Synthesis and Catalytic Activity in the Hydrogenation Reaction of Palladium-Doped Metal-Organic Frameworks Based on Oxo-Centered Zirconium Complexes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Techniques
2.3. Synthesis Techniques
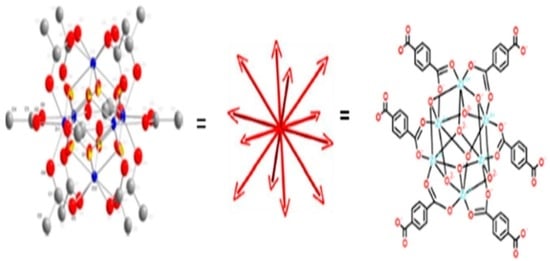
2.3.1. Synthesis of MOF of the UiO Type
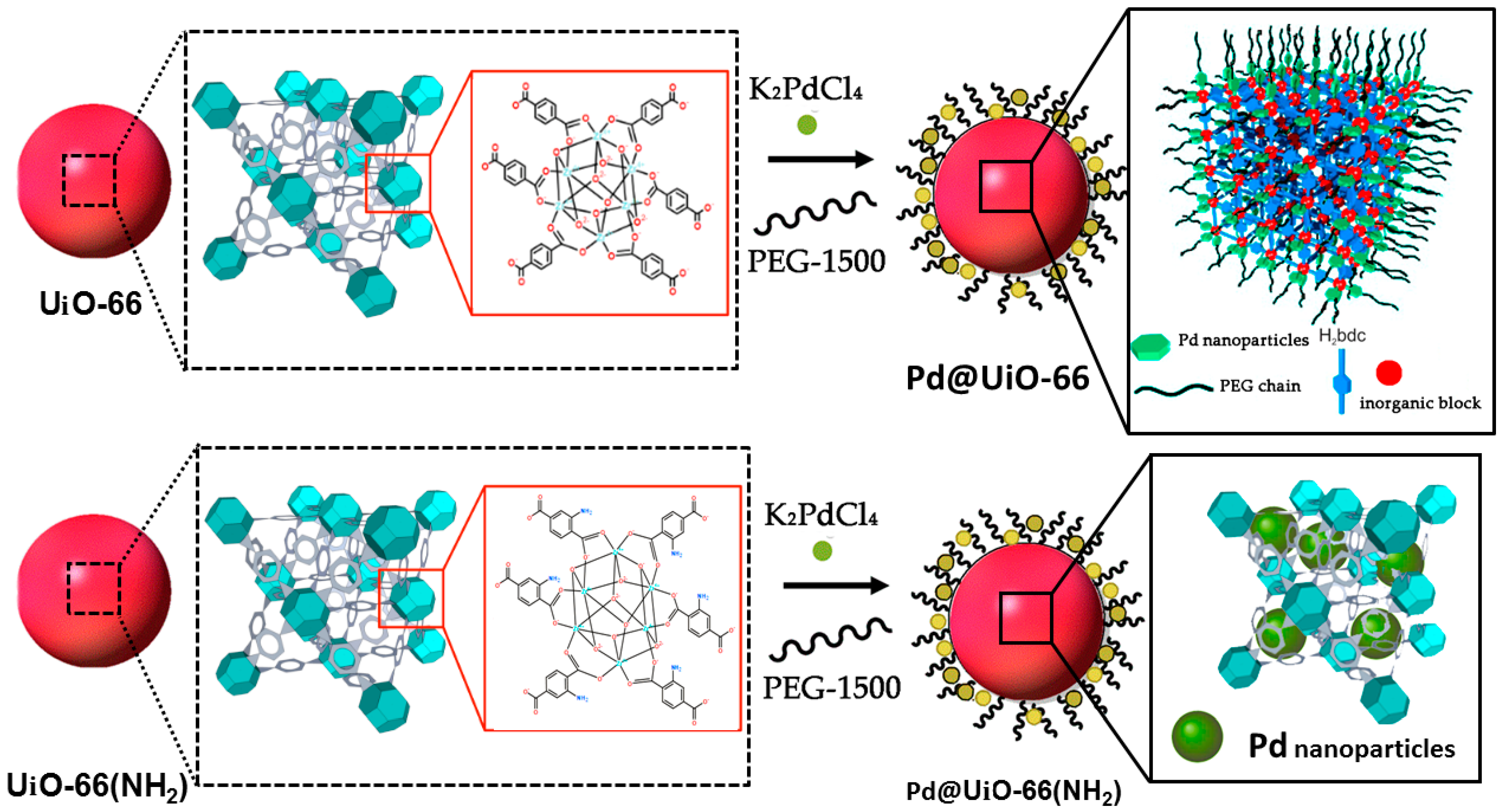
2.3.2. Synthesis of Pd Doped Heterogeneous Catalysts
2.4. Catalytic Tests
2.4.1. Hydrogenation of Cyclohexene
2.4.2. Hydrogenation of Phenylacetylene and Allyl Alcohol
3. Results
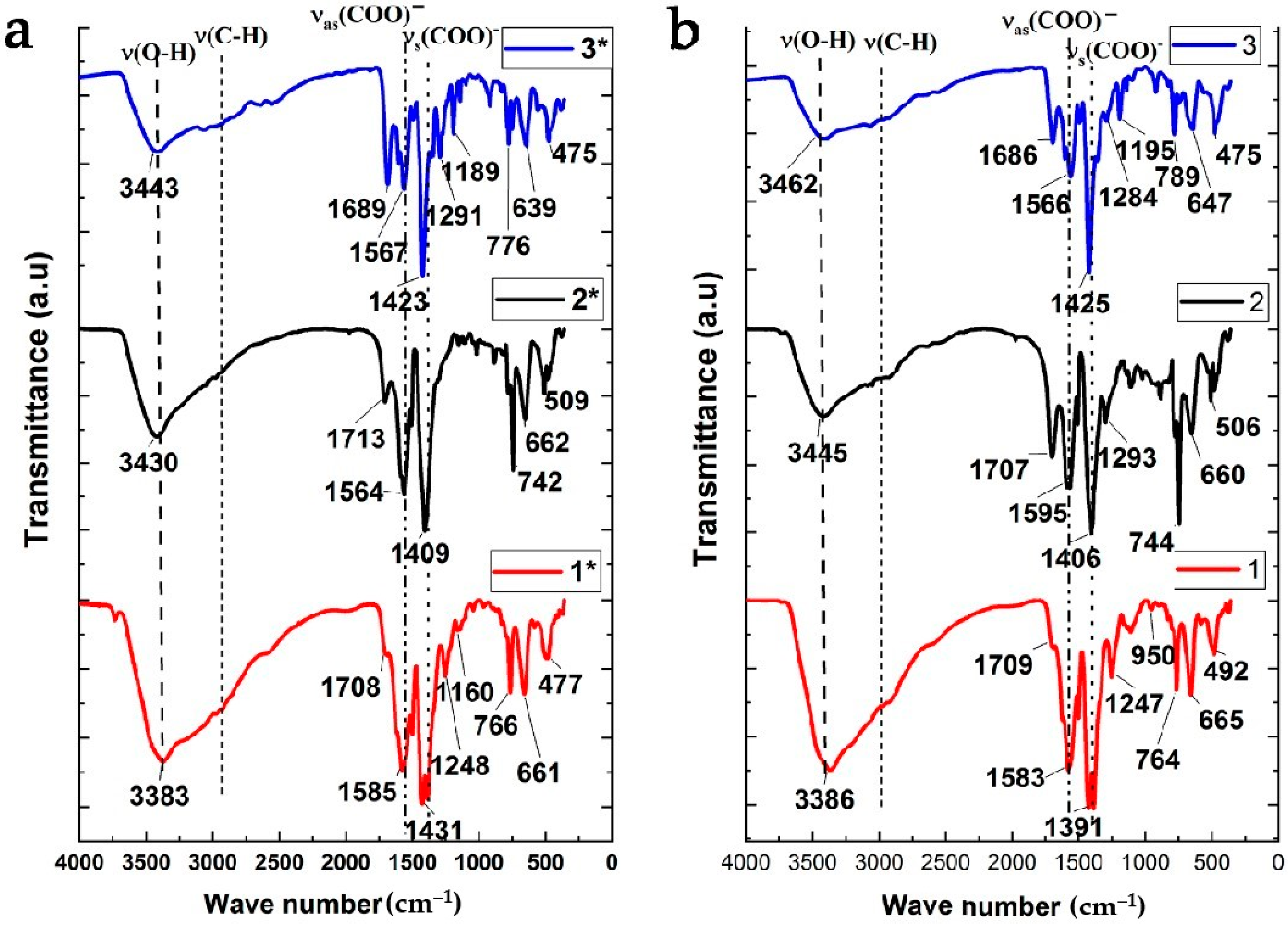
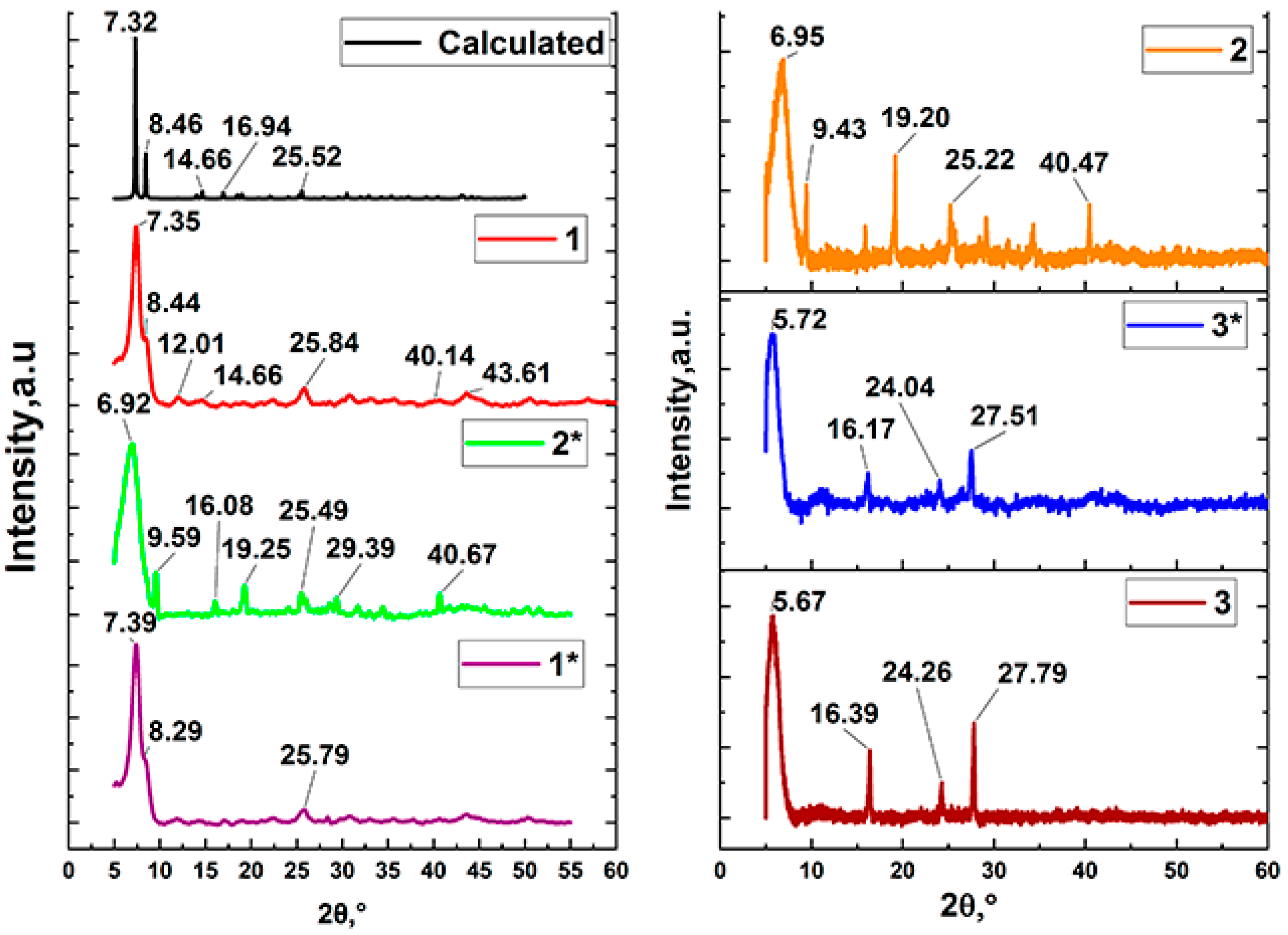
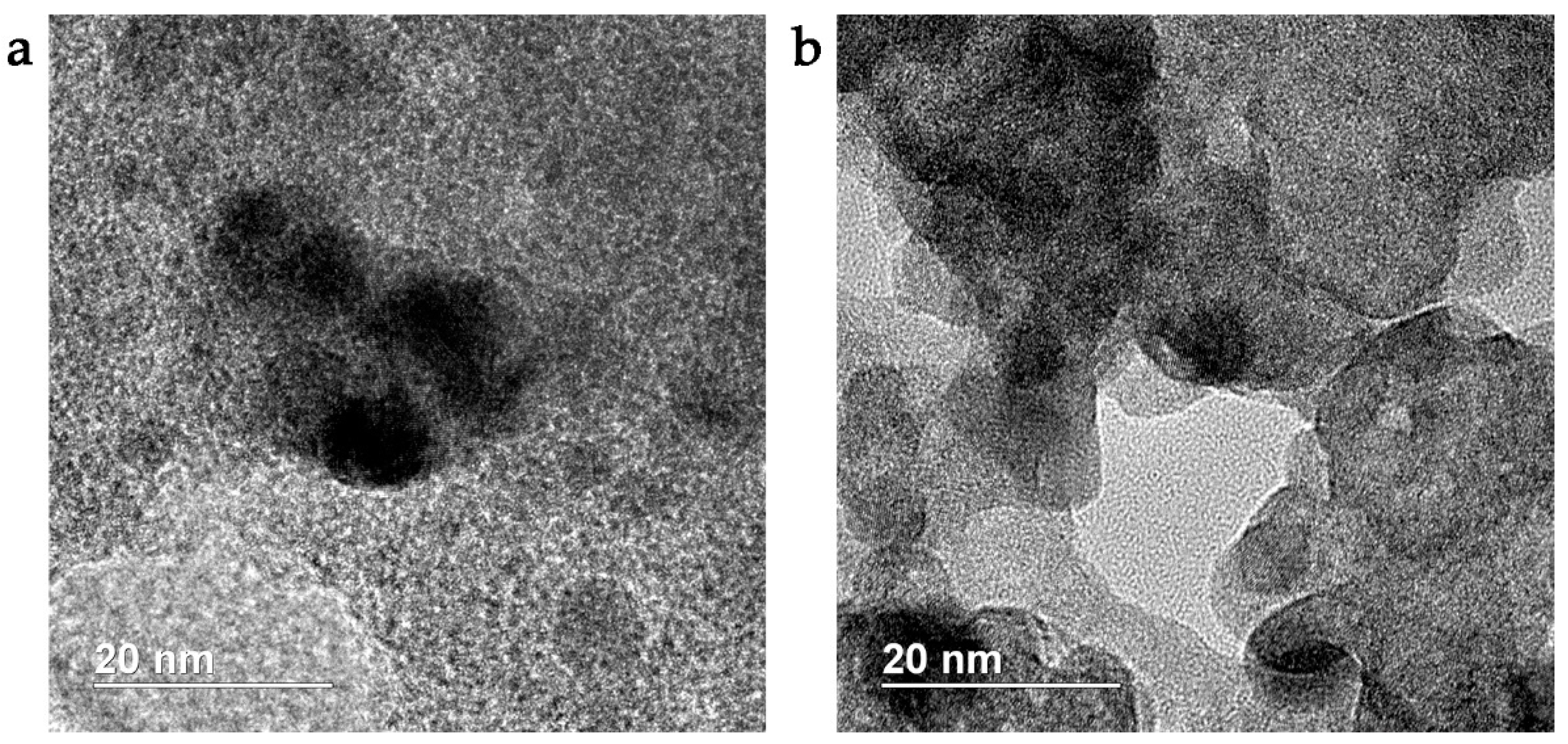
3.1. Synthesis of Pd-Doped Catalysts
| ZrCl4 + H2O → ZrOCl2 + 2HCl |
| 6ZrOCl2 + 12CH3COOH → Zr6O4(OH)4(CH3COO)12 + 12HCl pH = 1.5 |
| H2L + 2KOH → K2L |
| Zr6O4(OH)4(CH3COO)12 + K2L → Zr6O4(OH)4(L)6 |
3.2. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mironenko, R.M.; Likholobov, V.A.; Belskaya, O.B. Nanoglobular Carbon and Palladium–Nanoglobular Carbon Catalysts for Liquid-Phase Hydrogenation of Organic Compounds. Russ. Chem. Rev. 2022, 91, RCR5017. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X. Recent Development on Palladium Enhanced Photocatalytic Activity: A Review. J. Alloys Compd. 2020, 830, 154669. [Google Scholar] [CrossRef]
- Genuino, H.C.; van de Bovenkamp, H.H.; Wilbers, E.; Winkelman, J.G.M.; Goryachev, A.; Hofmann, J.P.; Hensen, E.J.M.; Weckhuysen, B.M.; Bruijnincx, P.C.A.; Heeres, H.J. Catalytic Hydrogenation of Renewable Levulinic Acid to γ-Valerolactone: Insights into the Influence of Feed Impurities on Catalyst Performance in Batch and Flow Reactors. ACS Sustain. Chem. Eng. 2020, 8, 5903–5919. [Google Scholar] [CrossRef]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Han, Y.; Xu, H.; Su, Y.; Xu, Z.; Wang, K.; Wang, W. Noble Metal (Pt, Au@Pd) Nanoparticles Supported on Metal Organic Framework (MOF-74) Nanoshuttles as High-Selectivity CO2 Conversion Catalysts. J. Catal. 2019, 370, 70–78. [Google Scholar] [CrossRef]
- Tshuma, P.; Makhubela, B.C.E.; Bingwa, N.; Mehlana, G. Palladium(II) Immobilized on Metal–Organic Frameworks for Catalytic Conversion of Carbon Dioxide to Formate. Inorg. Chem. 2020, 59, 6717–6728. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary Building Units as the Turning Point in the Development of the Reticular Chemistry of MOFs. Sci. Adv. 2018, 4, eaat9180. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kiskin, M.A.; Kovalenko, K.A.; Samsonenko, D.G.; Dybtsev, D.N.; Audebrand, N.; Sun, Y.; Fedin, V.P. Rational Synthesis and Dimensionality Tuning of MOFs from Preorganized Heterometallic Molecular Complexes. Dalton Trans. 2019, 48, 3676–3686. [Google Scholar] [CrossRef]
- Heu, R.; Ateia, M.; Awfa, D.; Punyapalakul, P.; Yoshimura, C. Photocatalytic Degradation of Organic Micropollutants in Water by Zr-MOF/GO Composites. J. Compos. Sci. 2020, 4, 54. [Google Scholar] [CrossRef]
- Wei, M.; Wan, Y.; Zhang, X. Metal-Organic Framework-Based Stimuli-Responsive Polymers. J. Compos. Sci. 2021, 5, 101. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Naumkina, V.N.; Zhinzhilo, V.A. Nanocomposites of Copper Trimesinate and Graphene Oxide as Sorbents for the Solid-Phase Extraction of Organic Dyes. J. Compos. Sci. 2022, 6, 215. [Google Scholar] [CrossRef]
- Qin, J.-S.; Yuan, S.; Lollar, C.; Pang, J.; Alsalme, A.; Zhou, H.-C. Stable Metal–Organic Frameworks as a Host Platform for Catalysis and Biomimetics. Chem. Commun. 2018, 54, 4231–4249. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mandal, S.K. A Microporous Metal–Organic Framework Catalyst for Solvent-Free Strecker Reaction and CO2 Fixation at Ambient Conditions. Inorg. Chem. 2020, 59, 4273–4281. [Google Scholar] [CrossRef] [PubMed]
- Pascanu, V.; González Miera, G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Liu, B.; Akita, T.; Haruta, M.; Sakurai, H.; Xu, Q. Au@ZIF-8: CO Oxidation over Gold Nanoparticles Deposited to Metal−Organic Framework. J. Am. Chem. Soc. 2009, 131, 11302–11303. [Google Scholar] [CrossRef]
- Yuan, B.; Pan, Y.; Li, Y.; Yin, B.; Jiang, H. A Highly Active Heterogeneous Palladium Catalyst for the Suzuki-Miyaura and Ullmann Coupling Reactions of Aryl Chlorides in Aqueous Media. Angew. Chem. Int. Ed. 2010, 49, 4054–4058. [Google Scholar] [CrossRef]
- Wang, W.; Chen, S.; Guisasola Cal, E.; Martínez Moro, M.; Moya, S.; Coy, E.; Wang, C.; Hamon, J.-R.; Astruc, D. ZIF-8-Based vs. ZIF-8-Derived Au and Pd Nanoparticles as Efficient Catalysts for the Ullmann Homocoupling Reaction. Inorg. Chem. Front. 2020, 7, 3945–3952. [Google Scholar] [CrossRef]
- Hicks, K.E.; Rosen, A.S.; Syed, Z.H.; Snurr, R.Q.; Farha, O.K.; Notestein, J.M. Zr6O8 Node-Catalyzed Butene Hydrogenation and Isomerization in the Metal–Organic Framework NU-1000. ACS Catal. 2020, 10, 14959–14970. [Google Scholar] [CrossRef]
- Nagendiran, A.; Pascanu, V.; Bermejo Gómez, A.; González Miera, G.; Tai, C.-W.; Verho, O.; Martín-Matute, B.; Bäckvall, J.-E. Mild and Selective Catalytic Hydrogenation of the C=C Bond in α,β-Unsaturated Carbonyl Compounds Using Supported Palladium Nanoparticles. Chem. Eur. J. 2016, 22, 7184–7189. [Google Scholar] [CrossRef]
- Bakuru, V.R.; Kalidindi, S.B. Synergistic Hydrogenation over Palladium through the Assembly of MIL-101(Fe) MOF over Palladium Nanocubes. Chem. Eur. J. 2017, 23, 16456–16459. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Eliseev, O.L.; Chernyshev, V.V.; Bondarenko, T.N.; Vergun, V.V.; Kapustin, G.I.; Lapidus, A.L.; Kustov, L.M. Palladium Nanoparticles Embedded in MOF Matrices: Catalytic Activity and Structural Stability in Iodobenzene Methoxycarbonylation. Polyhedron 2019, 158, 55–64. [Google Scholar] [CrossRef]
- Wu, H.Q.; Huang, L.; Li, J.Q.; Zheng, A.M.; Tao, Y.; Yang, L.X.; Yin, W.H.; Luo, F. Pd@Zn-MOF-74: Restricting a Guest Molecule by the Open-Metal Site in a Metal–Organic Framework for Selective Semihydrogenation. Inorg. Chem. 2018, 57, 12444–12447. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Cohen, S.M. Metalation of a Thiocatechol-Functionalized Zr(IV)-Based Metal–Organic Framework for Selective C–H Functionalization. J. Am. Chem. Soc. 2015, 137, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, L.A.; Terenina, M.V.; Kryazheva, I.Y.; Karakhanov, E.A. Unsaturated-Compound Hydrogenation Nanocatalysts Based on Palladium and Platinum Particles Immobilized in Pores of Mesoporous Aromatic Frameworks. Petr. Chem. 2017, 57, 222–229. [Google Scholar] [CrossRef]
- Zhang, K.; Kim, J.; Kirlikovali, K.O.; Wang, J.; Lee, T.H.; Kim, S.Y.; Varma, R.S.; Jang, H.W.; Farha, O.K.; Shokouhimehr, M. Magnetically Recyclable Nanocomposites via Lanthanide-Based MOFs Grown on Natural Sea Sponge: Screening Hydrogenation of Nitrophenol to Aminophenol. Mol. Catal. 2022, 528, 112459. [Google Scholar] [CrossRef]
- Aldoshin, S.M.; Dzhardimalieva, G.I.; Pomogailo, A.D.; Abuzin, Y.A. Reactivity of Metal-Containing Monomers 71. Synthesis of Nanosized Quasicrystals and Related Metallopolymer Composites. Russ. Chem. Bull. 2011, 60, 1871–1879. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Zharmagambetova, A.K.; Kudaibergenov, S.E.; Uflyand, I.E. Polymer-Immobilized Clusters and Metal Nanoparticles in Catalysis. Kinet. Catal. 2020, 61, 198–223. [Google Scholar] [CrossRef]
- Sharma, P.; Mukherjee, D.; Sarkar, S.; Mandler, D.; Varma, R.S.; Gawande, M.B.; Zbořil, R.; Sasson, Y. Pd Doped Carbon Nitride (Pd-g-C3N4): An Efficient Photocatalyst for Hydrogenation via an Al–H2O System and an Electrocatalyst towards Overall Water Splitting. Green Chem. 2022, 24, 5535–5546. [Google Scholar] [CrossRef]
- Rout, D.R.; Senapati, P.; Sutar, H.; Sau, D.C.; Murmu, R. Graphene Oxide (GO) Supported Palladium (Pd) Nanocomposites for Enhanced Hydrogenation. Graphene 2019, 8, 33–51. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, Thermal and Mechanical Stabilities of Metal–Organic Frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Godt, A.; Lippke, J.; Waltz, F.; Wiebcke, M.; Behrens, P. Modulated Synthesis of Zr-Based Metal-Organic Frameworks: From Nano to Single Crystals. Chem. Eur. J. 2011, 17, 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Hennig, C.; Weiss, S.; Kraus, W.; Kretzschmar, J.; Scheinost, A.C. Solution Species and Crystal Structure of Zr(IV) Acetate. Inorg. Chem. 2017, 56, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Harraz, F.A.; El-Hout, S.E.; Killa, H.M.; Ibrahim, I.A. Palladium Nanoparticles Stabilized by Polyethylene Glycol: Efficient, Recyclable Catalyst for Hydrogenation of Styrene and Nitrobenzene. J. Catal. 2012, 286, 184–192. [Google Scholar] [CrossRef]
- Yan, F.; Wang, Q.; Wang, F.; Huang, Z. Study on Energy Storage Properties of Metal-Organic Frameworks Nanofluids (UIO-67/Water and UIO-67/Methanol) by an Experimental and Theoretical Method. J. Mater. Sci. 2021, 56, 10008–10017. [Google Scholar] [CrossRef]
- Pangestu, A.; Lestari, W.W.; Wibowo, F.R.; Larasati, L. Green Electro-Synthesized MIL-101(Fe) and Its Aspirin Detoxification Performance Compared to MOF-808. J. Inorg. Organomet. Polym. 2022, 32, 1828–1839. [Google Scholar] [CrossRef]
- Di Pietrantonio, K.; Coccia, F.; Tonucci, L.; d’Alessandro, N.; Bressan, M. Hydrogenation of Allyl Alcohols Catalyzed by Aqueous Palladium and Platinum Nanoparticles. RSC Adv. 2015, 5, 68493–68499. [Google Scholar] [CrossRef]






| Sample | Estimated Formula | Elemental Content [wt. %] (Found/Calc.) | |||
|---|---|---|---|---|---|
| C | H | N | Pd | ||
| 1 | Pd/Zr6O4(OH)4(C8H4O4-NH2)6(H2O)12 | 27.6/28.9 | 3.7/3.8 | 3.3/4.2 | 0.79 |
| 2 | Pd/Zr6O4(OH)6(C8H4O4)6(H2O)4 | 33.4/33.0 | 2.8/2.8 | 0/0 | 0.50 |
| 3 | Pd/Zr6O4(OH)4(C12H6O4)6(H2O)18 | 37.8/37.8 | 3.7/3.4 | - | 0.61 |
| Sample | T5% [°C] | T10% [°C] | T20% [°C] | T30% [°C] | Tmax [°C] | Residual Weight at 600 °C [wt.%] |
|---|---|---|---|---|---|---|
| 1 | 112 | 146 | 272 | 433 | 364 | 64.87 |
| 2 | 235 | 285 | 308 | 318 | 330 | 35.87 |
| 3 | 365 | 380 | 395 | 405 | 543 | 36.52 |
| Sample | SBET [m2/g] | Vp [cm3/g] | Average Pore Diameter [nm] | t Method | ||
|---|---|---|---|---|---|---|
| Vmicro [cm3/g] | Smicro [m2/g] | Sexternal [m2/g] | ||||
| 1 | 558.9 | 0.47 | 5.68 | 0.11 | 257.0 | 302.0 |
| 2 | 359.6 | 0.86 | 3.78 | 0.014 | 39.1 | 320.5 |
| 3 | 400.0 | 0.65 | 4.73 | 0.025 | 64.8 | 335.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baimuratova, R.K.; Andreeva, A.V.; Uflyand, I.E.; Shilov, G.V.; Bukharbayeva, F.U.; Zharmagambetova, A.K.; Dzhardimalieva, G.I. Synthesis and Catalytic Activity in the Hydrogenation Reaction of Palladium-Doped Metal-Organic Frameworks Based on Oxo-Centered Zirconium Complexes. J. Compos. Sci. 2022, 6, 299. https://doi.org/10.3390/jcs6100299
Baimuratova RK, Andreeva AV, Uflyand IE, Shilov GV, Bukharbayeva FU, Zharmagambetova AK, Dzhardimalieva GI. Synthesis and Catalytic Activity in the Hydrogenation Reaction of Palladium-Doped Metal-Organic Frameworks Based on Oxo-Centered Zirconium Complexes. Journal of Composites Science. 2022; 6(10):299. https://doi.org/10.3390/jcs6100299
Chicago/Turabian StyleBaimuratova, Rose K., Anastasia V. Andreeva, Igor E. Uflyand, Gennadii V. Shilov, Farida U. Bukharbayeva, Alima K. Zharmagambetova, and Gulzhian I. Dzhardimalieva. 2022. "Synthesis and Catalytic Activity in the Hydrogenation Reaction of Palladium-Doped Metal-Organic Frameworks Based on Oxo-Centered Zirconium Complexes" Journal of Composites Science 6, no. 10: 299. https://doi.org/10.3390/jcs6100299
APA StyleBaimuratova, R. K., Andreeva, A. V., Uflyand, I. E., Shilov, G. V., Bukharbayeva, F. U., Zharmagambetova, A. K., & Dzhardimalieva, G. I. (2022). Synthesis and Catalytic Activity in the Hydrogenation Reaction of Palladium-Doped Metal-Organic Frameworks Based on Oxo-Centered Zirconium Complexes. Journal of Composites Science, 6(10), 299. https://doi.org/10.3390/jcs6100299