Iron in Lung Tissue
2.12. Immunohistochemistry in Lung Tissue
2.13. Measurement of Free Radical Activity and Calcium Concentration in Lung Tissue
2.14. Measurement of Malondialdehyde (MDA) Concentration in Lung Tissue
2.15. Terminal Deoxynucleotidyl Transferase dUTP Nick end Labeling (TUNEL) Assay
2.16. Western Blot Analysis
2.17. Statistics
3. Results
3.1. Contents of Ginsenoside Derivatives of GS and FAGS
3.2. FAGS and CK Reduced OVA-Induced Airway Hyperresponsiveness and IgE Production
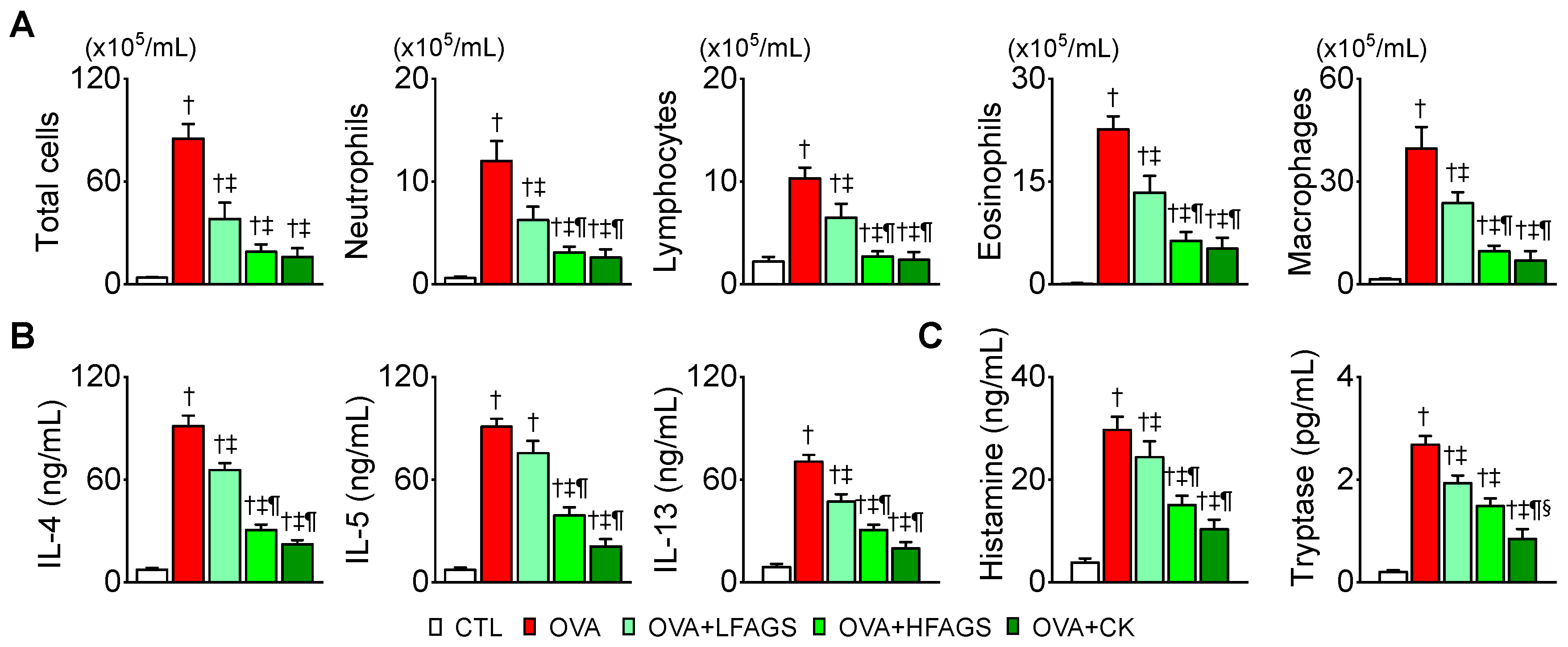
3.3. FAGS and CK Alleviated OVA-Induced Airway Inflammation in Bronchoalveolar Lavage Fluid
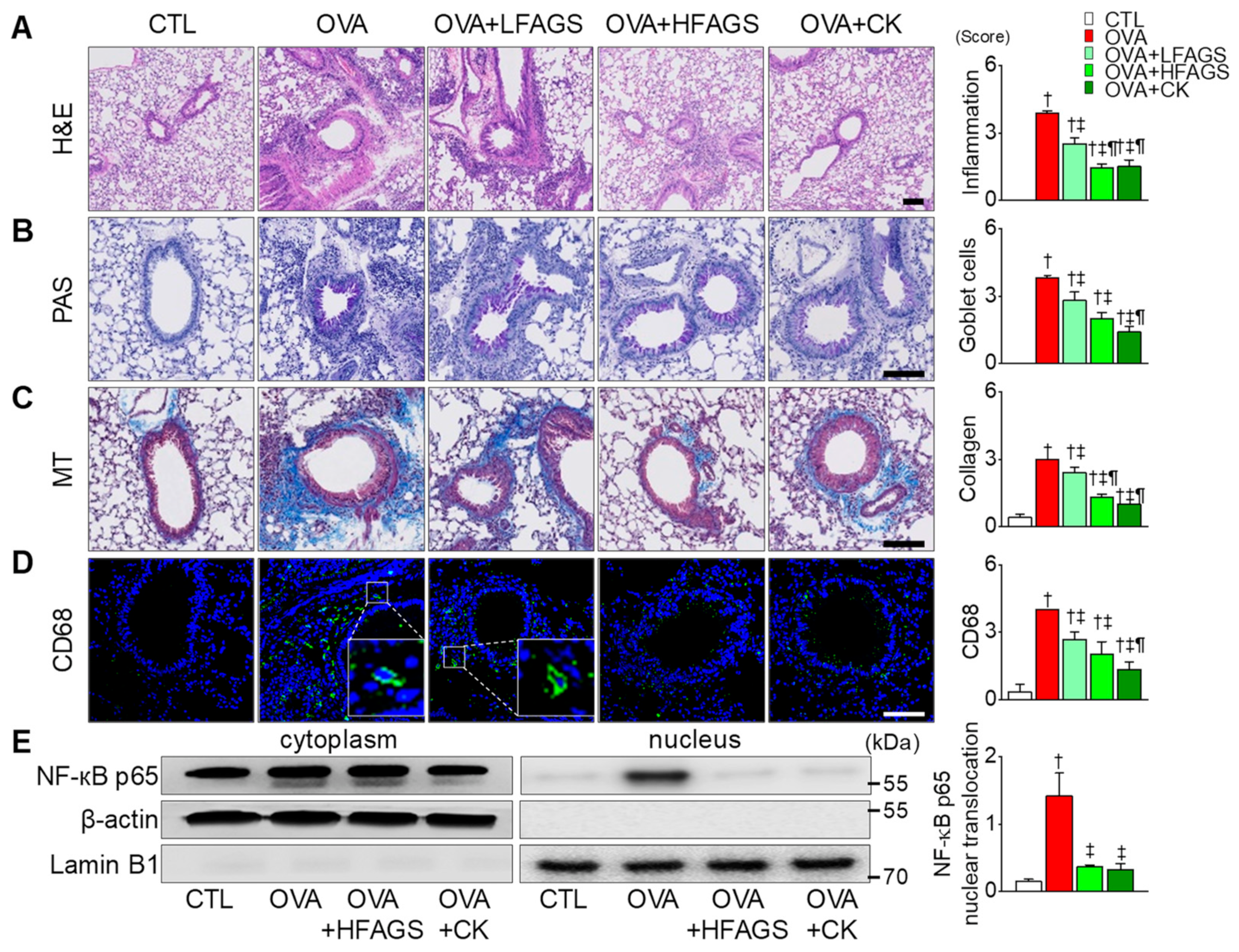
3.4. FAGS and CK Reduced OVA-Induced Airway Inflammation in Lung Tissues
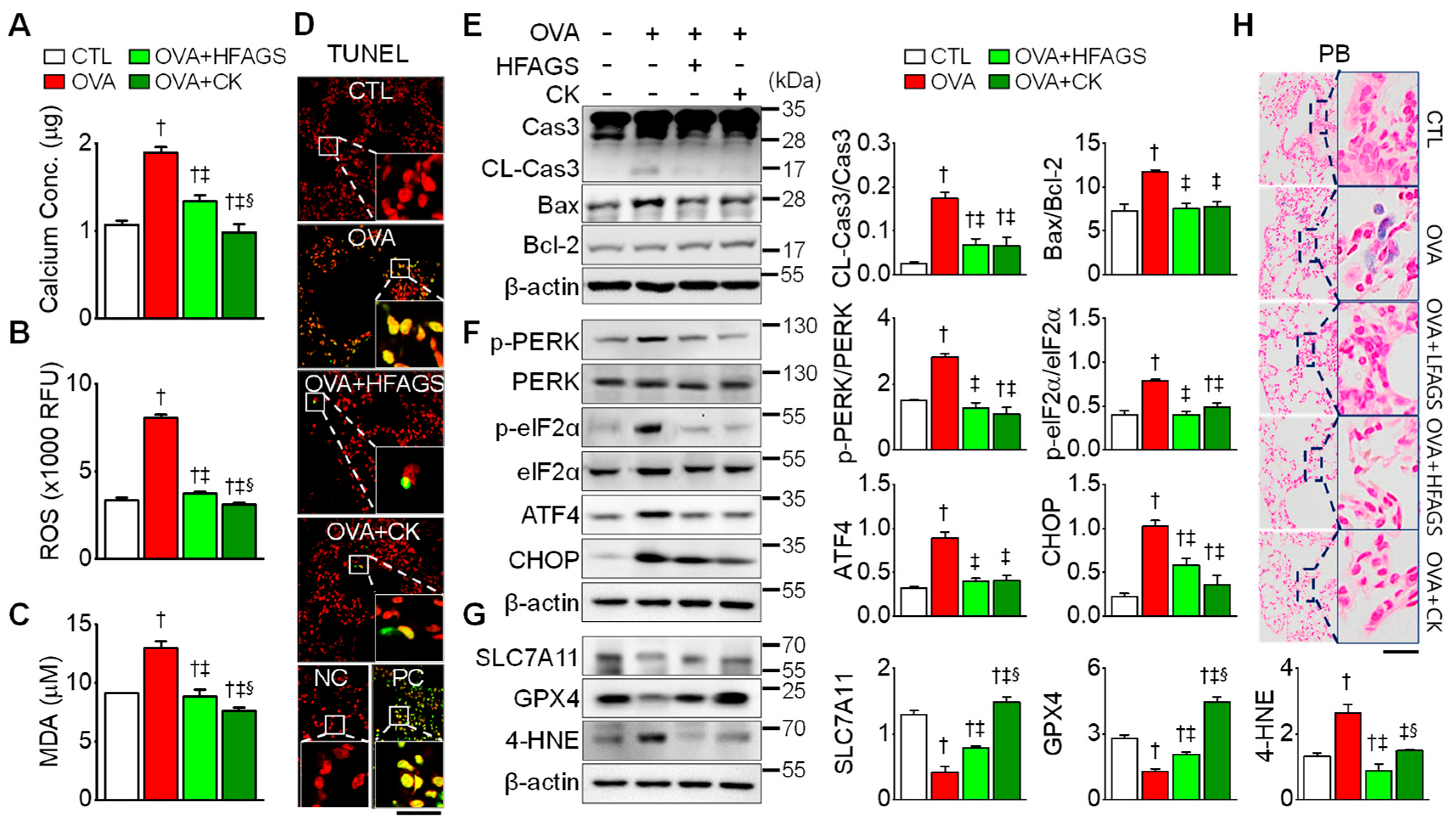
3.5. FAGS and CK Attenuated OVA-Induced Apoptosis, ER Stress, and Ferroptotic Signals in Lung Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiotiu, A. Biomarkers in asthma: State of the art. Asthma Res. Pract. 2018, 4, 10. [Google Scholar] [CrossRef]
- Agache, I.; Eguiluz-Gracia, I.; Cojanu, C.; Laculiceanu, A.; Del Giacco, S.; Zemelka-Wiacek, M.; Kosowska, A.; Akdis, C.A.; Jutel, M. Advances and highlights in asthma in 2021. Allergy 2021, 76, 3390–3407. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.W.; Ford, M.L.; Rogers, L.K.; Britt, R.D., Jr. Oxidative Stress Promotes Corticosteroid Insensitivity in Asthma and COPD. Antioxidants 2021, 10, 1335. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Abubakar-Waziri, H.; Lakhdar, R.; Raby, K.; Dixey, P.; Adcock, I.M.; Mumby, S.; Bhavsar, P.K.; Chung, K.F. Molecular mechanisms of oxidative stress in asthma. Mol. Asp. Med. 2022, 85, 101026. [Google Scholar] [CrossRef]
- Bazan-Socha, S.; Wojcik, K.; Olchawa, M.; Sarna, T.; Pieta, J.; Jakiela, B.; Soja, J.; Okon, K.; Zarychta, J.; Zareba, L.; et al. Increased Oxidative Stress in Asthma-Relation to Inflammatory Blood and Lung Biomarkers and Airway Remodeling Indices. Biomedicines 2022, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Jia, J.; Zheng, J.; Zhou, Y.; Jia, D.; Wang, J. Recent Progress of Ferroptosis in Lung Diseases. Front. Cell Dev. Biol. 2021, 9, 789517. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Wood, L.G.; Gibson, P.G.; Garg, M.L. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur. Respir. J. 2003, 21, 177–186. [Google Scholar] [CrossRef]
- Jesenak, M.; Zelieskova, M.; Babusikova, E. Oxidative Stress and Bronchial Asthma in Children-Causes or Consequences? Front. Pediatr. 2017, 5, 162. [Google Scholar] [CrossRef]
- Howell, E. Enzyme Nutrition; AVERY: Mentor, OH, USA, 1985. [Google Scholar]
- Kuo, Y.H.; Ikegami, F.; Lambein, F. Neuroactive and other free amino acids in seed and young plants of Panax ginseng. Phytochemistry 2003, 62, 1087–1091. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yang, H.; Lee, T.K.; Lee, C.H.; Seo, J.W.; Kim, J.E.; Kim, S.Y.; Park, J.H.Y.; Lee, K.W. A short-term, hydroponic-culture of ginseng results in a significant increase in the anti-oxidative activity and bioactive components. Food Sci. Biotechnol. 2020, 29, 1007–1012. [Google Scholar] [CrossRef]
- Kim, G.S.; Hyun, D.Y.; Kim, Y.O.; Lee, S.E.; Kwon, H.; Cha, S.W.; Park, C.B.; Kim, Y.B. Investigation of Ginsenosides in Different Parts of Panax ginseng Cultured by Hydroponics. Hortic. Sci. Technol. 2010, 28, 216–226. [Google Scholar]
- Hwang, C.E.; Kim, S.C.; Kim, D.H.; Lee, H.Y.; Suh, H.K.; Cho, K.M.; Lee, J.H. Enhancement of isoflavone aglycone, amino acid, and CLA contents in fermented soybean yogurts using different strains: Screening of antioxidant and digestive enzyme inhibition properties. Food Chem. 2021, 340, 128199. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.C.; Lee, H.Y.; Cho, D.Y.; Jung, J.G.; Kang, D.; Kang, S.; Cho, K.M. Changes in nutritional compositions of processed mountain-cultivated ginseng sprouts (Panax ginseng) and screening for their antioxidant and anti-inflammatory properties. J. Funct. Foods 2021, 86, 104668. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, H.Y.; Lee, Y.M.; Seo, E.Y.; Kim, D.H.; Son, K.-H.; Lee, J.; Cho, D.Y.; Lee, J.H. Comparative assessment of compositional constituents and antioxidant effects in ginseng sprouts (Panax ginseng) through aging and fermentation processes. LWT 2022, 164, 113644. [Google Scholar] [CrossRef]
- Sharma, A.; Lee, H.J. Ginsenoside Compound K: Insights into Recent Studies on Pharmacokinetics and Health-Promoting Activities. Biomolecules 2020, 10, 1028. [Google Scholar] [CrossRef]
- Yang, X.D.; Yang, Y.Y.; Ouyang, D.S.; Yang, G.P. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia 2015, 100, 208–220. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, M.S.; Jeung, W.; Ra, J.; Yoo, H.H.; Kim, D.H. Effects of gut microbiota on the pharmacokinetics of protopanaxadiol ginsenosides Rd, Rg3, F2, and compound K in healthy volunteers treated orally with red ginseng. J. Ginseng Res. 2020, 44, 611–618. [Google Scholar] [CrossRef]
- Theofani, E.; Xanthou, G. Autophagy: A Friend or Foe in Allergic Asthma? Int. J. Mol. Sci. 2021, 22, 6314. [Google Scholar] [CrossRef]
- Vignola, A.M.; Chiappara, G.; Gagliardo, R.; Gjomarkaj, M.; Merendino, A.; Siena, L.; Bousquet, J.; Bonsignore, G. Apoptosis and airway inflammation in asthma. Apoptosis 2000, 5, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Liu, R.; Wang, Y.; Ding, N.; Li, Y. Synthesis and biological evaluation of Ginsenoside Compound K analogues as a novel class of anti-asthmatic agents. Bioorg. Med. Chem. Lett. 2019, 29, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, E.; Xiao, X.; Wang, J.; Yang, X.; Yang, P.; Li, G.; Liu, Z. The role of IL-25 in the reduction of oxidative stress and the apoptosis of airway epithelial cells with specific immunotherapy in an asthma mouse model. Am. J. Transl. Res. 2017, 9, 4137–4148. [Google Scholar] [PubMed]
- Athari, S.S. Targeting cell signaling in allergic asthma. Signal Transduct. Target. Ther. 2019, 4, 45. [Google Scholar] [CrossRef]
- Miao, K.; Zhang, L.; Pan, T.; Wang, Y. Update on the role of endoplasmic reticulum stress in asthma. Am. J. Transl. Res. 2020, 12, 1168–1183. [Google Scholar] [PubMed]
- Ali, M.K.; Kim, R.Y.; Brown, A.C.; Mayall, J.R.; Karim, R.; Pinkerton, J.W.; Liu, G.; Martin, K.L.; Starkey, M.R.; Pillar, A.L.; et al. Crucial role for lung iron level and regulation in the pathogenesis and severity of asthma. Eur. Respir. J. 2020, 55, 1901340. [Google Scholar] [CrossRef]
- Lee, B.; Kim, Y.; Kim, Y.M.; Jung, J.; Kim, T.; Lee, S.-Y.; Shin, Y.-I.; Ryu, J.H. Anti-oxidant and anti-inflammatory effects of aquatic exercise in allergic airway inflammation in mice. Front. Physiol. 2019, 10, 1227. [Google Scholar] [CrossRef]
- Ryu, J.H.; Xie, C.; Kim, E.J.; Park, S.H.; Choi, Y.J.; Kang, S.S.; Shin, M.K.; Kang, D. Reduction of Asthmatic Parameters by Sea Hare Hydrolysates in a Mouse Model of Allergic Asthma. Nutrients 2017, 9, 699. [Google Scholar] [CrossRef]
- Myou, S.; Leff, A.R.; Myo, S.; Boetticher, E.; Tong, J.; Meliton, A.Y.; Liu, J.; Munoz, N.M.; Zhu, X. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase–TAT. J. Exp. Med. 2003, 198, 1573–1582. [Google Scholar] [CrossRef]
- Ford, J.G.; Rennick, D.; Donaldson, D.D.; Venkayya, R.; McArthur, C.; Hansell, E.; Kurup, V.P.; Warnock, M.; Grünig, G. IL-13 and IFN-γ: Interactions in lung inflammation. J. Immunol. 2001, 167, 1769–1777. [Google Scholar] [CrossRef]
- Di Valentin, E.; Crahay, C.; Garbacki, N.; Hennuy, B.; Guéders, M.; Noël, A.; Foidart, J.-M.; Grooten, J.; Colige, A.; Piette, J. New asthma biomarkers: Lessons from murine models of acute and chronic asthma. Am. J. Physiol. Renal. Physiol. 2009, 296, L185–L197. [Google Scholar] [CrossRef]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.J.; Shin, E.J.; Woo, M.S.; Kim, J.M.; Kim, J.H.; Lee, D.K.; Hahm, J.R.; Kim, H.J.; et al. Dipeptide YA is Responsible for the Positive Effect of Oyster Hydrolysates on Alcohol Metabolism in Single Ethanol Binge Rodent Models. Mar. Drugs 2020, 18, 512. [Google Scholar] [CrossRef]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.J.; Shin, E.J.; Woo, M.S.; Kim, J.M.; Park, S.H.; Hahm, J.R.; Choi, Y.J.; Kang, D. Oyster broth concentrate and its major component taurine alleviate acute alcohol-induced liver damage. Food Sci. Nutr. 2022, 10, 2390–2399. [Google Scholar] [CrossRef]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.J.; Cho, S.B.; Woo, M.S.; Lee, D.K.; Hong, S.G.; Han, J.; Kang, S.S.; Kim, D.R.; et al. Oyster-Derived Tyr-Ala (YA) Peptide Prevents Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Failure by Suppressing Inflammatory, Apoptotic, Ferroptotic, and Pyroptotic Signals. Mar. Drugs 2021, 19, 614. [Google Scholar] [CrossRef]
- Jian, Z.; Guo, H.; Liu, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Oxidative stress, apoptosis and inflammatory responses involved in copper-induced pulmonary toxicity in mice. Aging 2020, 12, 16867–16886. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, D.H.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Ferroptosis-Induced Endoplasmic Reticulum Stress: Cross-talk between Ferroptosis and Apoptosis. Mol. Cancer Res. MCR 2018, 16, 1073–1076. [Google Scholar] [CrossRef]
- Tao, N.; Li, K.; Liu, J. Molecular Mechanisms of Ferroptosis and Its Role in Pulmonary Disease. Oxid. Med. Cell. Longev. 2020, 2020, 9547127. [Google Scholar] [CrossRef]
- Yan, H.F.; Zou, T.; Tuo, Q.Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Sharma, A.; Bansal, S.; Nagpal, R.K. Lipid peroxidation in bronchial asthma. Indian J. Pediatr. 2003, 70, 715–717. [Google Scholar] [CrossRef]
- Alzoghaibi, M.A.; Bahammam, A.S. Lipid peroxides in stable asthmatics receiving inhaled steroids and long-acting beta2 -agonists. Respirology 2007, 12, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yu, D.; He, Z.; Bao, L.; Feng, L.; Chen, L.; Liu, Z.; Hu, X.; Zhang, N.; Wang, T.; et al. Endoplasmic reticulum stress-mediated autophagy activation is involved in cadmium-induced ferroptosis of renal tubular epithelial cells. Free Radic. Biol. Med. 2021, 175, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 2014, 3, e02523. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, H.; Liu, J.; Xu, L.; Yang, L.; Sun, Z.; Zhou, B. Tunicamycin aggravates endoplasmic reticulum stress and airway inflammation via PERK-ATF4-CHOP signaling in a murine model of neutrophilic asthma. J. Asthma Off. J. Assoc. Care Asthma 2017, 54, 125–133. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhou, L.; Lei, Y.; Zhang, Y.; Huang, C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019, 25, 101047. [Google Scholar] [CrossRef]
- Choi, I.D.; Ryu, J.H.; Lee, D.E.; Lee, M.H.; Shim, J.J.; Ahn, Y.T.; Sim, J.H.; Huh, C.S.; Shim, W.S.; Yim, S.V.; et al. Enhanced Absorption Study of Ginsenoside Compound K (20-O-beta-(D-Glucopyranosyl)-20(S)-protopanaxadiol) after Oral Administration of Fermented Red Ginseng Extract (HYFRG) in Healthy Korean Volunteers and Rats. Evid.-Based Complement. Altern. Med. eCAM 2016, 2016, 3908142. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, M.O.; Song, Y.N.; Min, J.H.; Kim, S.M.; Kang, M.J.; Oh, E.S.; Lee, R.W.; Jung, S.; Ro, H.; et al. Compound K ameliorates airway inflammation and mucus secretion through the regulation of PKC signaling in vitro and in vivo. J. Ginseng Res. 2022, 46, 496–504. [Google Scholar] [CrossRef]
- Liu, K.K.; Wang, Q.T.; Yang, S.M.; Chen, J.Y.; Wu, H.X.; Wei, W. Ginsenoside compound K suppresses the abnormal activation of T lymphocytes in mice with collagen-induced arthritis. Acta Pharmacol. Sin. 2014, 35, 599–612. [Google Scholar] [CrossRef]
- Huang, W.C.; Huang, T.H.; Yeh, K.W.; Chen, Y.L.; Shen, S.C.; Liou, C.J. Ginsenoside Rg3 ameliorates allergic airway inflammation and oxidative stress in mice. J. Ginseng Res. 2021, 45, 654–664. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, J.H.; Woo, M.S.; Cao, D.L.; Kim, E.-J.; Jeong, Y.Y.; Koh, E.-H.; Cho, K.M.; Kang, S.S.; Kang, D. Fermented and Aged Ginseng Sprouts (Panax ginseng) and Their Main Component, Compound K, Alleviate Asthma Parameters in a Mouse Model of Allergic Asthma through Suppression of Inflammation, Apoptosis, ER Stress, and Ferroptosis. Antioxidants 2022, 11, 2052. https://doi.org/10.3390/antiox11102052
Ryu JH, Woo MS, Cao DL, Kim E-J, Jeong YY, Koh E-H, Cho KM, Kang SS, Kang D. Fermented and Aged Ginseng Sprouts (Panax ginseng) and Their Main Component, Compound K, Alleviate Asthma Parameters in a Mouse Model of Allergic Asthma through Suppression of Inflammation, Apoptosis, ER Stress, and Ferroptosis. Antioxidants. 2022; 11(10):2052. https://doi.org/10.3390/antiox11102052
Chicago/Turabian StyleRyu, Ji Hyeon, Min Seok Woo, Dang Long Cao, Eun-Jin Kim, Yi Yeong Jeong, Eun-Ha Koh, Kye Man Cho, Sang Soo Kang, and Dawon Kang. 2022. "Fermented and Aged Ginseng Sprouts (Panax ginseng) and Their Main Component, Compound K, Alleviate Asthma Parameters in a Mouse Model of Allergic Asthma through Suppression of Inflammation, Apoptosis, ER Stress, and Ferroptosis" Antioxidants 11, no. 10: 2052. https://doi.org/10.3390/antiox11102052
APA StyleRyu, J. H., Woo, M. S., Cao, D. L., Kim, E.-J., Jeong, Y. Y., Koh, E.-H., Cho, K. M., Kang, S. S., & Kang, D. (2022). Fermented and Aged Ginseng Sprouts (Panax ginseng) and Their Main Component, Compound K, Alleviate Asthma Parameters in a Mouse Model of Allergic Asthma through Suppression of Inflammation, Apoptosis, ER Stress, and Ferroptosis. Antioxidants, 11(10), 2052. https://doi.org/10.3390/antiox11102052






