Serial Anti-GM-CSF Autoantibody Levels Reflect Disease Activity in Hypersensitivity Pneumonitis with Autoimmune Pulmonary Alveolar Proteinosis: Case Report
Abstract
1. Introduction
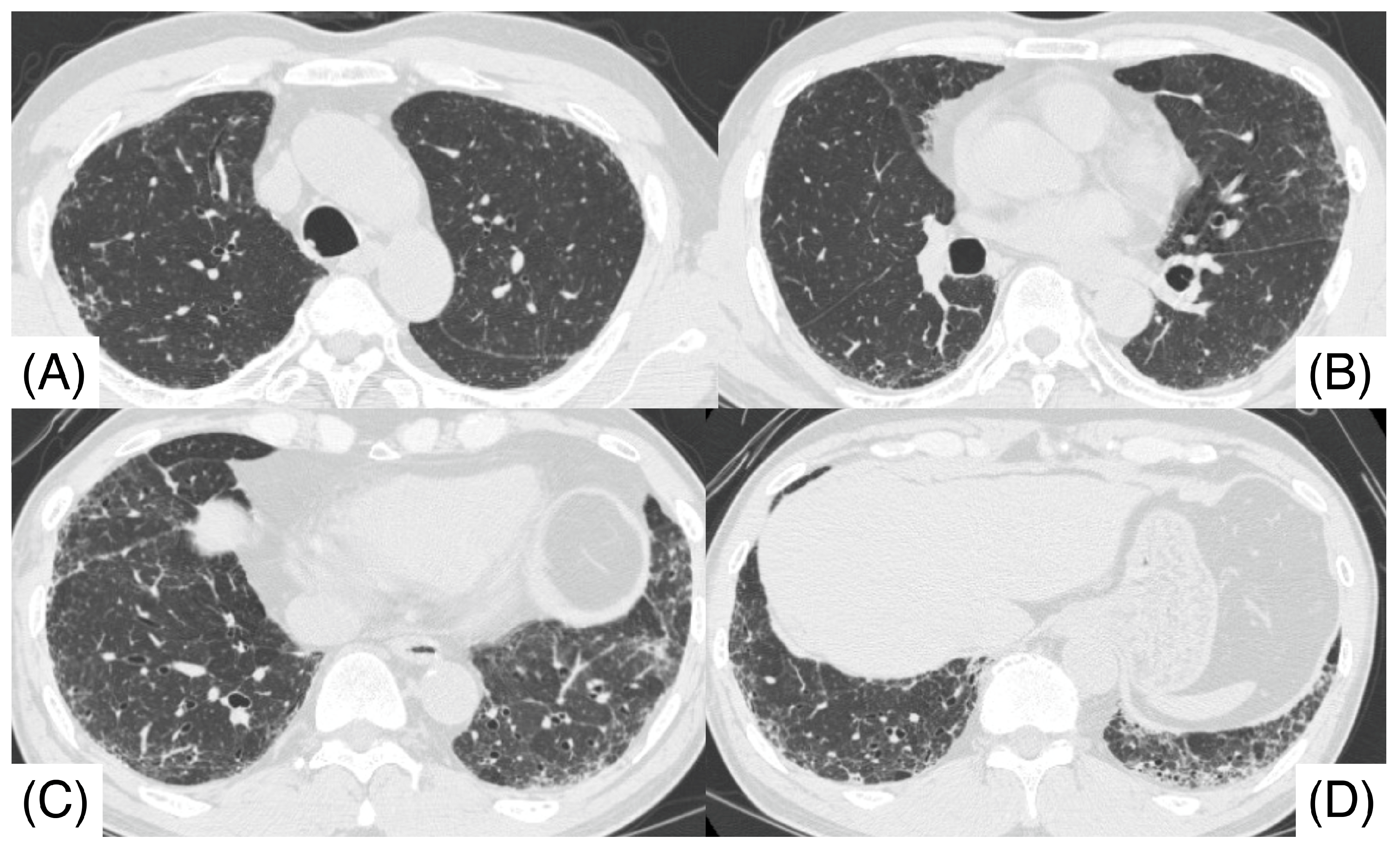
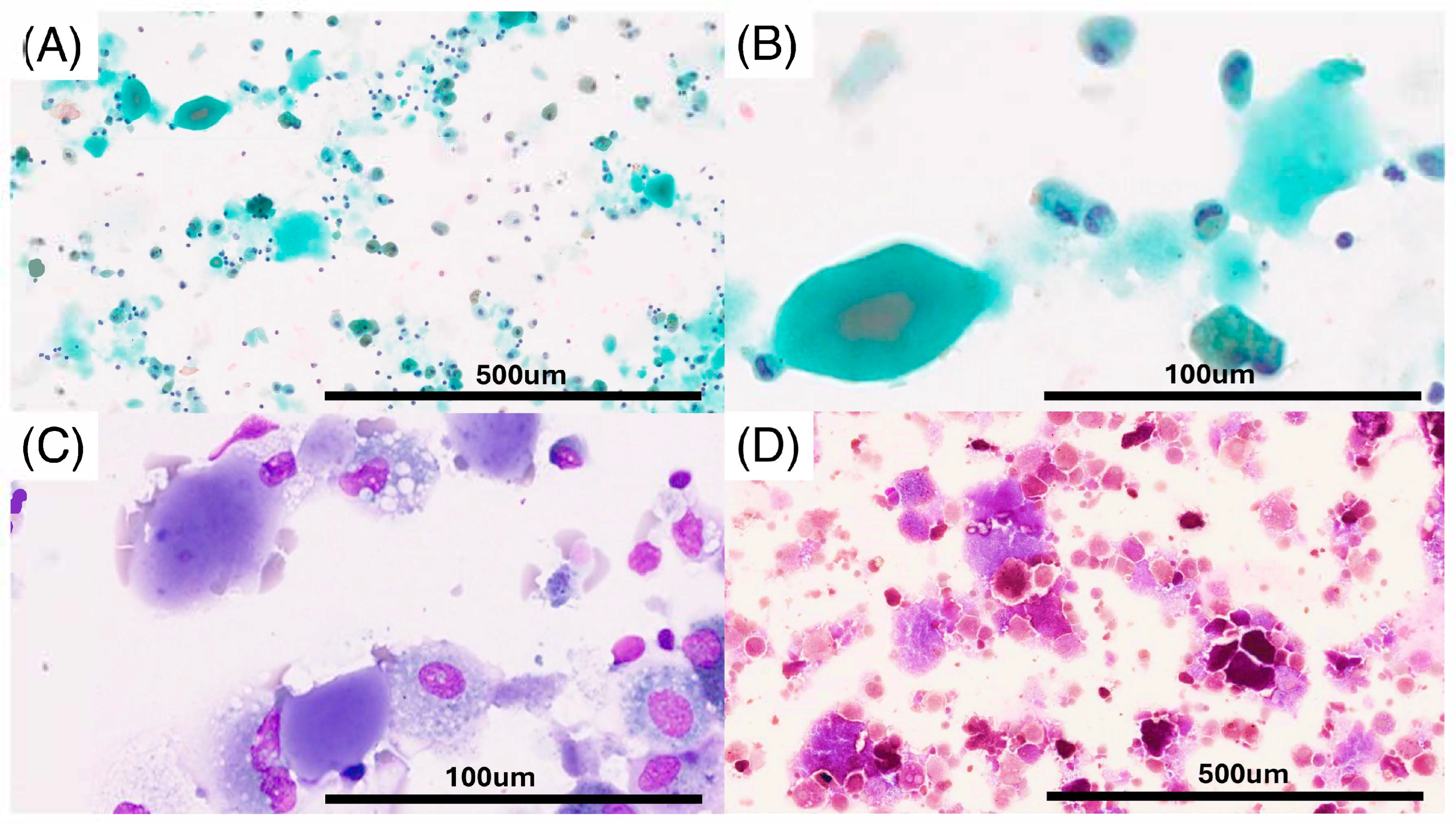
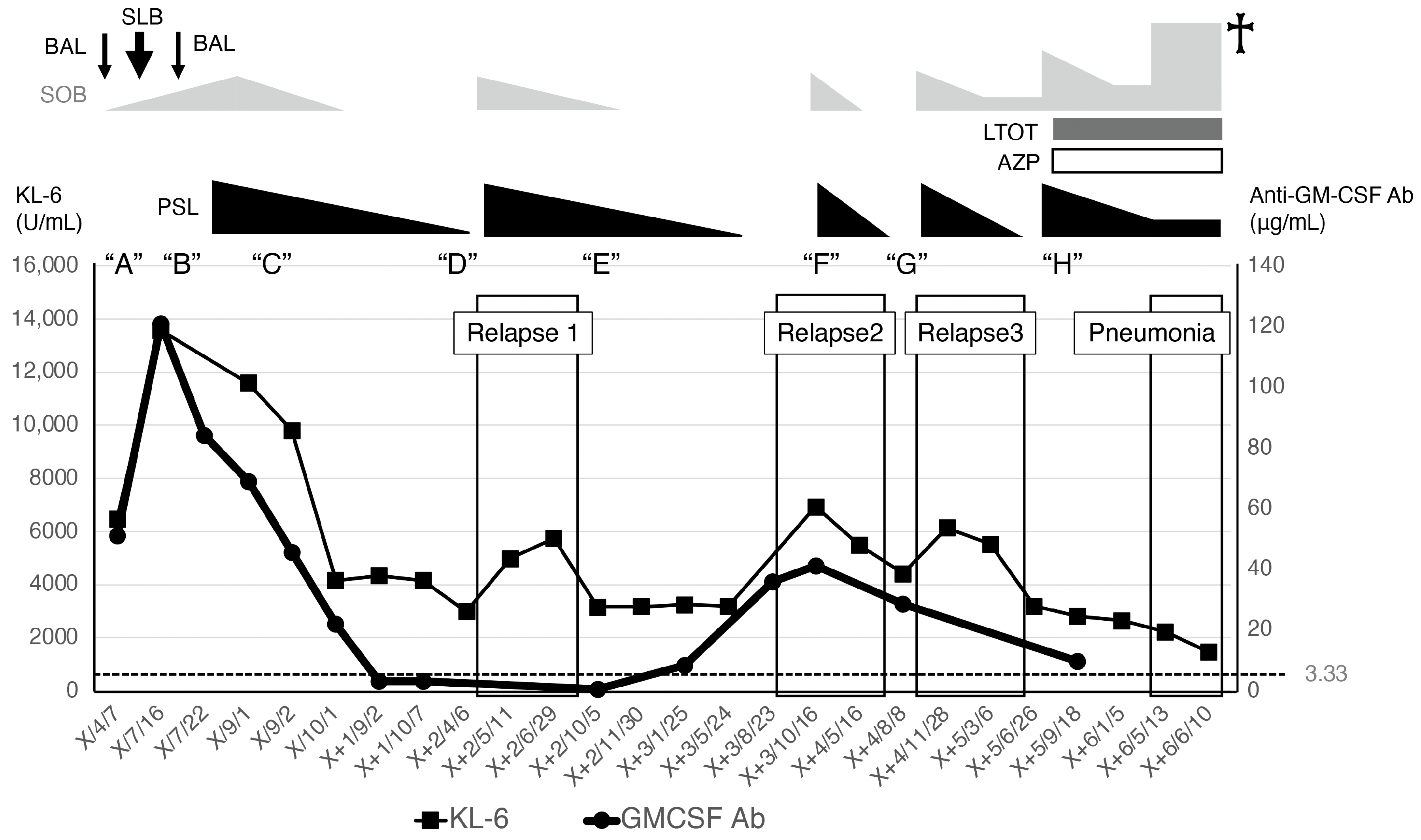
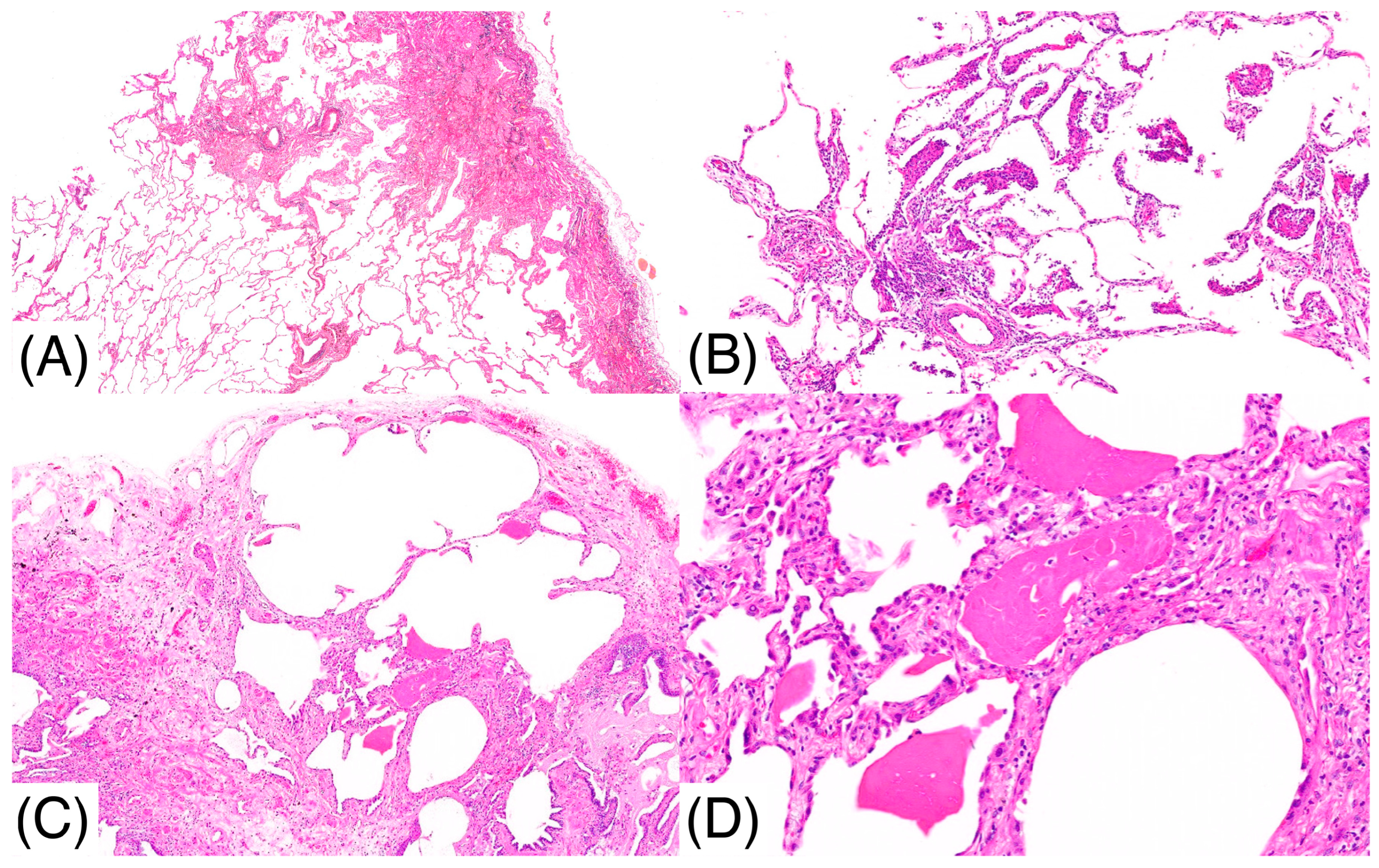
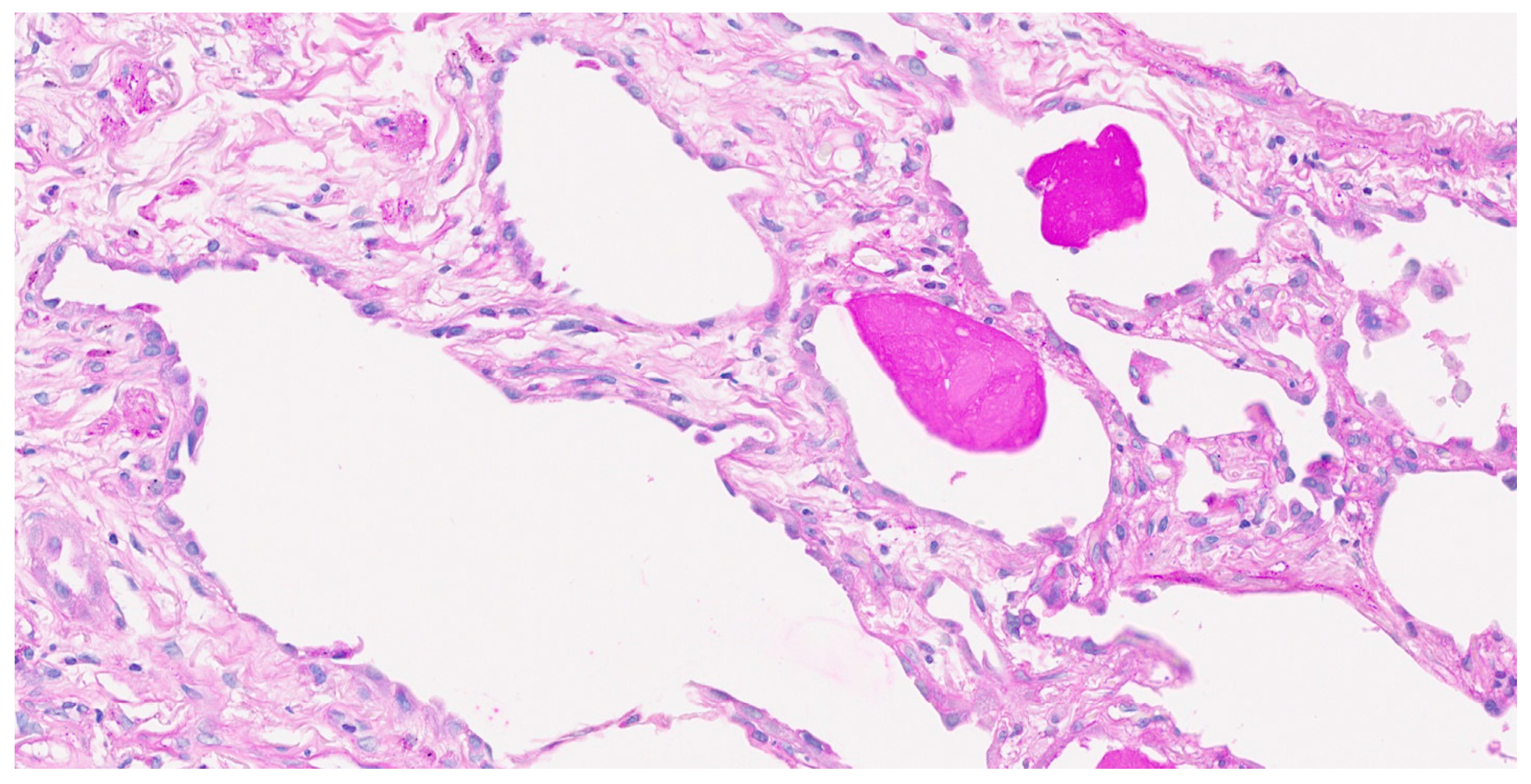
2. Case Report
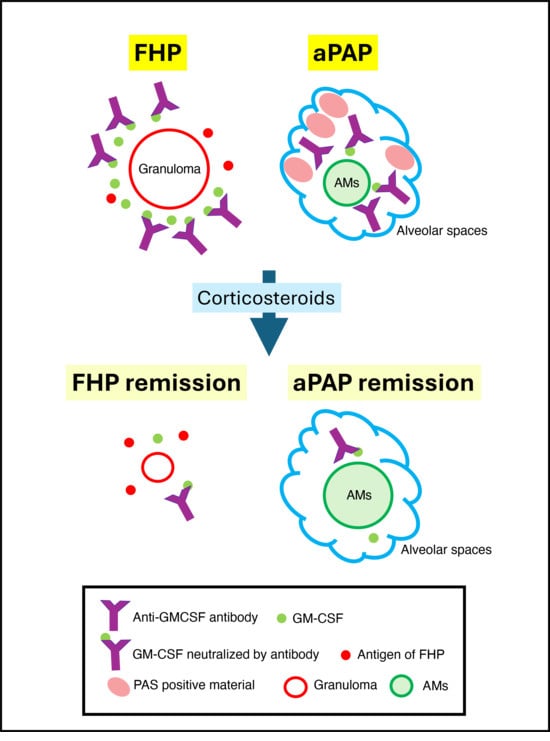
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aPAP | Autoimmune pulmonary alveolar proteinosis |
| BAL | Bronchoalveolar lavage |
| CT | Computed tomography |
| FHP | Fibrotic hypersensitivity pneumonitis |
| FVC | Forced vital capacity |
| GMAb | Anti-granulocyte-macrophage colony-stimulating factor autoantibodies |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GGO | Ground-glass opacity |
| HP | Hypersensitivity pneumonitis |
| PS | Periodic acid-Schiff |
| SLB | Surgical lung biopsy |
| SOB | Shortness of breath |
| KL-6 | Krebs von den Lungen-6 |
| PSL | Prednisolone |
| LTOT | Long-term oxygen therapy |
| AZP | Azathioprine |
| Ab | Antibody |
References
- Rosen, S.H.; Castleman, B.; Liebow, A.A. Pulmonary alveolar proteinosis. N. Engl. J. Med. 1958, 258, 1123–1142. [Google Scholar] [CrossRef]
- Inoue, Y.; Trapnell, B.C.; Tazawa, R.; Arai, T.; Takada, T.; Hizawa, N.; Kasahara, Y.; Tatsumi, K.; Hojo, M.; Ichiwata, T.; et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am. J. Respir. Crit. Care Med. 2008, 177, 752–762. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e36–e69. [Google Scholar] [CrossRef]
- Itoh, A.; Yamaguchi, E.; Kuzumaki, N.; Okazaki, N.; Furuya, K.; Abe, S.; Kawakami, Y. Expression of granulocyte-macrophage colony-stimulating factor mRNA by inflammatory cells in the sarcoid lung. Am. J. Respir. Cell Mol. Biol. 1990, 3, 245–249. [Google Scholar] [CrossRef]
- Chandra, H.; Yadav, E.; Yadav, J.S. Alveolar macrophage innate response to Mycobacterium immunogenum, the etiological agent of hypersensitivity pneumonitis: Role of JNK and p38 MAPK pathways. PLoS ONE 2013, 8, e83172. [Google Scholar] [CrossRef]
- Costa ESilva, M.; Campainha, S.; Souto Moura, C.; Marques, I.; Neves, S. Hypersensitivity pneumonitis in a patient with pulmonary alveolar proteinosis. Pulmonology 2021, 27, 464–466. [Google Scholar] [CrossRef]
- Asami-Noyama, M.; Ito, K.; Harada, M.; Hisamoto, Y.; Kunihiro, Y.; Ikeda, E.; Yamamoto, T.; Suizu, J.; Fukatsu, A.; Ohata, S.; et al. A case of development of autoimmune pulmonary alveolar proteinosis during the treatment of hypersensitivity pneumonitis. Respir. Med. Case Rep. 2023, 44, 101862. [Google Scholar] [CrossRef]
- Verma, H.; Nicholson, A.G.; Kerr, K.M.; Dempsey, O.J.; Gibbs, A.R.; Campbell, I.; Black, F.; Rassl, D.; Rice, A.J.; Renzoni, E.A.; et al. Alveolar proteinosis with hypersensitivity pneumonitis: A new clinical phenotype. Respirology 2010, 15, 1197–1202. [Google Scholar] [CrossRef]
- Katayama, K.; Hirose, M.; Arai, T.; Hatsuda, K.; Tachibana, K.; Sugawara, R.; Sugimoto, C.; Kasai, T.; Akira, M.; Inoue, Y. Clinical significance of serum anti-granulocyte-macrophage colony-stimulating factor autoantibodies in patients with sarcoidosis and hypersensitivity pneumonitis. Orphanet J. Rare Dis. 2020, 15, 272. [Google Scholar] [CrossRef]
- Arai, T.; Inoue, Y.; Sugimoto, C.; Inoue, Y.; Nakao, K.; Takeuchi, N.; Matsumuro, A.; Hirose, M.; Nakata, K.; Hayashi, S. CYFRA 21-1 as a disease severity marker for autoimmune pulmonary alveolar proteinosis. Respirology 2014, 19, 246–252. [Google Scholar] [CrossRef]
- Akasaka, K.; Tanaka, T.; Kitamura, N.; Ohkouchi, S.; Tazawa, R.; Takada, T.; Ichiwata, T.; Yamaguchi, E.; Hirose, M.; Arai, T.; et al. Outcome of corticosteroid administration in autoimmune pulmonary alveolar proteinosis: A retrospective cohort study. BMC Pulm. Med. 2015, 15, 88. [Google Scholar] [CrossRef]
- Imura, Y.; Yukawa, N.; Handa, T.; Nakashima, R.; Murakami, K.; Yoshifuji, H.; Ohmura, K.; Ishii, H.; Nakata, K.; Mimori, T. Two cases of autoimmune and secondary pulmonary alveolar proteinosis during immunosuppressive therapy in dermatomyositis with interstitial lung disease. Mod. Rheumatol. 2018, 28, 724–729. [Google Scholar] [CrossRef]
- Uchida, K.; Nakata, K.; Suzuki, T.; Nakata, K.; Trapnell, B. Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood 2009, 113, 2547–2556. [Google Scholar] [CrossRef]
- Rezende, C.M.; Goes, T.S.; Goes, V.S.; Azevedo, V.; Leite, M.F.; Goes, A.M. GM-CSF and TNF-alpha synergize to increase in vitro granuloma size of PBMC from humans induced by Schistosoma mansoni recombinant 28-kDa GST. Immunol. Lett. 2004, 95, 221–228. [Google Scholar] [CrossRef]
- Wynn, A.A.; Miyakawa, K.; Miyata, E.; Dranoff, G.; Takeya, M.; Takahashi, K. Role of granulocyte/macrophage colony-stimulating factor in zymocel-induced hepatic granuloma formation. Am. J. Pathol. 2001, 158, 131–145. [Google Scholar] [CrossRef]
- Walker, C.; Bauer, W.; Braun, R.K.; Menz, G.; Braun, P.; Schwarz, F.; Hansel, T.T.; Villiger, B. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am. J. Respir. Crit. Care Med. 1994, 150, 1038–1048. [Google Scholar] [CrossRef]
- Nunomura, S.; Tanaka, T.; Nakayama, T.; Otani, K.; Ishii, H.; Tabata, K.; Kondoh, Y.; Kataoka, K.; Johkoh, T.; Taniguchi, H.; et al. Pulmonary alveolar proteinosis-like change: A fairly common reaction associated with the severity of idiopathic pulmonary fibrosis. Respir. Investig. 2016, 54, 272–279. [Google Scholar] [CrossRef]
- Seymour, J.F.; Presneill, J.J. Pulmonary alveolar proteinosis: Progress in the first 44 years. Am. J. Respir. Crit. Care Med. 2002, 166, 215–235. [Google Scholar] [CrossRef]
- McCarthy, C.; Bonella, F.; O’Callaghan, M.; Dupin, C.; Alfaro, T.; Fally, M.; Borie, R.; Campo, I.; Cottin, V.; Fabre, A.; et al. European Respiratory Society guidelines for the diagnosis and management of pulmonary alveolar proteinosis. Eur. Respir. J. 2024, 64, 2400725. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, H.; Miyazaki, Y.; Inoue, Y.; Egashira, R.; Kawamura, T.; Sano, H.; Johkoh, T.; Takemura, T.; Hisada, T.; Fukuoka, J.; et al. Japanese clinical practice guide 2022 for hypersensitivity pneumonitis. Respir. Investig. 2024, 62, 16–43. [Google Scholar] [CrossRef]
- Iannuzzi, M.C.; Rybicki, B.A.; Teirstein, A.S. Sarcoidosis. N. Engl. J. Med. 2007, 357, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Yamaguchi, E.; Furuya, K.; Hizawa, N.; Ohnuma, N.; Kawakami, Y.; Kuzumaki, N. Correlation of GM-CSF mRNA in bronchoalveolar fluid with indices of clinical activity in sarcoidosis. Thorax 1993, 48, 1230–1234. [Google Scholar] [CrossRef]
- Arai, T.; Kasai, T.; Shimizu, K.; Kawahara, K.; Katayama, K.; Sugimoto, C.; Hirose, M.; Okamoto, H.; Tachibana, K.; Akira, M.; et al. Autoimmune Pulmonary Alveolar Proteinosis Complicated with Sarcoidosis: The Clinical Course and Serum Levels of Anti-granulocyte-macrophage colony-stimulating Factor Autoantibody. Intern. Med. 2020, 59, 2539–2546. [Google Scholar] [CrossRef]
- Gathungu, G.; Kim, M.O.; Ferguson, J.P.; Sharma, Y.; Zhang, W.; Ng, S.M.; Bonkowski, E.; Ning, K.; Simms, L.A.; Croft, A.R.; et al. Granulocyte-macrophage colony-stimulating factor autoantibodies: A marker of aggressive Crohn’s disease. Inflamm. Bowel Dis. 2013, 19, 1671–1680. [Google Scholar] [CrossRef]
- Ishikawa, N.; Hattori, N.; Yokoyama, A.; Kohno, N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir. Investig. 2012, 50, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Tsutsui, T.; Suhara, K.; Furusawa, H.; Miyazaki, Y.; Inase, N. Seasonal variation of serum KL-6 and SP-D levels in bird-related hypersensitivity pneumonitis. Sarcoidosis Vasc. Diffuse Lung Dis. 2015, 31, 364–367. [Google Scholar]
- Okamoto, T.; Fujii, M.; Furusawa, H.; Tsuchiya, K.; Miyazaki, Y.; Inase, N. The usefulness of KL-6 and SP-D for the diagnosis and management of chronic hypersensitivity pneumonitis. Respir. Med. 2015, 109, 1576–1581. [Google Scholar] [CrossRef]
- Takahashi, T.; Munakata, M.; Suzuki, I.; Kawakami, Y. Serum and bronchoalveolar fluid KL-6 levels in patients with pulmonary alveolar proteinosis. Am. J. Respir. Crit. Care Med. 1998, 158, 1294–1298. [Google Scholar] [CrossRef]
- Bonella, F.; Ohshimo, S.; Miaotian, C.; Griese, M.; Guzman, J.; Costabel, U. Serum KL-6 is a predictor of outcome in pulmonary alveolar proteinosis. Orphanet J. Rare Dis. 2013, 8, 53. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arai, T.; Hirose, M.; Sugimoto, E.; Takimoto, T.; Inoue, Y.; Sumikawa, H.; Takemura, T.; Shimizu, S. Serial Anti-GM-CSF Autoantibody Levels Reflect Disease Activity in Hypersensitivity Pneumonitis with Autoimmune Pulmonary Alveolar Proteinosis: Case Report. Pathophysiology 2025, 32, 47. https://doi.org/10.3390/pathophysiology32030047
Arai T, Hirose M, Sugimoto E, Takimoto T, Inoue Y, Sumikawa H, Takemura T, Shimizu S. Serial Anti-GM-CSF Autoantibody Levels Reflect Disease Activity in Hypersensitivity Pneumonitis with Autoimmune Pulmonary Alveolar Proteinosis: Case Report. Pathophysiology. 2025; 32(3):47. https://doi.org/10.3390/pathophysiology32030047
Chicago/Turabian StyleArai, Toru, Masaki Hirose, Eiji Sugimoto, Takayuki Takimoto, Yoshikazu Inoue, Hiromitsu Sumikawa, Tamiko Takemura, and Shigeki Shimizu. 2025. "Serial Anti-GM-CSF Autoantibody Levels Reflect Disease Activity in Hypersensitivity Pneumonitis with Autoimmune Pulmonary Alveolar Proteinosis: Case Report" Pathophysiology 32, no. 3: 47. https://doi.org/10.3390/pathophysiology32030047
APA StyleArai, T., Hirose, M., Sugimoto, E., Takimoto, T., Inoue, Y., Sumikawa, H., Takemura, T., & Shimizu, S. (2025). Serial Anti-GM-CSF Autoantibody Levels Reflect Disease Activity in Hypersensitivity Pneumonitis with Autoimmune Pulmonary Alveolar Proteinosis: Case Report. Pathophysiology, 32(3), 47. https://doi.org/10.3390/pathophysiology32030047