Dynamic Inverse Relationship Between Cell-Free DNA and Anti-dsDNA Antibodies in Experimental SLE Highlights the Potential for Targeted Immunomodulatory Therapy
Abstract
1. Introduction
2. Materials and Methods
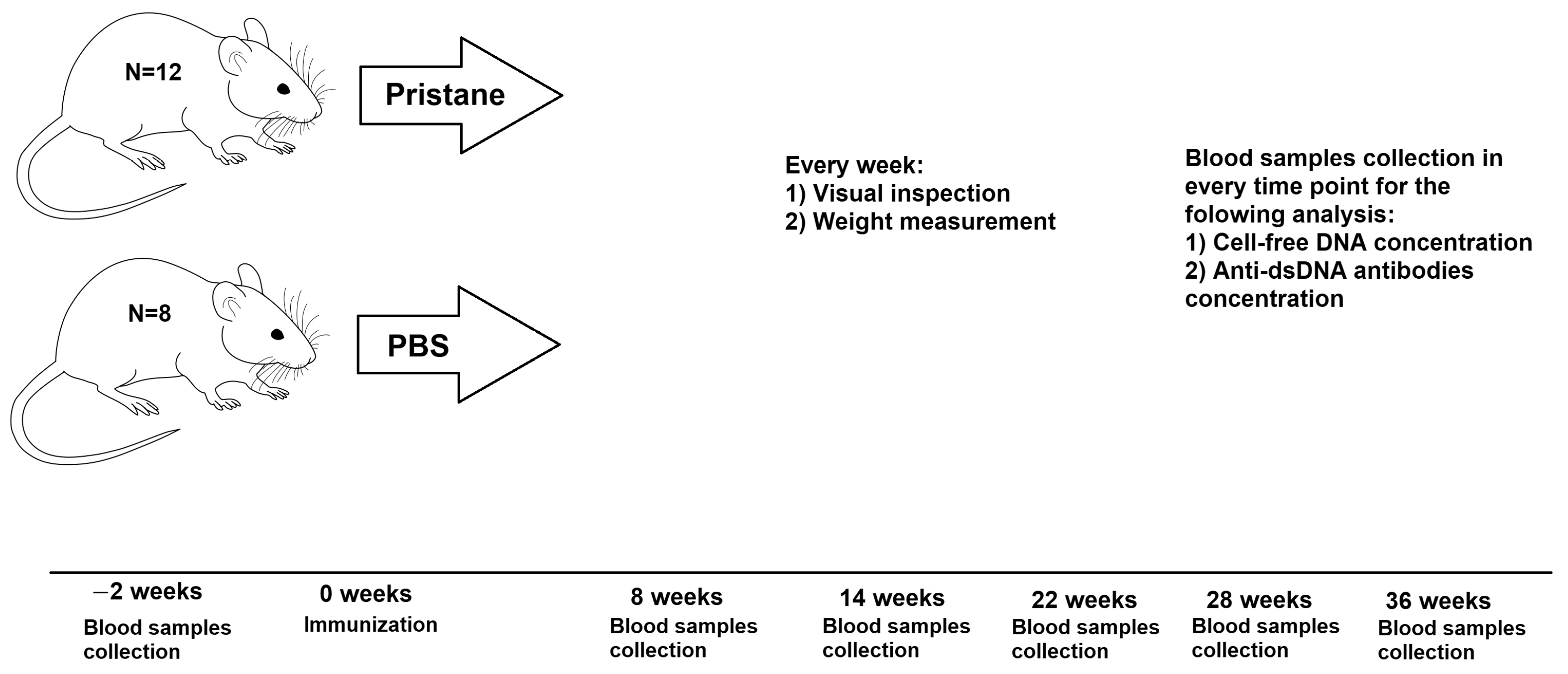
2.1. Animals and Immunization
2.2. Total cfDNA Isolation and Concentration Determination
2.3. Concentration of Anti-dsDNA Abs Determination
2.4. Statistical Analysis
3. Results
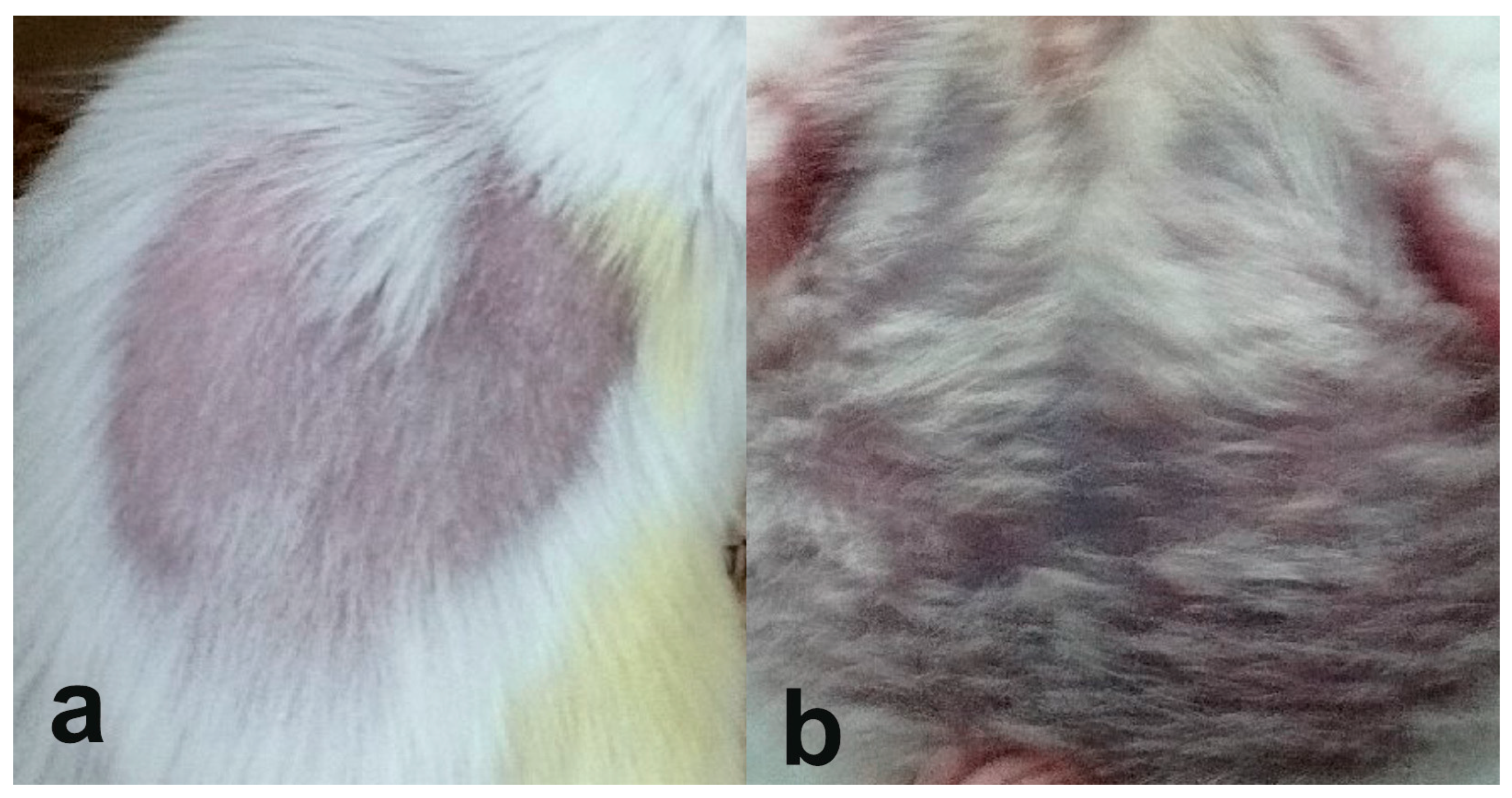
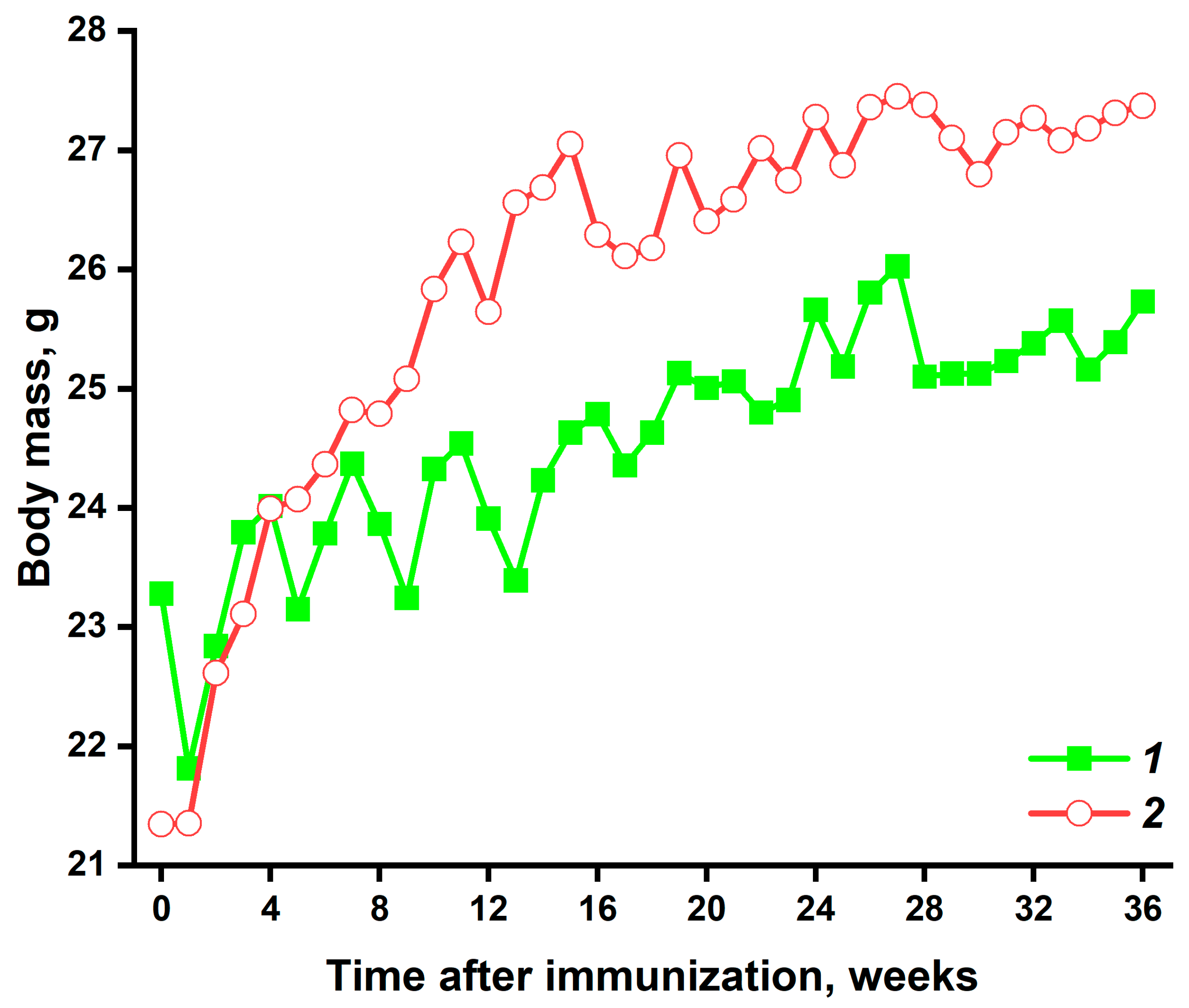
3.1. Visual Inspection and Weight Measurement
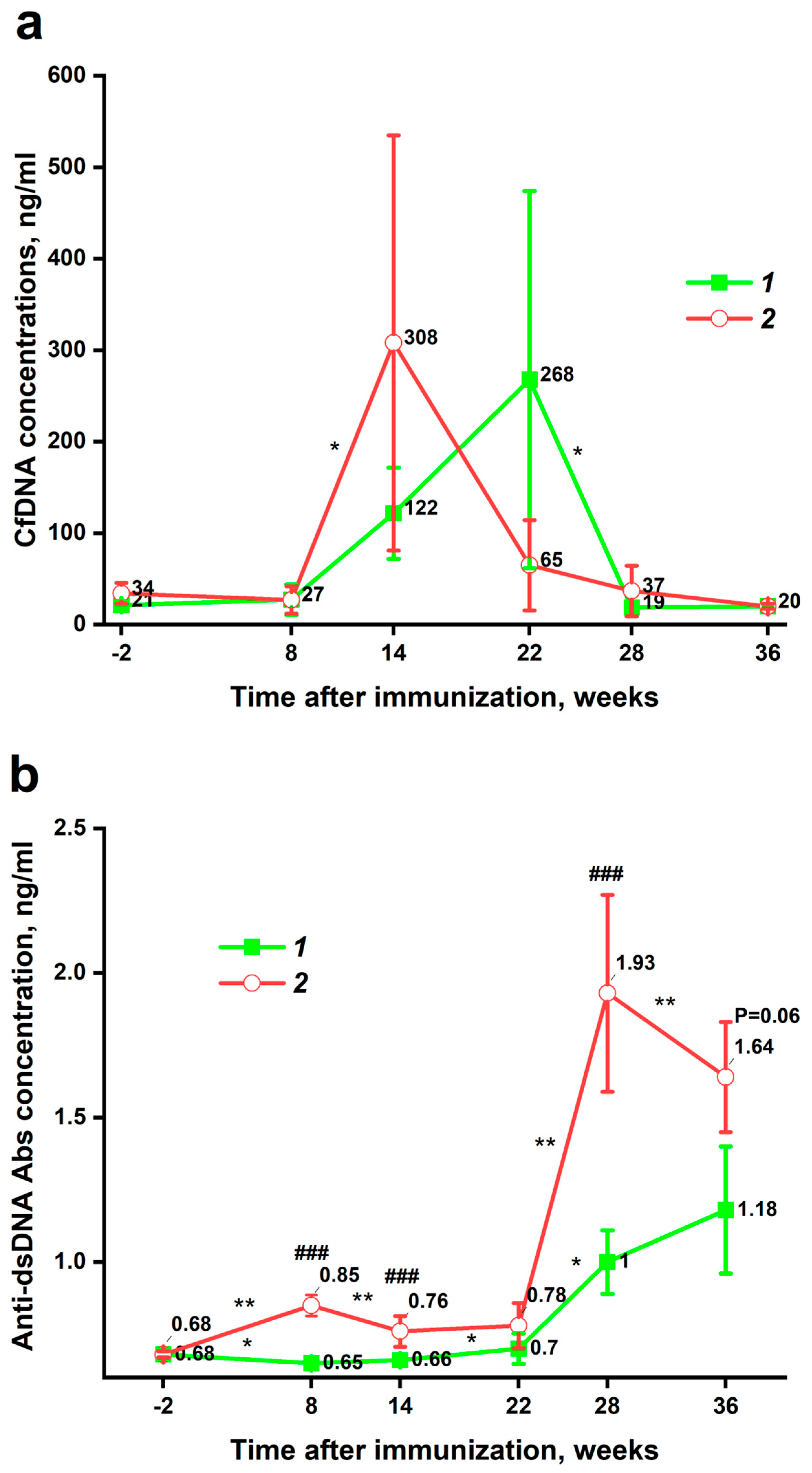
3.2. Dynamic Changes in Plasma cfDNA and Anti-dsDNA Abs Levels
3.3. Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cfDNA | Cell-free DNA |
| SLE | Systemic lupus erythematosus |
| Anti-dsDNA Abs | Antibodies to double-stranded DNA |
| DAMPs | Damage-associated molecular patterns |
| ELISA | Enzyme-linked immunosorbent assay |
References
- Carter, E.E.; Barr, S.G.; Clarke, A.E. The Global Burden of SLE: Prevalence, Health Disparities and Socioeconomic Impact. Nat. Rev. Rheumatol. 2016, 12, 605–620. [Google Scholar] [CrossRef]
- Baker, K.; Pope, J. Employment and Work Disability in Systemic Lupus Erythematosus: A Systematic Review. Rheumatology 2008, 48, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Huscher, D.; Merkesdal, S.; Thiele, K.; Zeidler, H.; Schneider, M.; Zink, A. Cost of Illness in Rheumatoid Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis and Systemic Lupus Erythematosus in Germany. Ann. Rheum. Dis. 2006, 65, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Infantino, M.; Nagy, E.; Bizzaro, N.; Fischer, K.; Bossuyt, X.; Damoiseaux, J. Anti-dsDNA Antibodies in the Classification Criteria of Systemic Lupus Erythematosus. J. Transl. Autoimmun. 2022, 5, 100139. [Google Scholar] [CrossRef]
- Kubota, T. An Emerging Role for Anti-DNA Antibodies in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 16499. [Google Scholar] [CrossRef]
- Duvvuri, B.; Lood, C. Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Front. Immunol. 2019, 10, 502. [Google Scholar] [CrossRef]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release Mechanisms of Major DAMPs. Apoptosis 2021, 26, 152–162. [Google Scholar] [CrossRef]
- Yang, D.; Han, Z.; Oppenheim, J.J. Alarmins and Immunity. Immunol. Rev. 2017, 280, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Hendy, O.M.; Motalib, T.A.; El Shafie, M.A.; Khalaf, F.A.; Kotb, S.E.; Khalil, A.; Ali, S.R. Circulating Cell Free DNA as a Predictor of Systemic Lupus Erythematosus Severity and Monitoring of Therapy. Egypt. J. Med. Hum. Genet. 2016, 17, 79–85. [Google Scholar] [CrossRef]
- Xu, Y.; Song, Y.; Chang, J.; Zhou, X.; Qi, Q.; Tian, X.; Li, M.; Zeng, X.; Xu, M.; Zhang, W.; et al. High Levels of Circulating Cell-free DNA Are a Biomarker of Active SLE. Eur. J. Clin. Investig. 2018, 48, e13015. [Google Scholar] [CrossRef]
- Diehl, F.; Schmidt, K.; Choti, M.A.; Romans, K.; Goodman, S.; Li, M.; Thornton, K.; Agrawal, N.; Sokoll, L.; Szabo, S.A.; et al. Circulating Mutant DNA to Assess Tumor Dynamics. Nat. Med. 2008, 14, 985–990. [Google Scholar] [CrossRef]
- Breitbach, S.; Sterzing, B.; Magallanes, C.; Tug, S.; Simon, P. Direct Measurement of Cell-Free DNA from Serially Collected Capillary Plasma during Incremental Exercise. J. Appl. Physiol. 2014, 117, 119–130. [Google Scholar] [CrossRef]
- Yao, W.; Mei, C.; Nan, X.; Hui, L. Evaluation and Comparison of in Vitro Degradation Kinetics of DNA in Serum, Urine and Saliva: A Qualitative Study. Gene 2016, 590, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Asano, H.; Tamaki, R.; Saito, Y.; Hosokawa, A.; Watari, H.; Umazume, T. Dynamics and Half-Life of Cell-Free DNA After Exercise: Insights from a Fragment Size-Specific Measurement Approach. Diagnostics 2025, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Aulova, K.S.; Toporkova, L.B.; Lopatnikova, J.A.; Alshevskaya, A.A.; Sedykh, S.E.; Buneva, V.N.; Budde, T.; Meuth, S.G.; Popova, N.A.; Orlovskaya, I.A.; et al. Changes in Cell Differentiation and Proliferation Lead to Production of Abzymes in EAE Mice Treated with DNA–Histone Complexes. J. Cell. Mol. Med. 2018, 22, 5816–5832. [Google Scholar] [CrossRef] [PubMed]
- Shuster, A.M.; Gololobov, G.V.; Kvashuk, O.A.; Bogomolova, A.E.; Smirnov, I.V.; Gabibov, A.G. DNA Hydrolyzing Autoantibodies. Science 1992, 256, 665–667. [Google Scholar] [CrossRef]
- Nevinsky, G.A.; Buneva, V.N. Catalytic Antibodies in Healthy Humans and Patients with Autoimmune and Viral Diseases. J. Cell. Mol. Med. 2003, 7, 265–276. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Nevinsky, G.A.; Buneva, V.N. Immunoglobulins with Non-Canonical Functions in Inflammatory and Autoimmune Disease States. Int. J. Mol. Sci. 2020, 21, 5392. [Google Scholar] [CrossRef]
- Novikova, T.S.; Ermakov, E.A.; Kostina, E.V.; Sinyakov, A.N.; Sizikov, A.E.; Nevinsky, G.A.; Buneva, V.N. Hydrolysis of Oligodeoxyribonucleotides on the Microarray Surface and in Solution by Catalytic Anti-DNA Antibodies in Systemic Lupus Erythematosus. Curr. Issues Mol. Biol. 2023, 45, 9887–9903. [Google Scholar] [CrossRef]
- Nevinsky, G.A.; Buneva, V.N.; Dmitrienok, P.S. Autoimmune Diseases: Enzymatic Cross Recognition and Hydrolysis of H2B Histone, Myelin Basic Protein, and DNA by IgGs against These Antigens. Int. J. Mol. Sci. 2022, 23, 8102. [Google Scholar] [CrossRef]
- Airlangga, D.I.; Rahmawati, H.R.; Susianti, H.; Handono, K. Exploring Systemic Lupus Erythematosus Pathogenesis through Animal Models: A Systematic Review of Humanized and Pristane-Induced Lupus Mice. J. Biomed. Transl. Res. 2023, 9, 140–156. [Google Scholar] [CrossRef]
- Tug, S.; Helmig, S.; Menke, J.; Zahn, D.; Kubiak, T.; Schwarting, A.; Simon, P. Correlation between Cell Free DNA Levels and Medical Evaluation of Disease Progression in Systemic Lupus Erythematosus Patients. Cell. Immunol. 2014, 292, 32–39. [Google Scholar] [CrossRef]
- Abdelal, I.T.; Zakaria, M.A.; Sharaf, D.M.; Elakad, G.M. Levels of Plasma Cell-Free DNA and Its Correlation with Disease Activity in Rheumatoid Arthritis and Systemic Lupus Erythematosus Patients. Egypt. Rheumatol. 2016, 38, 295–300. [Google Scholar] [CrossRef]
- Bartoloni, E.; Ludovini, V.; Alunno, A.; Pistola, L.; Bistoni, O.; Crinò, L.; Gerli, R. Increased Levels of Circulating DNA in Patients with Systemic Autoimmune Diseases: A Possible Marker of Disease Activity in Sjögren’s Syndrome. Lupus 2011, 20, 928–935. [Google Scholar] [CrossRef]
- Yun, Y.; Wang, X.; Xu, J.; Jin, C.; Chen, J.; Wang, X.; Wang, J.; Qin, L.; Yang, P. Pristane Induced Lupus Mice as a Model for Neuropsychiatric Lupus (NPSLE). Behav. Brain Funct. 2023, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Kridin, K.; Shalom, G.; Comaneshter, D.; Cohen, A.D. Is There an Association between Alopecia Areata and Systemic Lupus Erythematosus? A Population-Based Study. Immunol. Res. 2020, 68, 1–6. [Google Scholar] [CrossRef]
- Toller-Kawahisa, J.E.; Canicoba, N.C.; Venancio, V.P.; Kawahisa, R.; Antunes, L.M.G.; Cunha, T.M.; Marzocchi-Machado, C.M. Systemic Lupus Erythematosus Onset in Lupus-Prone B6.MRL/Lpr Mice Is Influenced by Weight Gain and Is Preceded by an Increase in Neutrophil Oxidative Burst Activity. Free Radic. Biol. Med. 2015, 86, 362–373. [Google Scholar] [CrossRef]
- Badr, W.H.; Loghmanee, D.; Karalus, R.J.; Murphy, T.F.; Thanavala, Y. Immunization of Mice with P6 of Nontypeable Haemophilus Influenzae: Kinetics of the Antibody Response and IgG Subclasses. Vaccine 1999, 18, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, P.-R.; Huang, L.-M.; Chen, P.-J.; Kao, C.-L.; Yang, P.-C. Chronological Evolution of IgM, IgA, IgG and Neutralisation Antibodies after Infection with SARS-Associated Coronavirus. Clin. Microbiol. Infect. 2004, 10, 1062–1066. [Google Scholar] [CrossRef]
- Shan, M.A.; Ishtiaq, W.; Kanwal, S.; Khan, M.U.; Iftikhar, A.; Khan, S. Cell-Free DNA as a Potential Diagnostic Biomarker in Academic Stress: A Case-Control Study in Young Adults. Saudi J. Biol. Sci. 2024, 31, 103933. [Google Scholar] [CrossRef]
- Ørntoft, M.W.; Jensen, S.Ø.; Øgaard, N.; Henriksen, T.V.; Ferm, L.; Christensen, I.J.; Reinert, T.; Larsen, O.H.; Nielsen, H.J.; Andersen, C.L. Age-stratified Reference Intervals Unlock the Clinical Potential of Circulating Cell-free DNA as a Biomarker of Poor Outcome for Healthy Individuals and Patients with Colorectal Cancer. Int. J. Cancer 2021, 148, 1665–1675. [Google Scholar] [CrossRef]
- He, M.; Hettinghouse, A.; Bi, Y.; Chen, Y.; Liu, C. Progranulin Mediates the Onset of Pristane Induced Systemic Lupus Erythematosus. Adv. Rheumatol. 2024, 64, 67. [Google Scholar] [CrossRef]
- Liu, S.; Li, Y.; Li, J.; Wang, S.; Ji, P.; Zhang, M.; Wang, Y. CD4+ T Cells Promote IgG Production in MHC-Independent and ICAM-1-Dependent Manners in Pristane-Induced Lupus Mice. Mediat. Inflamm. 2022, 2022, 9968847. [Google Scholar] [CrossRef]
- Steward, M.W.; Hay, F.C. Changes in Immunoglobulin Class and Subclass of Anti-DNA Antibodies with Increasing Age in N/ZBW F1 Hybrid Mice. Clin. Exp. Immunol. 1976, 26, 363–370. [Google Scholar]
- Pisetsky, D.S.; Caster, S.A.; Roths, J.B.; Murphy, E.D. Ipr Gene Control of the Anti-DNA Antibody Response. J. Immunol. 1982, 128, 2322–2325. [Google Scholar] [CrossRef] [PubMed]
- Howard, W.A.; Gibson, K.L.; Dunn-Walters, D.K. Antibody Quality in Old Age. Rejuvenation Res. 2006, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Manoussakis, M.N.; Tzioufas, A.G.; Silis, M.P.; Pange, P.J.; Goudevenos, J.; Moutsopoulos, H.M. High Prevalence of Anti-Cardiolipin and Other Autoantibodies in a Healthy Elderly Population. Clin. Exp. Immunol. 1987, 69, 557–565. [Google Scholar] [PubMed]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and Inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef]
- Freitas, D.F.; Colón, D.F.; Silva, R.L.; Santos, E.M.; Guimarães, V.H.D.; Ribeiro, G.H.M.; De Paula, A.M.B.; Guimarães, A.L.S.; Dos Reis, S.T.; Cunha, F.Q.; et al. Neutrophil Extracellular Traps (NETs) Modulate Inflammatory Profile in Obese Humans and Mice: Adipose Tissue Role on NETs Levels. Mol. Biol. Rep. 2022, 49, 3225–3236. [Google Scholar] [CrossRef]
- Li, J.; Yin, L.; Chen, S.; Li, Z.; Ding, J.; Wu, J.; Yang, K.; Xu, J. The Perspectives of NETosis on the Progression of Obesity and Obesity-Related Diseases: Mechanisms and Applications. Front. Cell Dev. Biol. 2023, 11, 1221361. [Google Scholar] [CrossRef]
- Feješ, A.; Šebeková, K.; Borbélyová, V. Pathophysiological Role of Neutrophil Extracellular Traps in Diet-Induced Obesity and Metabolic Syndrome in Animal Models. Nutrients 2025, 17, 241. [Google Scholar] [CrossRef] [PubMed]
- De Miranda Moura Dos Santos, F.; Borges, M.C.; Telles, R.W.; Correia, M.I.T.D.; Lanna, C.C.D. Excess Weight and Associated Risk Factors in Patients with Systemic Lupus Erythematosus. Rheumatol. Int. 2013, 33, 681–688. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melamud, M.M.; Ermakov, E.A.; Tolmacheva, A.S.; Nevinsky, G.A.; Buneva, V.N. Dynamic Inverse Relationship Between Cell-Free DNA and Anti-dsDNA Antibodies in Experimental SLE Highlights the Potential for Targeted Immunomodulatory Therapy. Pathophysiology 2025, 32, 48. https://doi.org/10.3390/pathophysiology32030048
Melamud MM, Ermakov EA, Tolmacheva AS, Nevinsky GA, Buneva VN. Dynamic Inverse Relationship Between Cell-Free DNA and Anti-dsDNA Antibodies in Experimental SLE Highlights the Potential for Targeted Immunomodulatory Therapy. Pathophysiology. 2025; 32(3):48. https://doi.org/10.3390/pathophysiology32030048
Chicago/Turabian StyleMelamud, Mark M., Evgeny A. Ermakov, Anna S. Tolmacheva, Georgy A. Nevinsky, and Valentina N. Buneva. 2025. "Dynamic Inverse Relationship Between Cell-Free DNA and Anti-dsDNA Antibodies in Experimental SLE Highlights the Potential for Targeted Immunomodulatory Therapy" Pathophysiology 32, no. 3: 48. https://doi.org/10.3390/pathophysiology32030048
APA StyleMelamud, M. M., Ermakov, E. A., Tolmacheva, A. S., Nevinsky, G. A., & Buneva, V. N. (2025). Dynamic Inverse Relationship Between Cell-Free DNA and Anti-dsDNA Antibodies in Experimental SLE Highlights the Potential for Targeted Immunomodulatory Therapy. Pathophysiology, 32(3), 48. https://doi.org/10.3390/pathophysiology32030048






