Decreased Heart Rate Variability Is Associated with Increased Fatigue Across Different Medical Populations: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Researchers
2.4. Quality Assessment
3. Results
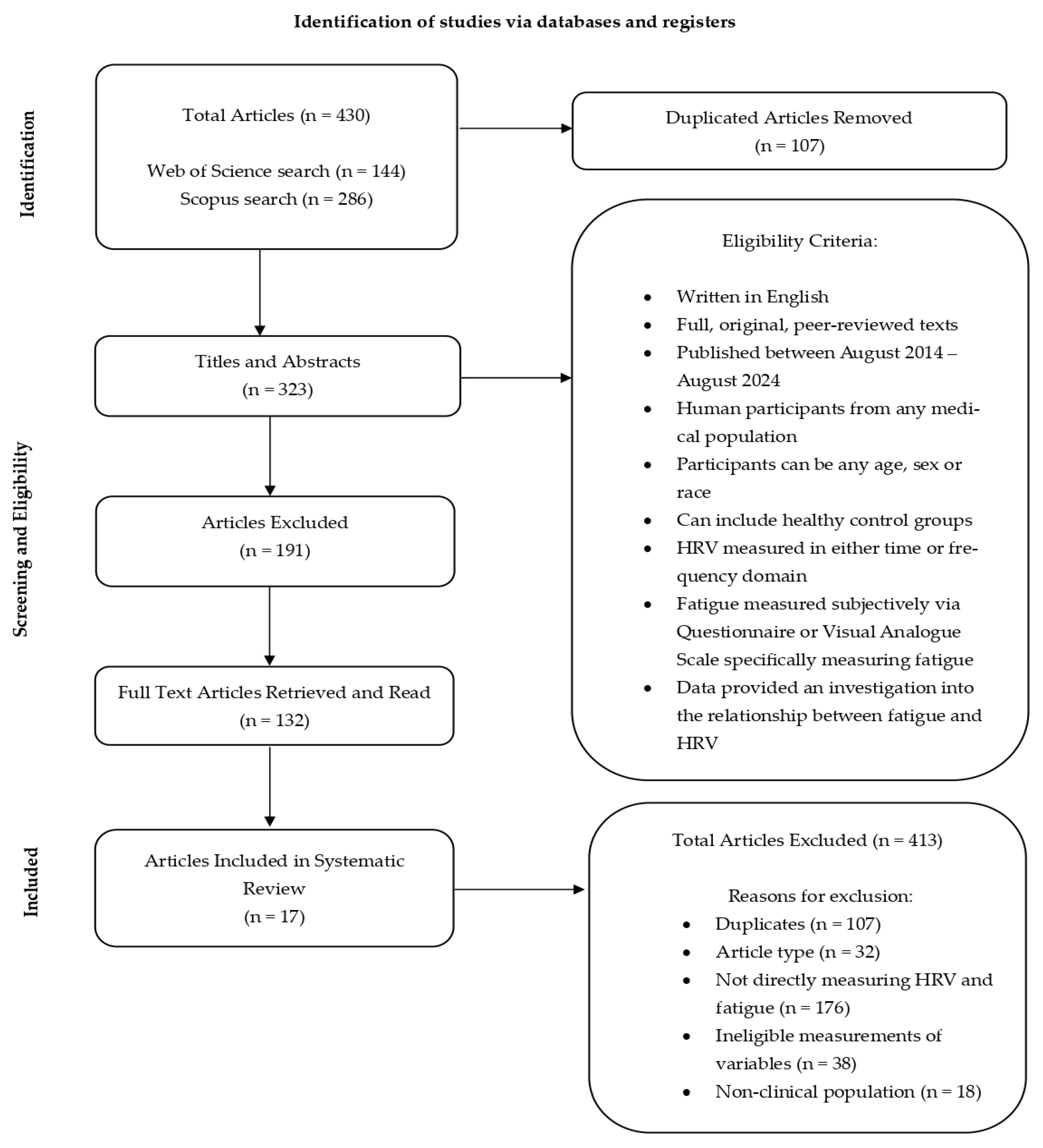
3.1. Study Process
3.2. Characteristics of Articles Included
3.3. Participant Population
3.4. Cancer
3.5. Multiple Sclerosis (MS)
3.6. Chronic Fatigue Syndrome (CFS)
3.7. Other Medical Populations
4. Discussion
4.1. Limitations
4.2. Future Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Billones, R.; Liwang, J.K.; Butler, K.; Graves, L.; Saligan, L.N. Dissecting the fatigue experience: A scoping review of fatigue definitions, dimensions, and measures in non-oncologic medical conditions. Brain Behav. Immun.-Health 2021, 15, 100266. [Google Scholar] [CrossRef]
- Cella, D.; Yount, S.; Rothrock, N.; Gershon, R.; Cook, K.; Reeve, B.; Ader, D.; Fries, J.F.; Bruce, B.; Rose, M. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap Cooperative Group During its First Two Years. Med. Care 2007, 45, S3–S11. [Google Scholar] [CrossRef]
- Matura, L.A.; Malone, S.; Jaime-Lara, R.; Riegel, B. A Systematic Review of Biological Mechanisms of Fatigue in Chronic Illness. Biol. Res. Nurs. 2018, 20, 410–421. [Google Scholar] [CrossRef]
- Behrens, M.; Gube, M.; Chaabene, H.; Prieske, O.; Zenon, A.; Broscheid, K.-C.; Schega, L.; Husmann, F.; Weippert, M. Fatigue and Human Performance: An Updated Framework. Sports Med. 2023, 53, 7–31. [Google Scholar] [CrossRef]
- Saligan, L.N.; Olson, K.; Filler, K.; Larkin, D.; Cramp, F.; Sriram, Y.; Escalante, C.P.; Del Giglio, A.; Kober, K.M.; Kamath, J.; et al. The biology of cancer-related fatigue: A review of the literature. Support. Care Cancer 2015, 23, 2461–2478. [Google Scholar] [CrossRef]
- Goërtz, Y.M.J.; Braamse, A.M.J.; Spruit, M.A.; Janssen, D.J.A.; Ebadi, Z.; Van Herck, M.; Burtin, C.; Peters, J.B.; Sprangers, M.A.G.; Lamers, F.; et al. Fatigue in patients with chronic disease: Results from the population-based Lifelines Cohort Study. Sci. Rep. 2021, 11, 20977. [Google Scholar] [CrossRef] [PubMed]
- Kluger, B.M.; Krupp, L.B.; Enoka, R.M. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology 2013, 80, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Van Langenberg, D.R.; Gibson, P.R. Systematic review: Fatigue in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2010, 32, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Knoop, V.; Cloots, B.; Costenoble, A.; Debain, A.; Vella Azzopardi, R.; Vermeiren, S.; Jansen, B.; Scafoglieri, A.; Bautmans, I.; Bautmans, I.; et al. Fatigue and the prediction of negative health outcomes: A systematic review with meta-analysis. Ageing Res. Rev. 2010, 67, 101261. [Google Scholar] [CrossRef]
- Ma, Y.; He, B.; Jiang, M.; Yang, Y.; Wang, C.; Huang, C.; Han, L. Prevalence and risk factors of cancer-related fatigue: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2020, 111, 103707. [Google Scholar] [CrossRef]
- Al Maqbali, M.; Al Sinani, M.; Al Naamani, Z.; Al Badi, K.; Tanash, M.I. Prevalence of Fatigue in Patients with Cancer: A Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2021, 61, 167–189.e14. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-J.; Ahn, Y.-C.; Jang, E.-S.; Lee, S.-W.; Lee, S.-H.; Son, C.-G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef]
- Machado, M.O.; Kang, N.C.; Tai, F.; Sambhi, R.D.S.; Berk, M.; Carvalho, A.F.; Chada, L.P.; Merola, J.F.; Piguet, V.; Alavi, A. Measuring fatigue: A meta-review. Int. J. Dermatol. 2021, 60, 1053–1069. [Google Scholar] [CrossRef]
- Georgiou, K.; Larentzakis, A.V.; Khamis, N.N.; Alsuhaibani, G.I.; Alaska, Y.A.; Giallafos, E.J. Can Wearable Devices Accurately Measure Heart Rate Variability? A Syst. Review. Folia Medica 2018, 60, 7–20. [Google Scholar] [CrossRef]
- Sammito, S.; Böckelmann, I. Factors influencing heart rate variability. Int. Cardiovasc. Forum J. 2016, 6, 18–22. [Google Scholar] [CrossRef]
- Jason, L.A.; Corradi, K.; Torres-Harding, S.; Taylor, R.R.; King, C. Chronic Fatigue Syndrome: The Need for Subtypes. Neuropsychol. Rev. 2005, 15, 29–58. [Google Scholar] [CrossRef] [PubMed]
- Meeus, M.; Goubert, D.; De Backer, F.; Struyf, F.; Hermans, L.; Coppieters, I.; De Wandele, I.; Da Silva, H.; Calders, P. Heart rate variability in patients with fibromyalgia and patients with chronic fatigue syndrome: A systematic review. Semin. Arthritis Rheum. 2013, 43, 279–287. [Google Scholar] [CrossRef]
- Cygankiewicz, I.; Zareba, W. Heart rate variability. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 117, pp. 379–393. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Electrophysiology, Task Force of the European Society of Cardiology the North American Society of Pacing. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [CrossRef]
- Buchman, T.G.; Stein, P.K.; Goldstein, B. Heart rate variability in critical illness and critical care. Curr. Opin. Crit. Care 2020, 8, 311–315. [Google Scholar] [CrossRef]
- Karmali, S.N.; Sciusco, A.; May, S.M.; Ackland, G.L. Heart rate variability in critical care medicine: A systematic review. Intensive Care Med. Exp. 2017, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Boissoneault, J.; Letzen, J.; Robinson, M.; Staud, R. Cerebral blood flow and heart rate variability predict fatigue severity in patients with chronic fatigue syndrome. Brain Imaging Behav. 2019, 13, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, L.; Castro-Marrero, J.; Alegre, J.; Ramos-Castro, J.; Escorihuela, R.M. Analysis of Gender Differences in HRV of Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Using Mobile-Health Technology. Sensors 2021, 21, 3746. [Google Scholar] [CrossRef]
- Deuring, G.; Kiss, A.; Halter, J.P.; Passweg, J.R.; Grossman, P. Cardiac autonomic functioning is impaired among allogeneic hematopoietic stem cell transplantation survivors: A controlled study. Bone Marrow Transplant. 2017, 52, 66–72. [Google Scholar] [CrossRef]
- Escorihuela, R.M.; Capdevila, L.; Castro, J.R.; Zaragozà, M.C.; Maurel, S.; Alegre, J.; Castro-Marrero, J. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J. Transl. Med. 2020, 18, 4. [Google Scholar] [CrossRef]
- Gashi, S.; Oldrati, P.; Moebus, M.; Hilty, M.; Barrios, L.; Ozdemir, F.; Kana, V.; Lutterotti, A.; Rätsch, G.; Holz, C. Modeling multiple sclerosis using mobile and wearable sensor data. npj Digit. Med. 2024, 7, 64. [Google Scholar] [CrossRef]
- Kitselaar, W.M.; de Morree, H.M.; Trompenaars, M.W.; Sitskoorn, M.M.; Rutten, G.-J.; Kop, W.J. Fatigue after neurosurgery in patients with a brain tumor: The role of autonomic dysregulation and disturbed sleep. J. Psychosom. Res. 2022, 156, 110766. [Google Scholar] [CrossRef]
- Kristiansen, M.S.; Stabursvik, J.; O’Leary, E.C.; Pedersen, M.; Asprusten, T.T.; Leegaard, T.; Osnes, L.T.; Tjade, T.; Skovlund, E.; Godang, K.; et al. Clinical symptoms and markers of disease mechanisms in adolescent chronic fatigue following Epstein-Barr virus infection: An exploratory cross-sectional study. Brain Behav. Immun. 2019, 80, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rosales, E.; Sola-Rodriguez, S.; Vargas-Hitos, J.A.; Gavilan-Carrera, B.; Rosales-Castillo, A.; Hernandez-Martinez, A.; Artero, E.G.; Sabio, J.M.; Soriano-Maldonado, A. Heart Rate Variability in Women with Systemic Lupus Erythematosus: Association with Health-Related Parameters and Effects of Aerobic Exercise. Int. J. Environ. Res. Public Health 2020, 17, 9501. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Tanahashi, T.; Lkhagvasuren, B.; Yamada, Y. The longitudinal effects of seated isometric yoga on blood biomarkers, autonomic functions, and psychological parameters of patients with chronic fatigue syndrome: A pilot study. Biopsychosoc. Med. 2019, 13, 28. [Google Scholar] [CrossRef]
- Olivera-Toro, A.; Fossion, R.; Li, L.; Lopez-Gomez, R.E.; Lopez-Espinosa, E.; Jimenez-Estrada, I.; Quiroz-Gonzalez, S. Changes in Heart Rate Variability in Patients with Spleen-Qi Deficiency Syndrome. J. Acupunct. Meridian Stud. 2019, 12, 111–121. [Google Scholar] [CrossRef]
- Park, H.Y.; Jeon, H.J.; Bang, Y.R.; Yoon, I.-Y. Multidimensional Comparison of Cancer-Related Fatigue and Chronic Fatigue Syndrome: The Role of Psychophysiological Markers. Psychiatry Investig. 2019, 16, 71–79. [Google Scholar] [CrossRef]
- Ryabkova, V.A.; Rubinskiy, A.V.; Marchenko, V.N.; Trofimov, V.I.; Churilov, L.P. Similar Patterns of Dysautonomia in Myalgic Encephalomyelitis/Chronic Fatigue and Post-COVID-19 Syndromes. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2024, 31, 1–17. [Google Scholar] [CrossRef]
- Rzepinski, L.; Zawadka-Kunikowska, M.; Newton, J.L.; Zalewski, P.; Slomko, J. Cardiovascular autonomic dysfunction in multiple sclerosis-findings and relationships with clinical outcomes and fatigue severity. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysi-Ology 2022, 43, 4829–4839. [Google Scholar] [CrossRef]
- Sander, C.; Modes, F.; Schlake, H.-P.; Eling, P.; Hildebrandt, H. Capturing fatigue parameters: The impact of vagal processing in multiple sclerosis related cognitive fatigue. Mult. Scler. Relat. Disord. 2019, 32, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.; Braun, N.; Modes, F.; Schlake, H.-P.; Eling, P.; Hildebrandt, H. Can biofeedback-based training alleviate fatigue and vigilance performance in fatigued MS patients? Neuropsychol. Rehabil. 2022, 32, 131–147. [Google Scholar] [CrossRef]
- Wheeler, C.; Pacheco, J.M.; Kim, A.C.; Camacho-Santiago, M.; Kalafut, M.A.; Ahern, T.; White, A.A.; Patay, B.; Criado, J.R. Cardiovascular Autonomic Regulation, ETCO2 and the Heart Rate Response to the Tilt Table Test in Patients with Orthostatic Intolerance. Appl. Psychophysiol. Biofeedback 2022, 47, 107–119. [Google Scholar] [CrossRef]
- Zhou, W.; Wan, Y.-H.; Chen, Q.; Qiu, Y.-R.; Luo, X.-M. Effects of Tai Chi Exercise on Cancer-Related Fatigue in Patients with Nasopharyngeal Carcinoma Undergoing Chemoradiotherapy: A Randomized Controlled Trial. J. Pain Symptom Manag. 2018, 55, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Yellen, S.B.; Cella, D.F.; Webster, K.; Blendowski, C.; Kaplan, E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manag. 1997, 13, 63–74. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Garis, G.; Haupts, M.; Duning, T.; Hildebrandt, H. Heart rate variability and fatigue in MS: Two parallel pathways representing disseminated inflammatory processes? Psychiatry Investig. 2023, 44, 83–98. [Google Scholar] [CrossRef]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the Functional Impact of Fatigue: Initial Validation of the Fatigue Impact Scale. Clin. Infect. Dis. 1994, 18 (Suppl. 1), S79–S83. [Google Scholar] [CrossRef]
- Malpas, S.C. Neural influences on cardiovascular variability: Possibilities and pitfalls. Am. J. Physiol.-Heart Circ. Physiol. 2002, 282, H6–H20. [Google Scholar] [CrossRef]
- Whitehead, L. The Measurement of Fatigue in Chronic Illness: A Systematic Review of Unidimensional and Multidimensional Fatigue Measures. J. Pain Symptom Manag. 2009, 37, 107–128. [Google Scholar] [CrossRef]
- Oka, T.; Tanahashi, T.; Chijiwa, T.; Lkhagvasuren, B.; Sudo, N.; Oka, K. Isometric yoga improves the fatigue and pain of patients with chronic fatigue syndrome who are resistant to conventional therapy: A randomized, controlled trial. Biopsychosoc. Med. 2014, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Pechnik, S.; Gross, D.; Sewell, L.; Goldstein, D.S. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin. Auton. Res. 2011, 21, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.J.; Bahl, J.S.; Buckley, J.D.; Thomson, R.L.; Davison, K. Evidence of altered cardiac autonomic regulation in myalgic encephalomyelitis/chronic fatigue syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e17600. [Google Scholar] [CrossRef]
- Ying-Chih, C.; Yu-Chen, H.; Wei-Lieh, H. Heart rate variability in patients with somatic symptom disorders and functional somatic syndromes: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 112, 336–344. [Google Scholar] [CrossRef]
- Vreijling, S.R.; Troudart, Y.; Brosschot, J.F. Reduced Heart Rate Variability in Patients with Medically Unexplained Physical Symptoms: A Meta-Analysis of HF-HRV and RMSSD. Psychosom. Med. 2021, 83, 2–15. [Google Scholar] [CrossRef]
- Arab, C.; Dias, D.P.M.; Barbosa, R.T.D.A.; Carvalho, T.D.D.; Valenti, V.E.; Crocetta, T.B.; Ferreira, M.; Abreu, L.C.D.; Ferreira, C. Heart rate variability measure in breast cancer patients and survivors: A systematic review. Psychoneuroendocrinology 2016, 68, 57–68. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.A.; Choi, Y.S.; Kim, S.H.; Lee, J.Y.; Kim, Y.E. Heart Rate Variability and Length of Survival in Hospice Cancer Patients. J. Korean Med. Sci. 2010, 25, 1140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ma, Z.; Zhang, L.; Zhou, S.; Wang, J.; Wang, B.; Fu, W. Heart rate variability in the prediction of survival in patients with cancer: A systematic review and meta-analysis. J. Psychosom. Res. 2016, 89, 20–25. [Google Scholar] [CrossRef]
- Crosswell, A.D.; Lockwood, K.G.; Ganz, P.A.; Bower, J.E. Low heart rate variability and cancer-related fatigue in breast cancer survivors. Psychoneuroendocrinology 2014, 45, 58–66. [Google Scholar] [CrossRef]
- Fagundes, C.P.; Murray, D.M.; Hwang, B.S.; Gouin, J.-P.; Thayer, J.F.; Sollers, J.J.; Shapiro, C.L.; Malarkey, W.B.; Kiecolt-Glaser, J.K. Sympathetic and parasympathetic activity in cancer-related fatigue: More evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology 2011, 36, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Haischer, M.H.; Opielinski, L.E.; Mirkes, L.M.; Uhrich, T.D.; Bollaert, R.E.; Danduran, M.; Bement, M.H.; Piacentine, L.B.; Papanek, P.E.; Hunter, S.K. Heart rate variability is reduced in COVID-19 survivors and associated with physical activity and fatigue. Physiol. Rep. 2024, 12, e15912. [Google Scholar] [CrossRef] [PubMed]
- Vigo, C.; Gatzemeier, W.; Sala, R.; Malacarne, M.; Santoro, A.; Pagani, M.; Lucini, D. Evidence of altered autonomic cardiac regulation in breast cancer survivors. J. Cancer Surviv. Res. Pract. 2015, 9, 699–706. [Google Scholar] [CrossRef]
- Windthorst, P.; Mazurak, N.; Kuske, M.; Hipp, A.; Giel, K.E.; Enck, P.; NieSS, A.; Zipfel, S.; Teufel, M. Heart rate variability biofeedback therapy and graded exercise training in management of chronic fatigue syndrome: An exploratory pilot study. J. Psychosom. Res. 2017, 93, 6–13. [Google Scholar] [CrossRef]
- Yamaguti, K.; Tajima, S.; Kuratsune, H. Autonomic Dysfunction in Chronic Fatigue Syndrome. Adv. Neuroimmune Biol. 2013, 4, 281–289. [Google Scholar] [CrossRef]
- De Maria, B.; Parati, M.; Dalla Vecchia, L.A.; La Rovere, M.T. Day and night heart rate variability using 24-h ECG recordings: A systematic review with meta-analysis using a gender lens. Clin. Auton. Res. 2023, 33, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Faro, M.; Sàez-Francás, N.; Castro-Marrero, J.; Aliste, L.; Fernández De Sevilla, T.; Alegre, J. Gender Differences in Chronic Fatigue Syndrome. Reumatol. Clínica 2016, 12, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Damoun, N.; Amekran, Y.; Taiek, N.; El Hangouche, A.J. Heart rate variability measurement and influencing factors: Towards the standardization of methodology. Glob. Cardiol. Sci. Pract. 2024, 2024, e202435. [Google Scholar] [CrossRef]
| Author and Year | Sample Size | Mean Age and Sex | Study Design | HRV Assessment | Fatigue Assessment | Main Results |
|---|---|---|---|---|---|---|
| Boissoneault et al. 2019 [25] | CRF = 14 HC = 14 | CRF = 48.57 ± 12.11 100% women HC = 49.57 ± 13.16 100% women | Cross-Sectional Clinical Trial | ECG | VAS 0-100 | Significant negative association between HRV TP and fatigue score (r = −0.70, p = 0.02). No other significant associations found between fatigue and LF, HF or VHF (p > 0.05). HC fatigue and HRV not investigated. |
| Capdevila et al. 2021 [26] | CFS = 32 HC = 19 | CFS = 47.38 ± 1.52 100% men HC = 47.32 ± 1.51 100% men | Cross-Sectional Cohort Study | Chest Strap Polar Band H7 and FitLab app | Fatigue Index Scale-40 (FIS-40) | Significant, negative association between physical fatigue score and pNN50 (r = −0.279, p = 0.049) n = 51. No other significant results found (p > 0.05). Simple linear regression analysis found significant relationship between physical fatigue score and HRV parameters SDNN (β = 0.487, p = 0.0047), RMSSD (β = −0.394, p = 0.0258), LF (β = −0.537, p = 0.0015), HF (β = −0.421, p = 0.0165) and pNN50 (β = −0.378, p = 0.033) in CFS patients (n = 32). No significant results found for HC (n = 19) (p > 0.05). |
| Deuring et al. 2017 [27] | Allogeneic Hematopoietic Stem Cell Transplantation Survivors = 104 HC = 45 | Survivors = 45.3 (median) 36.5% women HC = 41.4 (median) 44.4% women | Cross-Sectional Control Study | Vivo Metrics LifeShirt System | Functional Assessment of Chronic Illness-Fatigue (FACI-F) | GLM analysis found significant association between RSA and the fatigue scores of patients and controls (f = 6.57, p < 0.001). When controlling for V02 Max, a significant association between RSA and fatigue score of all patients and controls was still observed (p = 0.02). Contrast analysis revealed a significant association between HFP vs. NFP (t = −2.39, p = 0.02, d = 0.72). There was no significant association between MFP vs. NFP (t = −1.78, p = 0.08, d = 0.56) or NFP vs. CTL (t = 1.11, p = 0.27, d = 0.29). When controlling for V02 Max, a significant relationship was only detected in HFP vs. NFP (t = −2.74, p = 0.007, d = 0.72). |
| Escorihuela et al. 2020 [28] | CFS = 45 HC = 25 | CFS = 46.41 ± 0.84 100% women HC = 44.96 ± 1.30 100% women | Cross-Sectional Case Control Cohort Study | Chest Strap Polar Band H7 and FitLab app | Fatigue Index Scale-40 (FIS-40) | Significant relationship discovered between all fatigue scores and all the time domain parameters of HRV (correlation indices all < −0.423, all p < 0.05) for all participants (n = 70). Significant relationship between all fatigue score and some frequency domains; HF (all r < −0.451), LF–HF (all r < 0.360) and HFnu (all r < −0.476), all p < 0.05 (n = 70). LF only significant for physical fatigue score (r = 0.326) and cognitive fatigue score (r = 0.322), (p < 0.05). Simple linear regression analysis found significant association between total FIS-40 score and mean RR (r = −0.056, p = 0.005), RMSSD (r = −0.055, p = 0.0286) and HFnu (r = −0.365, p = 0.0067). No significant association found between fatigue and HRV for HC. |
| Gashi et al. 2024 [29] | MS = 55 HC = 24 | MS = 36.8 ± 9.5 64% women HC = 33.5 ± 10.6 54% women | Observational Study | Biovotion Eversion Armband (PPG Wearable Device) | Fatigue Scale for Motor and Cognitive Functions (FSMC) | HRV parameters correlated with FSMC in MS. pNN50 r = −0.40, p = 0.001. NN50 r = −0.41, p = 0.01. SD2 r = −0.47, p = 0.001. LF r = −0.26, p = 0.001. LF–HF r = 0.26, p = 0.001. RMSSD, SD1, HF was not significant. No fatigue and HRV were tested for HC. |
| Kitselaar et al. 2022 [30] | Brain Tumour Patients = 52 | 52.1 ± 15 44% women | Cross-Sectional Study | 24 h-Holter Monitor | Multidimensional Fatigue Inventory | No significant associations reported (r ≤ 0.16, p ≥ 0.25). |
| Kristiansen et al. 2019 [31] | Patients with Acute Epstein–Barr virus (EBV) = 195 Fatigued (EBV CF+) = 91 and non-fatigued (EBV NCF−) = 104) HC = 70 | CF = 17.4 ± 1.5 73.6% women NCF = 17.4 ± 1.7 57.7% women HC = 17 ± 1.8 62.9% women | Prospective Cohort Study 21-Month Follow Up | ECG (Task Force Monitor) | Chalder Fatigue Questionnaire | Weak negative correlation between LF–HF ratio response to controlled breathing and fatigue scores (T = 0.21, p = 0.005) in EBV CF+ group only (n = 91). |
| Martinez-Rosales et al. 2020 [32] | Systemic Lupus Erythematosus patients = 55 | 43.5 ± 14 100% women | Cross-Sectional Intervention Study | Polar V800 | Multidimensional Fatigue Inventory | Positive correlation between LF–HF and the physical domain fatigue scores (r = 0.30, p < 0.05). No other significant relationships discovered. |
| Oka et al. 2019 [33] | 15 CFS 15 CFS Control | CFS = 38.0 ± 11.1 80% women CFS Control = 39.1± 14.2 80% women | Pilot Study | ECG | Chalder Fatigue Scale | Yoga group fatigue scores and HRV (n = 15). Positive correlation between HF and fatigue scores (r = 0.705, p = 0.042). No other significant correlations detected. |
| Olivera-Toro et al. 2019 [34] | Spleen-Qi Deficiency Syndrome (SDS) = 67 HC = 37 | SDS = 56.2 ± 4.3 56.72% women HC = 52.5 ± 6.2 54.05% women | Cross-Sectional Study | ECG | Fatigue Impact Scale Spanish Version | Linear, positive correlation between HRV and fatigue in SDS patients (r = 0.48, p < 0.05) (n = 67). HF and fatigue score negative correlation (r = −0.37, p ≤ 0.01) (n = 67). |
| Park et al. 2019 [35] | CRF = 25 CFS = 20 | CRF = 55.52 ± 9.39 64% women CFS = 50.05 ± 9.25 65% women | Comparative Study | ECG | Fatigue Severity Scale (FSS) | CFS = FSS score significant correlation to RSSMD (r = −0.509, b = 0.032, p = 0.026), LF power (r = 0.484, b = 0.047, p = 0.036) and HF power (r = 0.508, b = 0.051, p = 0.026). No significant associations between FSS and pNN50, SDNN, LFnu, HFnu or HRV index. No significant associations discovered between FSS score and any of the HRV parameters CRF group. |
| Ryabkova et al. 2024 [36] | 34 CFS 29 post-COVID 19 Condition (PCC) 32 HC | CFS = 35.0 76% women PCC = 35.0 79% women HC = 34.50 69% women | Observational | ECG | Multidimensional Fatigue Inventory | No significant correlations discovered between fatigue scores and HRV parameters (p > 0.01). |
| Rzepinski et al. 2022 [37] | MS = 53 HC = 30 | MS = 45.8 ± 10.9 81.13% women HC = 40.8 ± 11.6 70% women | Cross-Sectional Study | ECG Task Force Monitor | Chalder Fatigue Scale (CFS) | MS = significant association between LF–HF ratio and physical fatigue score of CFS scale (β = −0.338, SE = 0.05, t = −2.672, p = 0.010). No analysis was conducted for fatigue and HRV in HC. |
| Sander et al. 2019 [38] | MS = 53 | 50.1 ± 8.7 79.2% women | Clinical Trial | NeXus-4- Biofeedback-System Blood Volume Pulse Sensor | Trait Fatigue = Fatigue Severity Scale (FSS) Tot Fatigue = VAS Trait Fatigue = Fatigue Scale for Motor and Cognitive Function (FSMC) | VLF and HF were significant predictors FSMC cognitive fatigue score (R2 = 0.218, f = 4.558, p = 0.007). No significant prediction for motor fatigue on FSMC and FSS. SDNN and pNN50 were significant predictors for tot fatigue (R2 = 0.241, f = 7.925, p ≤ 0.001). No correlation between tot fatigue and the cognitive scale of FSMC. No other significant correlations for fatigue scores found. |
| Sander et al. 2022 [39] | MS = 50 PMR Group = 24 SAT Group = 26 | 49.9 ± 8.0 80% women | Randomised Control Trial | NeXus-4- Biofeedback-System Blood Volume Pulse Sensor | Trait Fatigue = Fatigue Scale for Motor and Cognitive Function Trait Fatigue = Fatigue Severity Scale State fatigue = VAS | Mean and SD patients with weak–moderate fatigue vs. severe fatigue in SAT group pNN50 before (13.8 ± 20.1 vs. 6.0 ± 8.9) and after (12.5 ± 13.5 vs. 8.1 ± 10.1) and SDNN before (54.3 ± 33.9 vs. 36.3 ± 12.0) and after (55.4 ± 26.4 vs. 41.1 ± 14.2). Mean and SD patients with weak–moderate fatigue vs. severe fatigue in PMR group pNN50 before (8.8 ± 9.8 vs. 8.5 ± 16.4) and after (21.6 ± 18.4 vs. 10.1 ± 11.1) and SDNN before (50.5 ± 17.2 vs. 43.5 ± 28.2) and after (71.9 ± 33.7 vs. 47.7 ± 15.7). Dependent t-tests from post hoc testing found the PMR group with weak–moderate fatigue had a significant increase in SDNN (p = 0.001) and pNN50 (p = 0.008). No other significant differences were found. |
| Wheeler et al. 2022 [40] | Orthostatic Intolerance patients = 108 | NHR = 37.6 ± 16.9 77% women MHR = 27.9 ± 16.0 87% women EHR = 26.6 ± 14.0 84% women | Retrospective Study | ECG | Fatigue Severity Scale (FSS) | Multiple linear regression model discovered an association between low LF power and high FSS scores (t = 2.719, p = 0.008). No significant relationship reported between fatigue and RMSSD or HF power (p > 0.05). |
| Zhou et al. 2018 [41] | CFS = 114 57 = Tai Chi 57 = Control | Tai Chi = <30 years = 13 30–50 years = 34 >50 years = 10 33.33% women Control = <30 years = 7 30–50 years = 37 >50 years = 13 21.05% women | Randomised Control Trial | ECG | Multidimensional Fatigue Symptom Inventory Short Form | Linear regression model revealed a significant correlation between LF–HF ratio and CRF pre (r = 0.767, p ≤ 0.01) and post chemotherapy (r = 0.761, p ≤ 0.01). No significant relationship was documented between fatigue scores and nLF or nHF (p > 0.05). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penfold, S.M.; Cunningham, J.; Whelan, P.; McCabe, M.G.; Ainsworth, J. Decreased Heart Rate Variability Is Associated with Increased Fatigue Across Different Medical Populations: A Systematic Review. Pathophysiology 2025, 32, 46. https://doi.org/10.3390/pathophysiology32030046
Penfold SM, Cunningham J, Whelan P, McCabe MG, Ainsworth J. Decreased Heart Rate Variability Is Associated with Increased Fatigue Across Different Medical Populations: A Systematic Review. Pathophysiology. 2025; 32(3):46. https://doi.org/10.3390/pathophysiology32030046
Chicago/Turabian StylePenfold, Sophie Maria, James Cunningham, Pauline Whelan, Martin G. McCabe, and John Ainsworth. 2025. "Decreased Heart Rate Variability Is Associated with Increased Fatigue Across Different Medical Populations: A Systematic Review" Pathophysiology 32, no. 3: 46. https://doi.org/10.3390/pathophysiology32030046
APA StylePenfold, S. M., Cunningham, J., Whelan, P., McCabe, M. G., & Ainsworth, J. (2025). Decreased Heart Rate Variability Is Associated with Increased Fatigue Across Different Medical Populations: A Systematic Review. Pathophysiology, 32(3), 46. https://doi.org/10.3390/pathophysiology32030046






