Sleep Disorders in Climacteric Women: Glutathione, Glutathione S-Transferase P1 and Gut Microbiome Interrelation
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consent
2.2. Subjects
2.3. Methods
2.3.1. Questionnaire
2.3.2. Collection of Materials
2.3.3. GSH
2.3.4. GSTP1
2.3.5. Gut Microbiome
2.3.6. Statistical Analysis
3. Results
3.1. Characteristics of PSQI Groups
3.2. Characteristics of ISI Groups
3.3. Characteristics of ESS Groups
3.4. Correlation Analysis Between GSH in Serum and Bacterial Parameters
3.5. Correlation Analysis Between GSTP1 in Serum and Bacterial Parameters
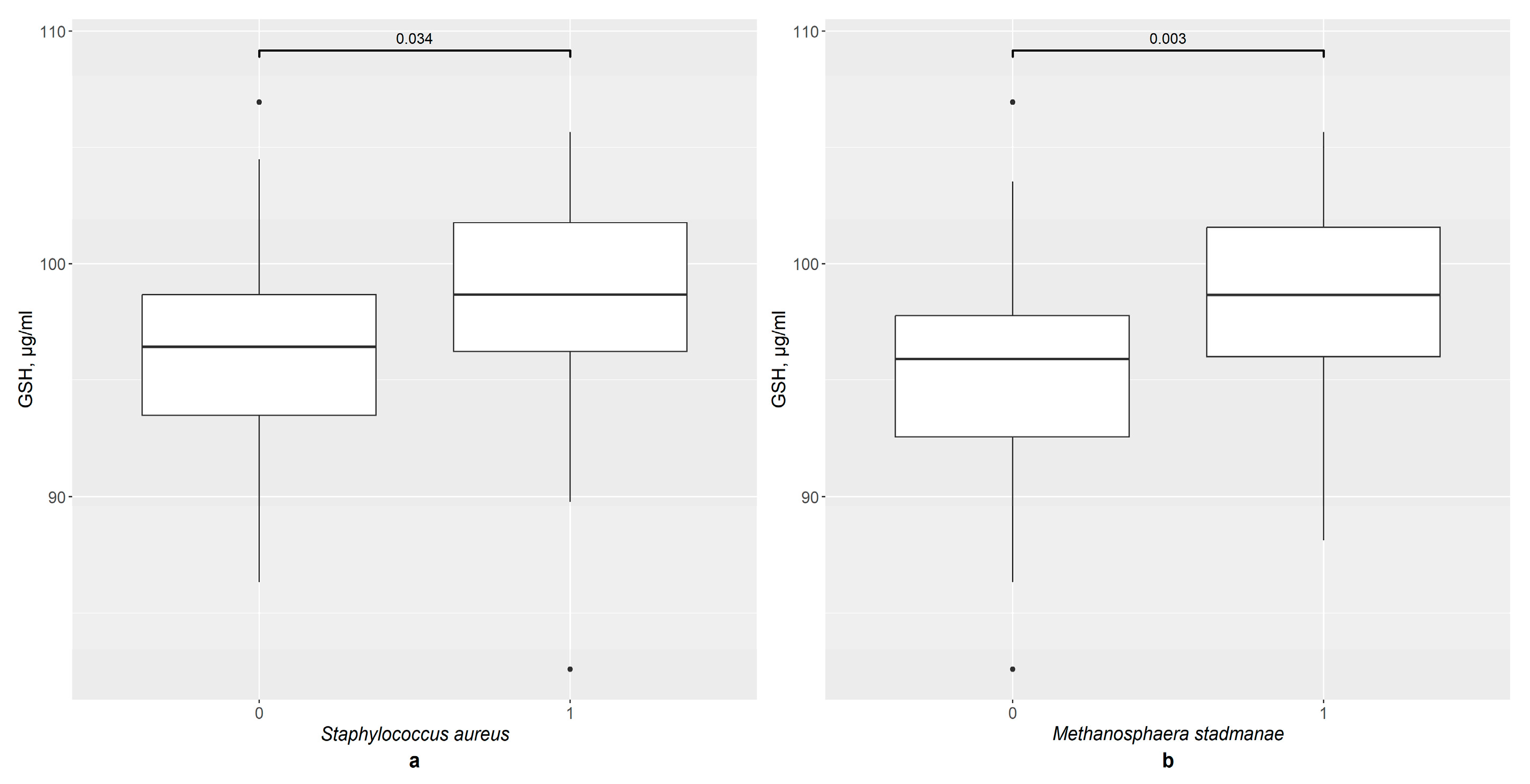
3.6. GSH and GSTP1 Levels in the Presence of Gut Bacteria in Total Group
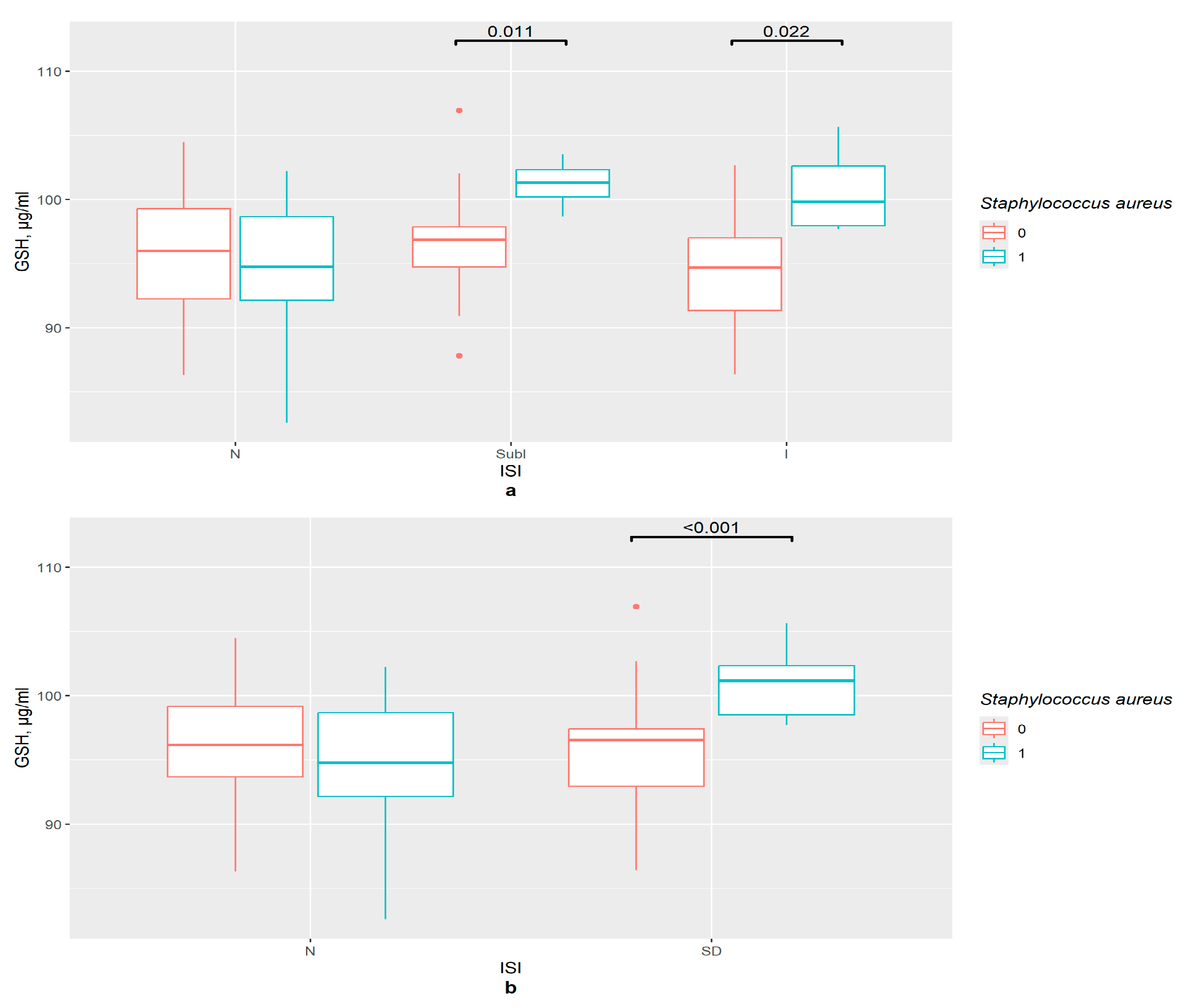
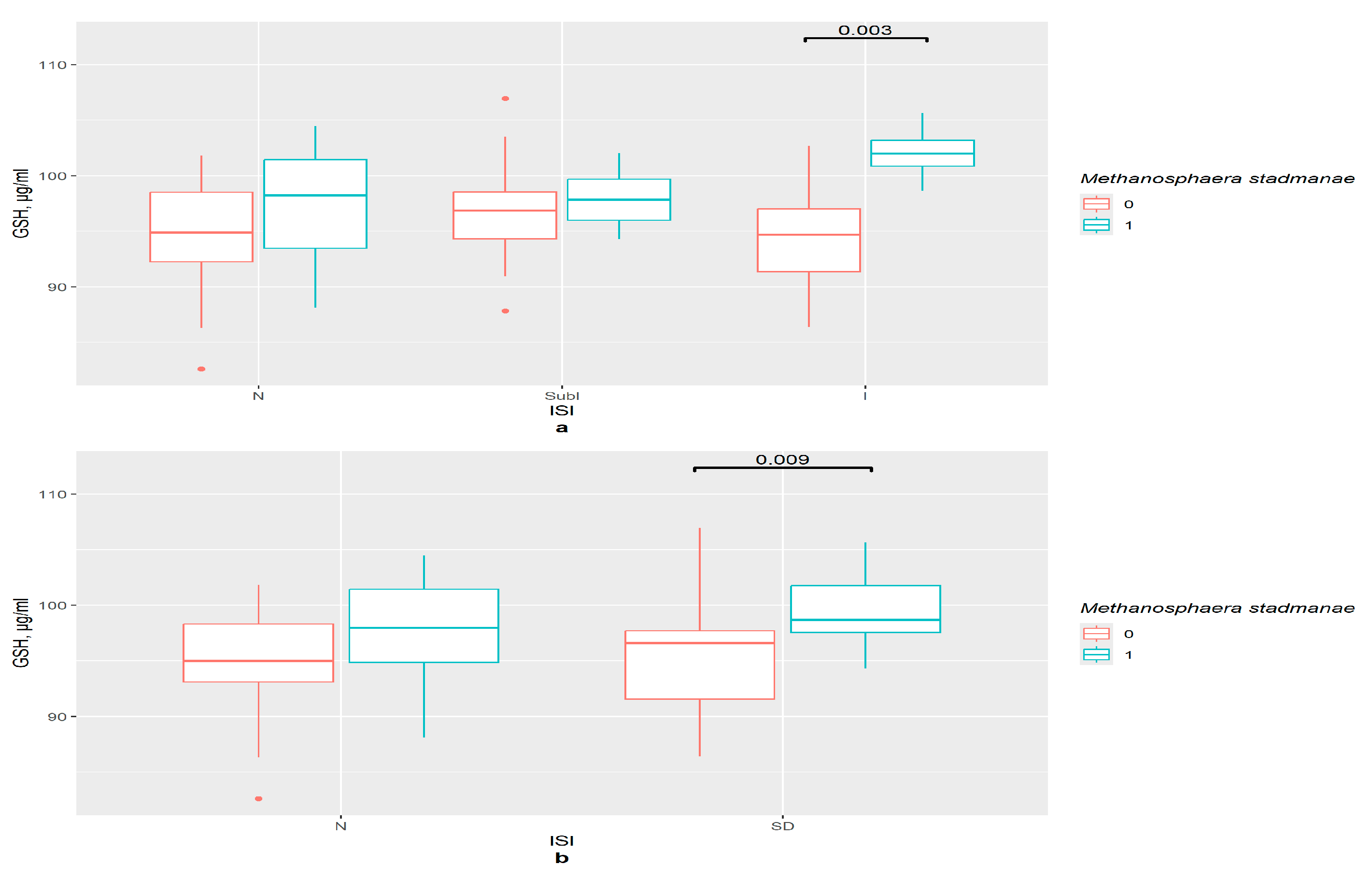
3.7. GSH and GSTP1 Levels in the Presence of Gut Bacteria in ISI Groups
3.8. GSH and GSTP1 Levels in the Presence of Gut Bacteria in PSQI Groups
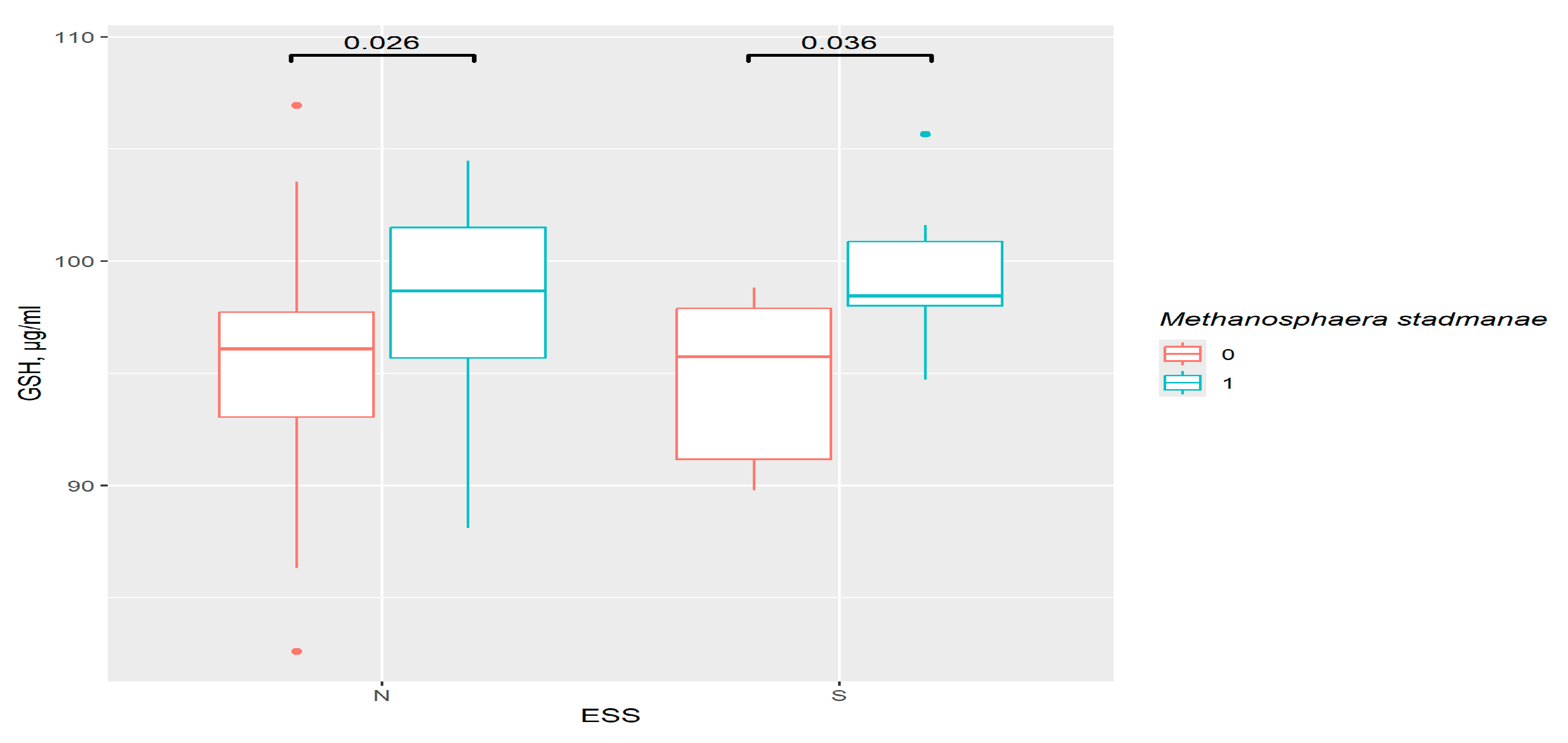
3.9. GSH and GSTP1 Levels in the Presence of Gut Bacteria in ESS Groups
3.10. GSH and GSTP1 Association with Bacteria in Different Groups with Sleep Disorders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semenova, N.V.; Madaeva, I.M.; Brichagina, A.S.; Kolesnikov, S.I.; Kolesnikova, L.I. 8-Hydroxy-2′-Deoxyguanosine as an Oxidative Stress Marker in Insomnia. Bull. Exp. Biol. Med. 2021, 171, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Xu, Z.; Chen, W. Association of oxidative balance score with sleep quality: NHANES 2007–2014. J. Affect. Disord. 2023, 339, 435–442. [Google Scholar] [CrossRef]
- Kanagasabai, T.; Riddell, M.C.; Ardern, C.I. Inflammation, Oxidative Stress, and Antioxidant Micronutrients as Mediators of the Relationship Between Sleep, Insulin Sensitivity, and Glycosylated Hemoglobin. Front. Public Health 2022, 10, 888331. [Google Scholar] [CrossRef]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1995; pp. 3–7. [Google Scholar] [CrossRef]
- Budkowska, M.; Cecerska-Heryć, E.; Marcinowska, Z.; Siennicka, A.; Dołęgowska, B. The Influence of Circadian Rhythm on the Activity of Oxidative Stress Enzymes. Int. J. Mol. Sci. 2022, 23, 14275. [Google Scholar] [CrossRef]
- Prokhorova, T.; Tereshkina, E.; Savushkina, O.; Boksha, I.; Vorobyova, E.; Burbaeva, G. Activity of Platelet Enzymes Glutathione Reductase and Glutathione-S-Transferase in Males and Females of Different Age Groups. Int. Res. J. 2020, 12, 137–141. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Semenova, N.; Garashchenko, N.; Kolesnikov, S.; Darenskaya, M.; Kolesnikova, L. Gut Microbiome Interactions with Oxidative Stress: Mechanisms and Consequences for Health. Pathophysiology 2024, 31, 309–330. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Fan, F.; Zhang, B. The Role of Microbiome in Insomnia, Circadian Disturbance and Depression. Front. Psychiatry 2018, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Khalyfa, A.; Ericsson, A.; Gozal, D. Fecal microbiota transplantation from mice exposed to chronic intermittent hypoxia elicits sleep disturbances in naïve mice. Exp. Neurol. 2020, 334, 113439. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, B.; Sheng, D.; Yang, J.; Fu, S.; Wang, J.; Zhao, C.; Wang, Y.; Gai, X.; Wang, J.; et al. Multiomics Analysis Reveals Aberrant Metabolism and Immunity Linked Gut Microbiota with Insomnia. Microbiol. Spectr. 2022, 10, e00998-22. [Google Scholar] [CrossRef]
- Vaccaro, A.; Dor, Y.K.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar] [CrossRef]
- Semenova, N.V.; Madaeva, I.M.; Kolesnikov, S.I.; Solodova, E.I.; Kolesnikova, L.I. Insomnia in Peri- and Postmenopausal Women: Plasma Lipids, Lipid Peroxidation and Some Antioxidant System Parameters. Neuropsychiatry 2018, 8, 1452–1460. [Google Scholar] [CrossRef]
- Hachul, H.; De Campos, B.H.; Lucena, L.; Tufik, S. Sleep During Menopause. Sleep Med. Clin. 2023, 18, 423–433. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Al-Rawas, A.M.; Al-Maqbali, M.; Al-Saleh, M.; Enriquez, M.B.; Al-Siyabi, S.; Al-Hashmi, K.; Al-Lawati, I.; Bulthuis, M.L.C.; et al. Systemic Oxidative Stress Is Increased in Postmenopausal Women and Independently Associates with Homocysteine Levels. Int. J. Mol. Sci. 2020, 21, 314. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Sciarrino, R.; Bianchi, S.; Battaglia, D.; Mercuri, A.; Maffei, S. Sex-related differences in association of oxidative stress status with coronary artery disease. Fertil. Steril. 2012, 97, 414–419.e2. [Google Scholar] [CrossRef]
- Leanza, G.; Conte, C.; Cannata, F.; Isgrò, C.; Piccoli, A.; Strollo, R.; Quattrocchi, C.C.; Papalia, R.; Denaro, V.; Maccarrone, M.; et al. Oxidative Stress in Postmenopausal Women with or without Obesity. Cells 2023, 12, 1137. [Google Scholar] [CrossRef]
- Semenova, N.V.; Garashchenko, N.E.; Kolesnikov, S.I.; Nikitina, O.A.; Novikova, E.A.; Smurova, N.E.; Klimenko, E.S.; Kolesnikov, S.I.; Madaeva, I.M.; Kolesnikova, L.I. Methanogen Methanosphaera stadtmanae in women intestine. Influence on the free radical oxidation and sleep quality in menopause. Bull. Exp. Biol. Med. 2025, 179, 602–606. [Google Scholar] [CrossRef]
- Semenova, N.V.; Madaeva, I.M.; Brichagina, A.S.; Kolesnikov, S.I.; Kolesnikova, L.I. Glutathione Component of Antioxidant Status in Menopausal Women with Insomnia. Bull. Exp. Biol. Med. 2022, 173, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Zitser, J.; Allen, I.E.; Falgàs, N.; Le, M.M.; Neylan, T.C.; Kramer, J.H.; Walsh, C.M. Pittsburgh Sleep Quality Index (PSQI) responses are modulated by total sleep time and wake after sleep onset in healthy older adults. PLoS ONE 2022, 17, e0270095. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Omachi, T.A. Measures of sleep in rheumatologic diseases: Epworth Sleepiness Scale (ESS), Functional Outcome of Sleep Questionnaire (FOSQ), Insomnia Severity Index (ISI), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Care Res. 2011, 63, S287–S296. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Lechat, B.; Hirotsu, C.; Appleton, S.; Younes, M.; Adams, R.J.; Vakulin, A.; Hansen, K.; Zajamsek, B.; Wittert, G.; Catcheside, P.; et al. A novel EEG marker predicts perceived sleepiness and poor sleep quality. Sleep 2022, 45, zsac051. [Google Scholar] [CrossRef]
- Wang, L.; Fang, X.; Xu, C.; Pan, N.; Wang, Y.; Xue, T.; Zhang, M.; Cao, J.; Zhang, J. Epworth sleepiness scale is associated with hypothyroidism in male patients with obstructive sleep apnea. Front. Endocrinol. 2022, 13, 1010646. [Google Scholar] [CrossRef]
- Bermingham, K.M.; Linenberg, I.; Hall, W.L.; Kadé, K.; Franks, P.W.; Davies, R.; Wolf, J.; Hadjigeorgiou, G.; Asnicar, F.; Segata, N.; et al. Menopause is associated with postprandial metabolism, metabolic health and lifestyle: The ZOE PREDICT study. EBioMedicine 2022, 85, 104303. [Google Scholar] [CrossRef]
- Dai, Y.; Vgontzas, A.N.; Chen, L.; Zheng, D.; Chen, B.; Wu, J.; Shao, R.; Li, Y. A multi-omics study of the association between insomnia with objective short sleep duration phenotype and high blood pressure. Sleep 2025, 48, zsaf030. [Google Scholar] [CrossRef] [PubMed]
- Chern, Y.-B.; Tsai, J.-P.; Liu, C.-H.; Lin, Y.-L.; Wang, C.-H.; Hsu, B.-G. Serum Indoxyl Sulfate as a Potential Biomarker of Peripheral Arterial Stiffness in Patients with Non-Dialysis Chronic Kidney Disease Stages 3 to 5. Toxins 2025, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, Q.; Liu, Z. The relationship between gut microbiota and insomnia: A bi-directional two-sample Mendelian randomization research. Front. Cell. Infect. Microbiol. 2023, 13, 1296417. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, S.; Chen, S.; Li, C.; Chan, Y.L.; Chan, N.Y.; Wing, Y.K.; Chan, F.K.L.; Su, Q.; Ng, S.C. The Role of Gut Microbiota in Insomnia: A Systematic Review of Case–Control Studies. Life 2025, 15, 1086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, X.; Li, Z.; Zou, Z.; Dou, S.; Li, G.; Yan, F.; Chen, B.; Li, Y. Alterations in Gut Microbiota Are Correlated With Serum Metabolites in Patients with Insomnia Disorder. Front. Cell. Infect. Microbiol. 2022, 12, 722662. [Google Scholar] [CrossRef] [PubMed]
- Botin, T.; Ramirez-Chamorro, L.; Vidic, J.; Langella, P.; Martin-Verstraete, I.; Chatel, J.-M.; Auger, S. The Tolerance of Gut Commensal Faecalibacterium to Oxidative Stress Is Strain Dependent and Relies on Detoxifying Enzymes. Appl. Environ. Microbiol. 2023, 89, e00606-23. [Google Scholar] [CrossRef]
- Wang, L.; Qi, X.; Wang, S.; Tian, C.; Zou, T.; Liu, Z.; Chen, Q.; Chen, Y.; Zhao, Y.; Li, S.; et al. Banxia-Yiyiren alleviates insomnia and anxiety by regulating the gut microbiota and metabolites of PCPA-induced insomnia model rats. Front. Microbiol. 2024, 15, 1405566. [Google Scholar] [CrossRef]
- Qi, X.; Ye, J.; Wen, Y.; Liu, L.; Cheng, B.; Cheng, S.; Yao, Y.; Zhang, F. Evaluating the Effects of Diet-Gut Microbiota Interactions on Sleep Traits Using the UK Biobank Cohort. Nutrients 2022, 14, 1134. [Google Scholar] [CrossRef]
- Stamation, R. Endogenous Ethanol Production in the Human Alimentary Tract: A Literature Review. J. Gastroenterol. Hepatol. 2025, 40, 783–790. [Google Scholar] [CrossRef] [PubMed]
- AL-Khikani, F.O.; Abadi, R.; Ayit, A. Emerging carbapenemase Klebsiella oxytoca with multidrug resistance implicated in urinary tract infection. Biomed. Biotechnol. Res. J. 2020, 4, 148. [Google Scholar] [CrossRef]
- Özaslan, M.S. Investigation of Potential Effects of Some Indole Compounds on the Glutathione S-Transferase Enzyme. Biochemistry 2024, 89, 553–561. [Google Scholar] [CrossRef]
- Chiu, Y.-J.; Lin, C.-H.; Lin, C.-Y.; Yang, P.-N.; Lo, Y.-S.; Chen, Y.-C.; Chen, C.-M.; Wu, Y.-R.; Yao, C.-F.; Chang, K.-H.; et al. Investigating Therapeutic Effects of Indole Derivatives Targeting Inflammation and Oxidative Stress in Neurotoxin-Induced Cell and Mouse Models of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 2642. [Google Scholar] [CrossRef]
- Park, C.; Lee, H.; Park, S.-H.; Hong, S.H.; Song, K.S.; Cha, H.-J.; Kim, G.-Y.; Chang, Y.-C.; Kim, S.; Kim, H.-S.; et al. Indole-6-carboxaldehyde prevents oxidative stress-induced mitochondrial dysfunction, DNA damage and apoptosis in C2C12 skeletal myoblasts by regulating the ROS-AMPK signaling pathway. Mol. Cell. Toxicol. 2020, 16, 455–467. [Google Scholar] [CrossRef]
- Gaike, A.H.; Kalamkar, S.D.; Gajjar, V.; Divate, U.; Karandikar-Iyer, S.; Goel, P.; Shouche, Y.S.; Ghaskadbi, S.S. Effect of long-term oral glutathione supplementation on gut microbiome of type 2 diabetic individuals. FEMS Microbiol. Lett. 2023, 370, fnad116. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, J.; Zhuge, A.; Li, L.; Ni, S. Gut microbiota modulates osteoclast glutathione synthesis and mitochondrial biogenesis in mice subjected to ovariectomy. Cell Prolif. 2022, 55, e13194. [Google Scholar] [CrossRef]
- Frazier, K.; Chang, E.B. Intersection of the gut microbiome and circadian rhythms in metabolism. Trends Endocr. Metab. 2020, 1, 25–36. [Google Scholar] [CrossRef]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut microbiome–brain alliance: A landscape view into mental and gastrointestinal health and disorders. ACS Chem. Neurosci. 2023, 10, 1717–1763. [Google Scholar] [CrossRef]
- Peters, B.A.; Lin, J.; Qi, Q.; Usyk, M.; Isasi, C.R.; Mossavar-Rahmani, Y.; Derby, C.A.; Santoro, N.; Perreira, K.M.; Daviglus, M.L.; et al. Menopause Is Associated with an Altered Gut Microbiome and Estrobolome, with Implications for Adverse Cardiometabolic Risk in the Hispanic Community Health Study/Study of Latinos. mSystems 2022, 7, e0027322. [Google Scholar] [CrossRef]
- Kwa, M.; Plottel, C.S.; Blaser, M.J.; Adams, S. The Intestinal Microbiome and Estrogen Receptor-Positive Female Breast Cancer. J. Natl. Cancer Inst. 2016, 108, djw029. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Vich Vila, A.; Garmaeva, S.; Jankipersadsing, S.A.; Imhann, F.; Collij, V.; Bonder, M.J.; Jiang, X.; Gurry, T.; Alm, E.J.; et al. Analysis of 1135 gut metagenomes identifies sex-specific resistome profiles. Gut Microbes 2019, 10, 358–366. [Google Scholar] [CrossRef]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Dohi, O.; Yoshida, N.; Kamada, K.; et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J. Gastroenterol. 2019, 54, 53–63. [Google Scholar] [CrossRef] [PubMed]
- de la Cuesta-Zuluaga, J.; Kelley, S.T.; Chen, Y.; Escobar, J.S.; Mueller, N.T.; Ley, R.E.; McDonald, D.; Huang, S.; Swafford, A.D.; Knight, R.; et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems 2019, 4, e00261-19. [Google Scholar] [CrossRef] [PubMed]
- Garashchenko, N.E.; Semenova, N.V.; Kolesnikova, L.I. Melatonin and gut microbiome. Acta Biomed. Sci. 2024, 9, 12–23. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, T.; Lee, T.H. Cellular Mechanisms of Melatonin: Insight from Neurodegenerative Diseases. Biomolecules 2020, 10, 1158. [Google Scholar] [CrossRef] [PubMed]
- Michurina, S.V.; Ishchenko, I.Y.; Bochkareva, A.L.; Arkhipov, S.A.; Kolesnikov, S.I. Effect of melatonin on expression of apoptosis regulator proteins BCL-2 and BAD in ovarian follicular apparatus after high temperature exposure. Bull. Exp. Biol. Med. 2021, 170, 598–603. [Google Scholar] [CrossRef]
- Ahmadi, S.; Taghizadieh, M.; Mehdizadehfar, E.; Hasani, A.; Fard, J.K.; Feizi, H.; Hamishehkar, H.; Ansarin, M.; Yekani, M.; Memar, M.Y. Gut microbiota in neurological diseases: Melatonin plays an important regulatory role. Biomed. Pharmacother. 2024, 174, 116487. [Google Scholar] [CrossRef]
- Bonmatí-Carrión, M.-Á.; Rol, M.-A. Melatonin as a Mediator of the Gut Microbiota–Host Interaction: Implications for Health and Disease. Antioxidants 2023, 13, 34. [Google Scholar] [CrossRef]





| Parameter 1 | ISI N | ISI SubI | rho CI | ISI I | rho CI | ISI SevI | ISI2 N | ISI2 SD | rho CI | ISI I+SevI | rho CI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | 0.14 | 0.79 | 0.46 | 0.33 | 0.31 | 0.59 | 0.48 | ||||
| K. oxytoca | 0.61 | 0.54 | 0.04 | 0.53 [0.31; 0.76] | NA | 0.27 | 0.11 | 0.04 | 0.46 [0.22; 0.69] | ||
| S. aureus | 0.75 | 0.01 | 0.45 [0.24; 0.68] | 0.01 | 0.60 [0.25; 0.86] | NA | 0.67 | 0.00 | 0.52 [0.32; 0.70] | 0.02 | 0.53 [0.23; 0.79] |
| Enterobacter spp. | 0.21 | 0.56 | 0.01 | 0.61 [0.10; 0.84] | 0.74 | 0.22 | 0.02 | 0.36 [0.06; 0.60] | 0.05 | ||
| Shigella spp. | 0.11 | 0.07 | 0.04 | 0.53 [0.31; 0.76] | NA | 0.11 | 0.00 | 0.44 [0.25; 0.63] | 0.04 | 0.46 [0.22; 0.69] | |
| Streptococcus spp. | 0.66 | 0.82 | 0.06 | 0.42 | 0.80 | 0.11 | 0.10 | ||||
| Prevotella spp. | 0.54 | 0.99 | 0.01 | 0.62 [0.30; 0.84] | 0.26 | 0.56 | 0.14 | 0.05 | |||
| M. stadmanae | 0.21 | 0.19 | 0.00 | 0.76 [0.42; 0.91] | NA | 0.07 | 0.01 | 0.43 [0.15; 0.65] | 0.00 | 0.64 [0.37; 0.89] |
| Parameter 1 | Total | rho CI | PSQI N | PSQI SD | rho CI | ESS N | rho CI | ESS S | rho CI |
|---|---|---|---|---|---|---|---|---|---|
| E. coli | 0.21 | 0.72 | 0.07 | 0.63 | 0.04 | −0.52 [−0.85; 0.08] | |||
| K. oxytoca | 0.75 | 0.07 | 0.23 | 0.86 | 0.34 | ||||
| S. aureus | 0.04 | 0.22 [−0.03; 0.41] | 0.34 | 0.00 | 0.43 [0.12; 0.61] | 0.14 | 0.29 | ||
| Enterobacter spp. | 0.75 | 0.56 | 0.33 | 0.85 | 0.39 | ||||
| Shigella spp. | 0.00 | 0.34 [0.21; 0.49] | NA | 0.00 | 0.44 [0.28. 0.61] | 0.02 | 0.27 [0.15; 0.45] | 0.02 | 0.59 [0.43; 0.75] |
| Streptococcus spp. | 0.20 | 0.80 | 0.20 | 0.88 | 0.01 | −0.67 [−0.90; −0.14] | |||
| Prevotella spp. | 0.64 | 0.70 | 0.29 | 0.82 | 0.18 | ||||
| M. stadmanae | 0.00 | 0.33 [0.12; 0.51] | 0.08 | 0.02 | 0.31 [0.06; 0.54] | 0.02 | 0.27 [0.03; 0.47] | 0.01 | 0.63 [0.18; 0.86] |
| Parameter 1 | ISIsc N | ISIsc SubI | rho CI | ISIsc I | rho CI | ISIsc SevI | ISI2 N | ISI2 SD | rho CI | ISI3 I | rho CI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| F. prausnitzii | 0.11 | 0.26 | 0.23 | 0.35 | 0.10 | 0.83 | 0.09 | ||||
| S. aureus | 0.25 | 0.01 | 0.45 [0.27; 0.70] | 0.05 | 0.18 | 0.17 | 0.09 | 0.37 | |||
| P. micra | 0.80 | 0.22 | 0.34 | 0.18 | 0.69 | 0.03 | 0.32 [0.03; 0.56] | 0.76 | |||
| Acinetobacter spp. | 0.92 | 0.84 | 0.03 | 0.50 [0.11; 0.75] | 0.17 | 0.68 | 0.18 | 0.19 | |||
| E. rectale | 0.06 | 0.47 | 0.07 | 0.55 | 0.07 | 0.63 | 0.04 | 0.42 [−0.04; 0.71] |
| Parameter 1 | Total | PSQI N | rho CI | PSQI SD | ESS N | ESS S | rho CI |
|---|---|---|---|---|---|---|---|
| F. prausnitzii | 0.39 | 0.01 | −0.45 [−0.67; −0.14] | 0.61 | 0.57 | 0.63 | |
| S. aureus | 0.63 | 0.16 | 0.15 | 0.69 | 0.66 | ||
| P. micra | 0.07 | 0.46 | 0.13 | 0.22 | 0.05 | 0.49 [0.06; 0.77] | |
| Acinetobacter spp. | 0.21 | 0.63 | 0.09 | 0.21 | 0.59 | ||
| E. rectale | 0.37 | 0.10 | 0.80 | 0.48 | 0.52 |
| Parameter | PSQI | ISI 4 Groups | ISI 3 Groups | ISI 2 Groups | ESS |
|---|---|---|---|---|---|
| E. coli | Negative, moderate in S group | ||||
| K. oxytoca | Positive, moderate in Insomnia group | Positive, moderate in combining group | |||
| S. aureus | Positive, moderate in SD group | Positive, moderate in SubI group; positive, strong in I group | Positive, moderate in combining group | Positive, moderate in I group | |
| Enterobacter spp. | Positive, strong in I group | Positive, weak in I group | |||
| Shigella spp. | Positive, moderate in SD group | Positive, moderate in Insomnia group | Positive, moderate in combining group | Positive, moderate in I group | Positive, weak in control; positive, moderate in S group |
| Streptococcus spp. | Negative, strong in S group | ||||
| Prevotella spp. | Positive, strong in I group | ||||
| M. stadmanae | Positive, moderate in SD group | Positive, strong in I group | Positive, strong in combining group | Positive, moderate in I group | Positive, weak in control; positive, strong in S group |
| K. oxytoca bi | Higher in 1 than in 0 in I group | Higher in 1 than in 0 in combining group | |||
| S. aureus bi | Higher in 1 than in 0 in SD group | Higher in 1 than in 0 in I and SubI groups | Higher in 1 than in 0 in combining and SubI groups | Higher in 1 than in 0 in I group | |
| Enterococcus spp. bi | Higher in I than in control in 1 group | ||||
| K. pneumoniae bi | Lower in SD than in control in 1 group | ||||
| M. stadmanae bi | Higher in 1 than in 0 in I group | Higher in 1 than in 0 in combining group | Higher in 1 than in 0 in I group |
| Parameter | PSQI | ISI 4 Groups | ISI 3 Groups | ISI 2 Groups | ESS |
|---|---|---|---|---|---|
| F. prausnitzii | Negative, moderate in Control | Positive, moderate in I group | |||
| S. aureus | Positive, moderate in SubI group; positive, moderate in I group | ||||
| P. micra | Positive, weak in I group | Positive, moderate in S group | |||
| Acinetobacter spp. | Positive, moderate in I group | ||||
| E. rectale | Positive, moderate in combining group | ||||
| F. nucleatum bi | Lower in 1 than 0 in Control | ||||
| P. micra bi | Lower in S than in control in 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Semenova, N.; Garashchenko, N.; Nikitina, O.; Kolesnikov, S.; Belkova, N.; Klimenko, E.; Smurova, N.; Novikova, E.; Madaeva, I.; Kolesnikova, L. Sleep Disorders in Climacteric Women: Glutathione, Glutathione S-Transferase P1 and Gut Microbiome Interrelation. Pathophysiology 2026, 33, 3. https://doi.org/10.3390/pathophysiology33010003
Semenova N, Garashchenko N, Nikitina O, Kolesnikov S, Belkova N, Klimenko E, Smurova N, Novikova E, Madaeva I, Kolesnikova L. Sleep Disorders in Climacteric Women: Glutathione, Glutathione S-Transferase P1 and Gut Microbiome Interrelation. Pathophysiology. 2026; 33(1):3. https://doi.org/10.3390/pathophysiology33010003
Chicago/Turabian StyleSemenova, Natalya, Nadezhda Garashchenko, Olga Nikitina, Sergey Kolesnikov, Natalia Belkova, Elizaveta Klimenko, Nadezhda Smurova, Elizaveta Novikova, Irina Madaeva, and Liubov Kolesnikova. 2026. "Sleep Disorders in Climacteric Women: Glutathione, Glutathione S-Transferase P1 and Gut Microbiome Interrelation" Pathophysiology 33, no. 1: 3. https://doi.org/10.3390/pathophysiology33010003
APA StyleSemenova, N., Garashchenko, N., Nikitina, O., Kolesnikov, S., Belkova, N., Klimenko, E., Smurova, N., Novikova, E., Madaeva, I., & Kolesnikova, L. (2026). Sleep Disorders in Climacteric Women: Glutathione, Glutathione S-Transferase P1 and Gut Microbiome Interrelation. Pathophysiology, 33(1), 3. https://doi.org/10.3390/pathophysiology33010003






