Current Evidence on the Involvement of RAGE–Diaph1 Signaling in the Pathology and Treatment of Neurodegenerative Diseases—An Overview
Abstract
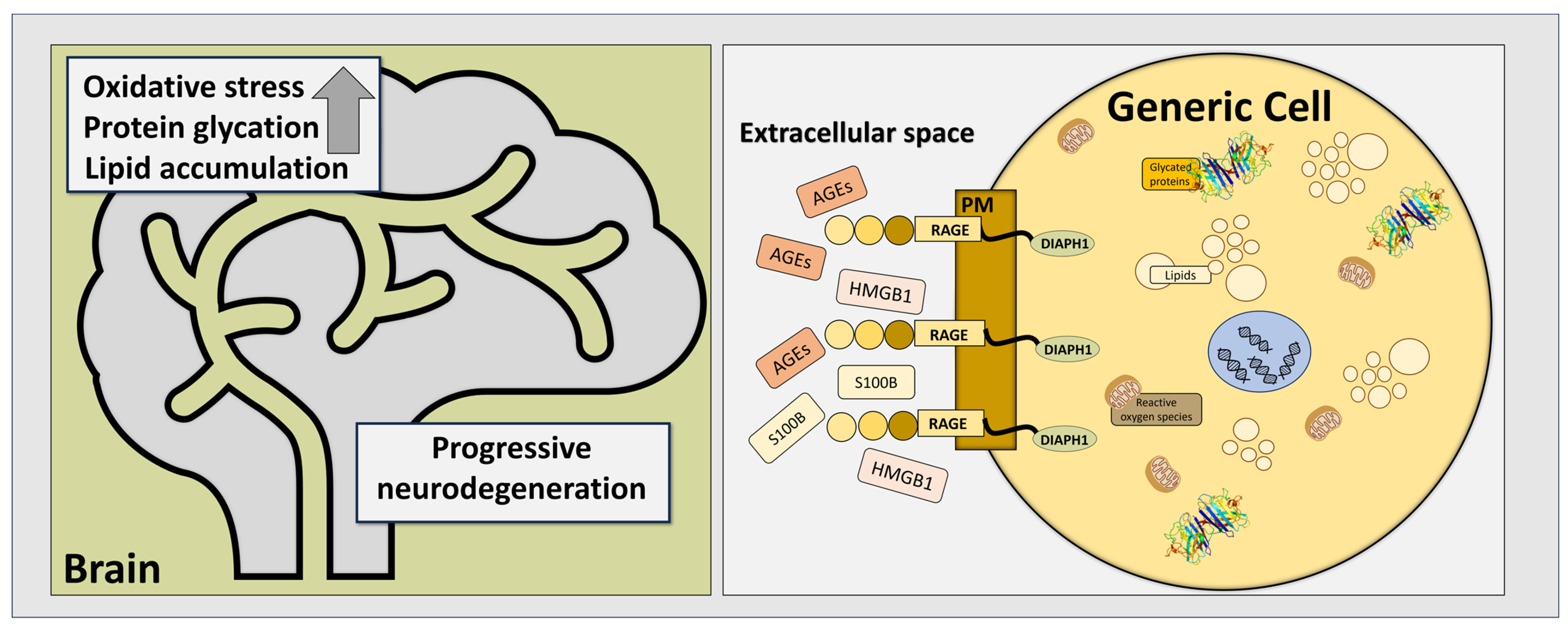
1. Introduction
2. RAGE and Its Most Prominent Ligands in Neurodegenerative Disorders
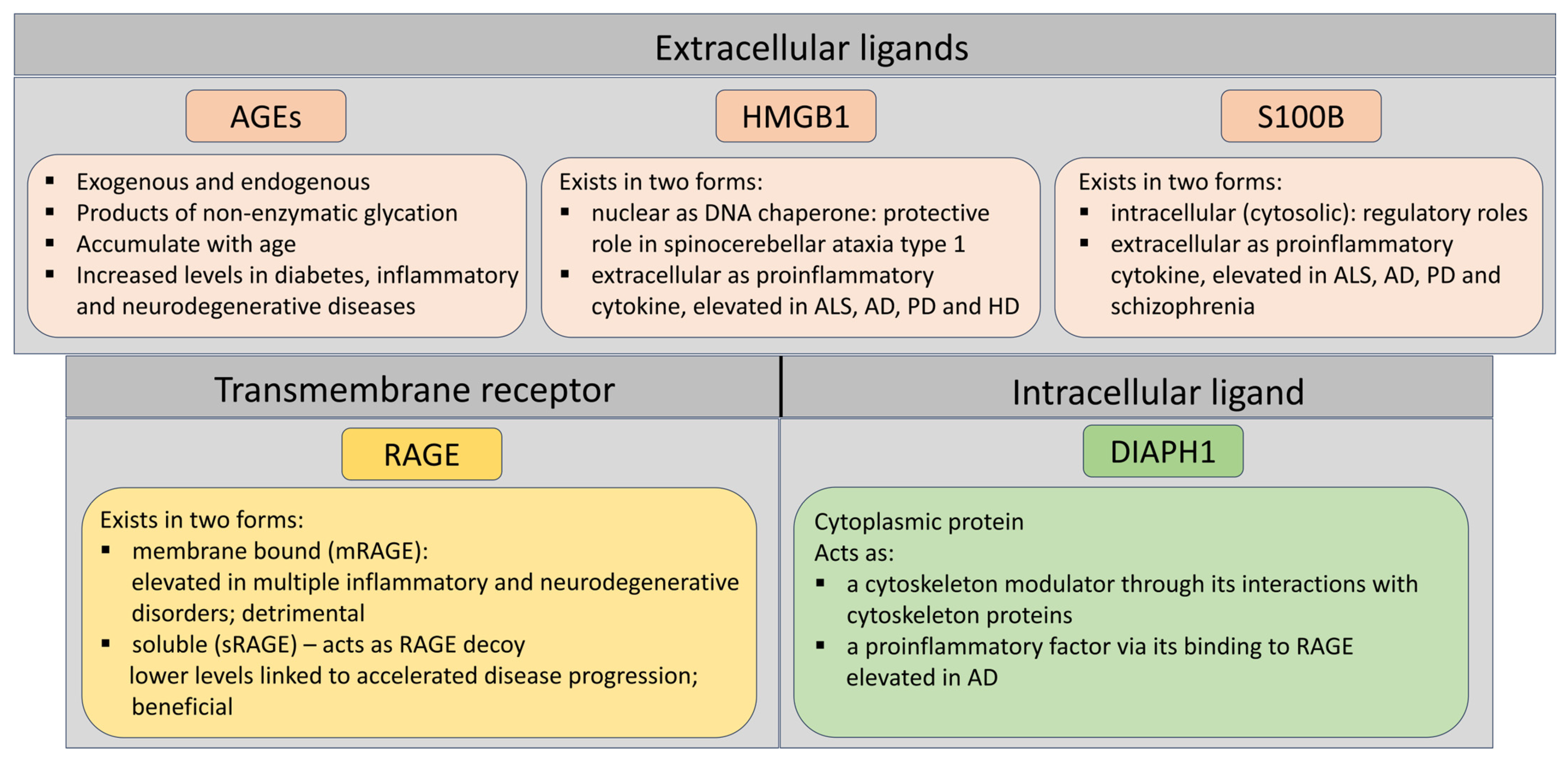
2.1. RAGE
2.2. HMGB1
2.3. S100B
2.4. Diaph1—An Intracellular Protein with RAGE-Binding Domain
3. RAGE–Diaph1 Proteomic and Transcriptomic Expression Patterns in Neurodegenerative Disorders
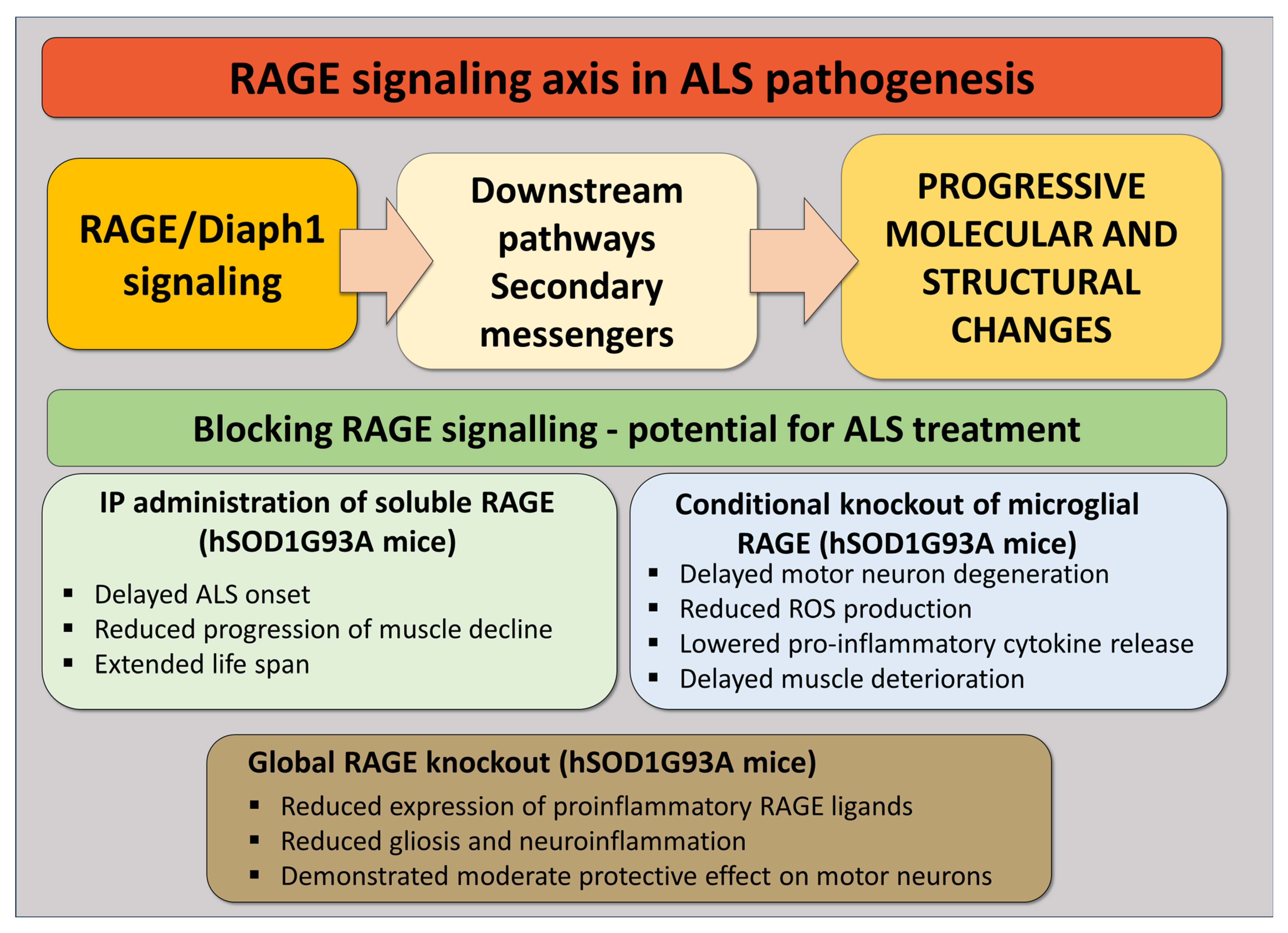
3.1. ALS
3.2. AD
3.3. PD
3.4. RAGE–Diaph1 Gene Differential Expression
4. AGER and DIAPH1 Gene Polymorphisms and Epigenetic Factors in Neurodegenerative Diseases
4.1. AGER
4.2. DIAPH1
4.3. AGER-DIAPH1 Epigenetics
5. RAGE Signaling Inhibition as a Potential ALS Treatment—Lessons from Our Studies
6. Limitations and Challenges in Targeting the RAGE–Diaph1 Pathway
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic lateral sclerosis |
| RAGE | Receptor for advanced glycation end-products |
| AGEs | Advanced glycation end-products |
| ROS | Reactive oxygen species |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| HMGB1 | High mobility group protein B1 |
| MS | Multiple sclerosis |
| PPR | Pattern recognition receptor |
| DAMPs | Damage-associated molecular patterns |
| mRAGE | Membrane-bound RAGE |
| sRAGE | Soluble RAGE |
| SCA1 | Spinocerebellar ataxia type 1 |
| DRFs | Diaphanous-related formins |
| CML | N-ε-carboxy-methyllysine |
| AMD | Age-related macular degeneration |
| TLR | Toll-like receptor |
References
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 145, pp. 301–307. [Google Scholar]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: Modelling study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef]
- Tesco, G.; Lomoio, S. Pathophysiology of neurodegenerative diseases: An interplay among axonal transport failure, oxidative stress, and inflammation? Semin. Immunol. 2022, 59, 101628. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Hofmann, M.; Taguchi, A.; Yan, S.D.; Stern, D.M. RAGE: A multiligand receptor contributing to the cellular response in diabetic vasculopathy and inflammation. Semin. Thromb. Hemost. 2000, 26, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Neeper, M.; Schmidt, A.M.; Brett, J.; Yan, S.D.; Wang, F.; Pan, Y.C.; Elliston, K.; Stern, D.; Shaw, A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 1992, 267, 14998–15004. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Hasu, M.; Popov, D.; Zhang, J.H.; Chen, J.; Yan, S.D.; Brett, J.; Cao, R.; Kuwabara, K.; Costache, G.; et al. Receptor for advanced glycation end products (AGEs) has a central role in vessel wall interactions and gene activation in response to circulating AGE proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 8807–8811. [Google Scholar] [CrossRef]
- Mao, Y. FORMIN a link between kinetochores and microtubule ends. Trends Cell Biol. 2011, 21, 625–629. [Google Scholar] [CrossRef]
- Rai, V.; Maldonado, A.Y.; Burz, D.S.; Reverdatto, S.; Yan, S.F.; Schmidt, A.M.; Shekhtman, A. Signal transduction in receptor for advanced glycation end products (RAGE): Solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J. Biol. Chem. 2012, 287, 5133–5144. [Google Scholar] [CrossRef]
- Brett, J.; Schmidt, A.M.; Yan, S.D.; Zou, Y.S.; Weidman, E.; Pinsky, D.; Nowygrod, R.; Neeper, M.; Przysiecki, C.; Shaw, A.; et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am. J. Pathol. 1993, 143, 1699–1712. [Google Scholar]
- Juranek, J.; Mukherjee, K.; Kordas, B.; Zalecki, M.; Korytko, A.; Zglejc-Waszak, K.; Szuszkiewicz, J.; Banach, M. Role of RAGE in the Pathogenesis of Neurological Disorders. Neurosci. Bull 2022, 38, 1248–1262. [Google Scholar] [CrossRef]
- Ferrari, S.; Finelli, P.; Rocchi, M.; Bianchi, M. The active gene that encodes human high mobility group 1 protein (HMG1) contains introns and maps to chromosome 13. Genomics 1996, 35, 367–371. [Google Scholar] [CrossRef]
- Chou, D.K.; Evans, J.E.; Jungalwala, F.B. Identity of nuclear high-mobility-group protein, HMG-1, and sulfoglucuronyl carbohydrate-binding protein, SBP-1, in brain. J. Neurochem. 2001, 77, 120–131. [Google Scholar]
- Thul, P.J.; Åkesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Björk, L.; Breckels, L.M.; et al. A subcellular map of the human proteome. Science 2017, 356, eaal3321. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Andersson, U. Targeting Inflammation Driven by HMGB1. Front. Immunol. 2020, 11, 484. [Google Scholar] [CrossRef]
- Juranek, J.K.; Daffu, G.K.; Wojtkiewicz, J.; Lacomis, D.; Kofler, J.; Schmidt, A.M. Receptor for Advanced Glycation End Products and its Inflammatory Ligands are Upregulated in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2015, 9, 485. [Google Scholar] [CrossRef]
- Son, S.; Bowie, L.E.; Maiuri, T.; Hung, C.L.K.; Desmond, C.R.; Xia, J.; Truant, R. High-mobility group box 1 links sensing of reactive oxygen species by huntingtin to its nuclear entry. J. Biol. Chem. 2019, 294, 1915–1923. [Google Scholar] [CrossRef]
- Ito, H.; Fujita, K.; Tagawa, K.; Chen, X.; Homma, H.; Sasabe, T.; Shimizu, J.; Shimizu, S.; Tamura, T.; Muramatsu, S.; et al. HMGB1 facilitates repair of mitochondrial DNA damage and extends the lifespan of mutant ataxin-1 knock-in mice. EMBO Mol. Med. 2015, 7, 78–101. [Google Scholar] [CrossRef]
- Fujita, K.; Motoki, K.; Tagawa, K.; Chen, X.; Hama, H.; Nakajima, K.; Homma, H.; Tamura, T.; Watanabe, H.; Katsuno, M.; et al. HMGB1, a pathogenic molecule that induces neurite degeneration via TLR4-MARCKS, is a potential therapeutic target for Alzheimer’s disease. Sci. Rep. 2016, 6, 31895. [Google Scholar] [CrossRef]
- Michetti, F.; Clementi, M.E.; Di Liddo, R.; Valeriani, F.; Ria, F.; Rende, M.; Di Sante, G.; Romano Spica, V. The S100B Protein: A Multifaceted Pathogenic Factor More Than a Biomarker. Int. J. Mol. Sci. 2023, 24, 9605. [Google Scholar] [CrossRef]
- Sathe, K.; Maetzler, W.; Lang, J.D.; Mounsey, R.B.; Fleckenstein, C.; Martin, H.L.; Schulte, C.; Mustafa, S.; Synofzik, M.; Vukovic, Z.; et al. S100B is increased in Parkinson’s disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-α pathway. Brain A J. Neurol. 2012, 135, 3336–3347. [Google Scholar] [CrossRef]
- Edwards, M.M.; Robinson, S.R. TNF alpha affects the expression of GFAP and S100B: Implications for Alzheimer’s disease. J. Neural Transm. 2006, 113, 1709–1715. [Google Scholar] [CrossRef]
- Schmitt, A.; Bertsch, T.; Henning, U.; Tost, H.; Klimke, A.; Henn, F.A.; Falkai, P. Increased serum S100B in elderly, chronic schizophrenic patients: Negative correlation with deficit symptoms. Schizophr. Res. 2005, 80, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, N.; Wang, C.; Qin, B.; Zhou, Y.; Xiao, M.; Chang, L.; Yan, L.-J.; Zhao, B. Role of RAGE in Alzheimer’s disease. Cell. Mol. Neurobiol. 2016, 36, 483–495. [Google Scholar] [CrossRef]
- Kamo, H.; Haebara, H.; Akiguchi, I.; Kameyama, M.; Kimura, H.; McGeer, P.L. A distinctive distribution of reactive astroglia in the precentral cortex in amyotrophic lateral sclerosis. Acta Neuropathol. 1987, 74, 33–38. [Google Scholar] [CrossRef]
- Migheli, A.; Cordera, S.; Bendotti, C.; Atzori, C.; Piva, R.; Schiffer, D. S-100beta protein is upregulated in astrocytes and motor neurons in the spinal cord of patients with amyotrophic lateral sclerosis. Neurosci. Lett. 1999, 261, 25–28. [Google Scholar] [CrossRef]
- Süssmuth, S.D.; Sperfeld, A.D.; Hinz, A.; Brettschneider, J.; Endruhn, S.; Ludolph, A.C.; Tumani, H. CSF glial markers correlate with survival in amyotrophic lateral sclerosis. Neurology 2010, 74, 982–987. [Google Scholar] [CrossRef]
- Cifra, A.; Nani, F.; Nistri, A. Riluzole is a potent drug to protect neonatal rat hypoglossal motoneurons in vitro from excitotoxicity due to glutamate uptake block. Eur. J. Neurosci. 2011, 33, 899–913. [Google Scholar] [CrossRef]
- Shobha, K.; Alladi, P.A.; Nalini, A.; Sathyaprabha, T.N.; Raju, T.R. Exposure to CSF from sporadic amyotrophic lateral sclerosis patients induces morphological transformation of astroglia and enhances GFAP and S100beta expression. Neurosci. Lett. 2010, 473, 56–61. [Google Scholar] [CrossRef]
- Díaz-Amarilla, P.; Olivera-Bravo, S.; Trias, E.; Cragnolini, A.; Martínez-Palma, L.; Cassina, P.; Beckman, J.; Barbeito, L. Phenotypically aberrant astrocytes that promote motoneuron damage in a model of inherited amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2011, 108, 18126–18131. [Google Scholar] [CrossRef]
- Casula, M.; Iyer, A.M.; Spliet, W.G.; Anink, J.J.; Steentjes, K.; Sta, M.; Troost, D.; Aronica, E. Toll-like receptor signaling in amyotrophic lateral sclerosis spinal cord tissue. Neuroscience 2011, 179, 233–243. [Google Scholar] [CrossRef]
- Kühn, S.; Geyer, M. Formins as effector proteins of Rho GTPases. Small GTPases 2014, 5, e983876. [Google Scholar] [CrossRef]
- Jarosławska, J.; Kordas, B.; Miłowski, T.; Juranek, J.K. Mammalian Diaphanous1 signalling in neurovascular complications of diabetes. Eur. J. Neurosci. 2024, 59, 2628–2645. [Google Scholar] [CrossRef]
- Alasmari, B.G.; Alpakra, M.; Hassanien, S.S.; Elmugadam, A.A.; Elzubair, L.; Suliman, E.A.; Alghubishi, S.A. A Novel Variant in the DIAPH1 Gene Causing Macrothrombocytopenia and Non-syndromic Hearing Loss in a Pediatric Saudi Girl. Cureus 2024, 16, e61044. [Google Scholar] [CrossRef]
- Neuhaus, C.; Lang-Roth, R.; Zimmermann, U.; Heller, R.; Eisenberger, T.; Weikert, M.; Markus, S.; Knipper, M.; Bolz, H.J. Extension of the clinical and molecular phenotype of DIAPH1-associated autosomal dominant hearing loss (DFNA1). Clin. Genet. 2017, 91, 892–901. [Google Scholar] [CrossRef]
- Ercan-Sencicek, A.G.; Jambi, S.; Franjic, D.; Nishimura, S.; Li, M.; El-Fishawy, P.; Morgan, T.M.; Sanders, S.J.; Bilguvar, K.; Suri, M.; et al. Homozygous loss of DIAPH1 is a novel cause of microcephaly in humans. Eur. J. Hum. Genet. 2015, 23, 165–172. [Google Scholar] [CrossRef]
- Kaustio, M.; Nayebzadeh, N.; Hinttala, R.; Tapiainen, T.; Astrom, P.; Mamia, K.; Pernaa, N.; Lehtonen, J.; Glumoff, V.; Rahikkala, E.; et al. Loss of DIAPH1 causes SCBMS, combined immunodeficiency, and mitochondrial dysfunction. J. Allergy Clin. Immunol. 2021, 148, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.J.; Funes, S.; McKeon, J.E.; Morgan, B.R.; Boopathy, S.; O’Connor, L.C.; Bilsel, O.; Massi, F.; Jégou, A.; Bosco, D.A. ALS-linked PFN1 variants exhibit loss and gain of functions in the context of formin-induced actin polymerization. Proc. Natl. Acad. Sci. USA. 2021, 118, e2024605118. [Google Scholar] [CrossRef]
- Alexander, G.M.; Heiman-Patterson, T.D.; Bearoff, F.; Sher, R.B.; Hennessy, L.; Terek, S.; Caccavo, N.; Cox, G.A.; Philip, V.M.; Blankenhorn, E.A. Identification of quantitative trait loci for survival in the mutant dynactin p150Glued mouse model of motor neuron disease. PLoS ONE 2022, 17, e0274615. [Google Scholar] [CrossRef]
- Hudson, B.I.; Kalea, A.Z.; Del Mar Arriero, M.; Harja, E.; Boulanger, E.; D’Agati, V.; Schmidt, A.M. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J. Biol. Chem. 2008, 283, 34457–34468. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Rabbani, P.; Egana-Gorrono, L.; Quadri, N.; Frye, L.; Zhou, B.; Reverdatto, S.; Ramirez, L.S.; Dansereau, S.; Pan, J.; et al. Small-molecule antagonism of the interaction of the RAGE cytoplasmic domain with DIAPH1 reduces diabetic complications in mice. Sci. Transl. Med. 2021, 13, eabf7084. [Google Scholar] [CrossRef]
- Manigrasso, M.B.; Pan, J.; Rai, V.; Zhang, J.; Reverdatto, S.; Quadri, N.; DeVita, R.J.; Ramasamy, R.; Shekhtman, A.; Schmidt, A.M. Small molecule inhibition of ligand-stimulated RAGE-DIAPH1 signal transduction. Sci. Rep. 2016, 6, 22450. [Google Scholar] [CrossRef]
- Nowicka, N.; Szymańska, K.; Juranek, J.; Zglejc-Waszak, K.; Korytko, A.; Załęcki, M.; Chmielewska-Krzesińska, M.; Wąsowicz, K.; Wojtkiewicz, J. The involvement of RAGE and its ligands during progression of ALS in SOD1 G93A transgenic mice. Int. J. Mol. Sci. 2022, 23, 2184. [Google Scholar] [CrossRef]
- MacLean, M.; Juranek, J.; Cuddapah, S.; Lopez-Diez, R.; Ruiz, H.H.; Hu, J.; Frye, L.; Li, H.; Gugger, P.F.; Schmidt, A.M. Microglia RAGE exacerbates the progression of neurodegeneration within the SOD1(G93A) murine model of amyotrophic lateral sclerosis in a sex-dependent manner. J. Neuroinflammation 2021, 18, 139. [Google Scholar] [CrossRef]
- Nowicka, N.; Zglejc-Waszak, K.; Juranek, J.; Korytko, A.; Wąsowicz, K.; Chmielewska-Krzesińska, M.; Wojtkiewicz, J. Novel insights into RAGE signaling pathways during the progression of amyotrophic lateral sclerosis in RAGE-deficient SOD1 G93A mice. PLoS ONE 2024, 19, e0299567. [Google Scholar] [CrossRef]
- Takuma, K.; Fang, F.; Zhang, W.; Yan, S.; Fukuzaki, E.; Du, H.; Sosunov, A.; McKhann, G.; Funatsu, Y.; Nakamichi, N. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-β and neuronal dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 20021–20026. [Google Scholar] [CrossRef]
- Wang, M.Y.; Ross-Cisneros, F.N.; Aggarwal, D.; Liang, C.-Y.; Sadun, A.A. Receptor for advanced glycation end products is upregulated in optic neuropathy of Alzheimer’s disease. Acta Neuropathol. 2009, 118, 381–389. [Google Scholar] [CrossRef]
- Miller, M.C.; Tavares, R.; Johanson, C.E.; Hovanesian, V.; Donahue, J.E.; Gonzalez, L.; Silverberg, G.D.; Stopa, E.G. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer’s disease. Brain Res. 2008, 1230, 273–280. [Google Scholar] [CrossRef]
- Bhogal, I.; Pankaj, V.; Roy, S. Identifying RAGE inhibitors as potential therapeutics for Alzheimer’s disease via integrated in-silico approaches. Sci. Rep. 2025, 15, 17730. [Google Scholar] [CrossRef]
- Derk, J.; MacLean, M.; Juranek, J.; Schmidt, A.M. The receptor for advanced glycation endproducts (RAGE) and mediation of inflammatory neurodegeneration. J. Alzheimer’s Dis. Park. 2018, 8, 421. [Google Scholar] [CrossRef]
- Gasparotto, J.; Ribeiro, C.T.; Bortolin, R.C.; Somensi, N.; Rabelo, T.K.; Kunzler, A.; Souza, N.C.; Pasquali, M.A.d.B.; Moreira, J.C.F.; Gelain, D.P. Targeted inhibition of RAGE in substantia nigra of rats blocks 6-OHDA–induced dopaminergic denervation. Sci. Rep. 2017, 7, 8795. [Google Scholar] [CrossRef]
- Long, H.; Zhang, S.; Zeng, S.; Tong, Y.; Liu, J.; Liu, C.; Li, D. Interaction of RAGE with α-synuclein fibrils mediates inflammatory response of microglia. Cell Rep. 2022, 40, 111401. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Niu, M.; Zhang, X.; Wang, J.; Zhou, C.; Xie, A. RAGE silencing ameliorates neuroinflammation by inhibition of p38-NF-κB signaling pathway in mouse model of Parkinson’s disease. Front. Neurosci. 2020, 14, 353. [Google Scholar] [CrossRef]
- Santos, G.; Barateiro, A.; Brites, D.; Fernandes, A. S100B impairs oligodendrogenesis and myelin repair following demyelination through RAGE engagement. Front. Cell. Neurosci. 2020, 14, 279. [Google Scholar] [CrossRef]
- Senatus, L.; Egaña-Gorroño, L.; López-Díez, R.; Bergaya, S.; Aranda, J.F.; Amengual, J.; Arivazhagan, L.; Manigrasso, M.B.; Yepuri, G.; Nimma, R. DIAPH1 mediates progression of atherosclerosis and regulates hepatic lipid metabolism in mice. Commun. Biol. 2023, 6, 280. [Google Scholar] [CrossRef]
- Zglejc-Waszak, K.; Mukherjee, K.; Korytko, A.; Lewczuk, B.; Pomianowski, A.; Wojtkiewicz, J.; Banach, M.; Załęcki, M.; Nowicka, N.; Jarosławska, J. Novel insights into the nervous system affected by prolonged hyperglycemia. J. Mol. Med. 2023, 101, 1015–1028. [Google Scholar] [CrossRef]
- Ruiz, H.H.; Nguyen, A.; Wang, C.; He, L.; Li, H.; Hallowell, P.; McNamara, C.; Schmidt, A.M. AGE/RAGE/DIAPH1 axis is associated with immunometabolic markers and risk of insulin resistance in subcutaneous but not omental adipose tissue in human obesity. Int. J. Obes. 2021, 45, 2083–2094. [Google Scholar] [CrossRef]
- Kwok, J.B. Role of Epigenetics in Alzheimer‘S and Parkinson‘S Disease. Epigenomics 2010, 2, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Coppedè, F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2009, 667, 82–97. [Google Scholar] [CrossRef]
- Marques, S.; Outeiro, T.F. Epigenetics in Parkinson’s and Alzheimer’s diseases. Epigenetics Dev. Dis. 2012, 61, 507–525. [Google Scholar]
- Imahara, S.D.; O’Keefe, G.E. Genetic determinants of the inflammatory response. Curr. Opin. Crit. Care 2004, 10, 318–324. [Google Scholar] [CrossRef]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, M.J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef]
- Schmidt, A.M. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory disease. Vasc. Pharmacol. 2015, 72, 1–8. [Google Scholar]
- Mahayana, N.P.K.; Yadmika, N.P.W.P.; Aryaweda, M.D.W.; Mahardana, M.D.P.; Mamangdean, C.T.; Dewi, N.N.A.; Wirawan, C.; Laksmidewi, A.A.A.P. Decoying the enemy: Soluble receptor for advanced glycation end products and cognitive impairment in neurodegenerative diseases—A systematic review and meta-analysis. Egypt. J. Neurol. Psychiatry Neurosurg. 2024, 60, 93. [Google Scholar] [CrossRef]
- Geroldi, D.; Falcone, C.; Emanuele, E.; D’Angelo, A.; Calcagnino, M.; Buzzi, M.P.; Scioli, G.A.; Fogari, R. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J. Hypertens. 2005, 23, 1725–1729. [Google Scholar] [CrossRef]
- Son, M.; Oh, S.; Park, H.; Ahn, H.; Choi, J.; Kim, H.; Lee, H.S.; Lee, S.; Park, H.-J.; Kim, S.U. Protection against RAGE-mediated neuronal cell death by sRAGE-secreting human mesenchymal stem cells in 5xFAD transgenic mouse model. Brain Behav. Immun. 2017, 66, 347–358. [Google Scholar] [CrossRef]
- Zhu, S.D. sRAGE: Potential therapeutic target for Alzheimer’s disease. In Proceedings of the Second International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Oxford, UK, 7–13 November 2022; pp. 1147–1153. [Google Scholar]
- Kinjo, T.; Kitaguchi, Y.; Droma, Y.; Yasuo, M.; Wada, Y.; Ueno, F.; Ota, M.; Hanaoka, M. The Gly82Ser mutation in AGER contributes to pathogenesis of pulmonary fibrosis in combined pulmonary fibrosis and emphysema (CPFE) in Japanese patients. Sci. Rep. 2020, 10, 12811. [Google Scholar] [CrossRef]
- Faiz, A.; Rathnayake, S.N.; Ten Hacken, N.H.; Guryev, V.; van den Berge, M.; Pouwels, S.D. Single-nucleotide polymorphism rs2070600 regulates AGER splicing and the sputum levels of the COPD biomarker soluble receptor for advanced glycation end-products. ERJ Open Res. 2021, 7, 00947-2020. [Google Scholar] [CrossRef]
- Serveaux-Dancer, M.; Jabaudon, M.; Creveaux, I.; Belville, C.; Blondonnet, R.; Gross, C.; Constantin, J.-M.; Blanchon, L.; Sapin, V. Pathological implications of receptor for advanced glycation end-product (AGER) gene polymorphism. Dis. Markers 2019, 2019, 2067353. [Google Scholar] [CrossRef]
- Miller, S.; Henry, A.P.; Hodge, E.; Kheirallah, A.K.; Billington, C.K.; Rimington, T.L.; Bhaker, S.K.; Obeidat, M.; Melen, E.; Merid, S.K. The Ser82 RAGE variant affects lung function and serum RAGE in smokers and sRAGE production in vitro. PLoS ONE 2016, 11, e0164041. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, F.; Qiao, Y.; Wang, P.; Du, H.; Si, C.; Wang, X.; Chen, K.; Song, F. Genetically modified circulating levels of advanced glycation end-products and their soluble receptor (AGEs-RAGE Axis) with risk and mortality of breast cancer. Cancers 2022, 14, 6124. [Google Scholar] [CrossRef]
- Daborg, J.; von Otter, M.; Sjölander, A.; Nilsson, S.; Minthon, L.; Gustafson, D.R.; Skoog, I.; Blennow, K.; Zetterberg, H. Association of the RAGE G82S polymorphism with Alzheimer’s disease. J. Neural Transm. 2010, 117, 861–867. [Google Scholar] [CrossRef]
- Bennet, A.M.; Reynolds, C.A.; Eriksson, U.K.; Hong, M.G.; Blennow, K.; Gatz, M.; Alexeyenko, A.; Pedersen, N.L.; Prince, J.A. Genetic association of sequence variants near AGER/NOTCH4 and dementia. J. Alzheimers Dis. 2011, 24, 475–484. [Google Scholar] [CrossRef]
- Kolonaite, G.; Vilkeviciute, A.; Kriauciuniene, L.; Gedvilaite, G.; Liutkeviciene, R. Association of RAGE rs1800624 and rs1800625 gene polymorphisms with predisposition to optic neuritis and optic neuritis together with multiple sclerosis. Ophthalmic Genet. 2021, 42, 685–690. [Google Scholar] [CrossRef]
- Banevicius, M.; Vilkeviciute, A.; Kriauciuniene, L.; Liutkeviciene, R.; Deltuva, V.P. The Association Between Variants of Receptor for Advanced Glycation End Products (RAGE) Gene Polymorphisms and Age-Related Macular Degeneration. Med. Sci. Monit. 2018, 24, 190–199. [Google Scholar] [CrossRef]
- Al-Maawali, A.; Barry, B.J.; Rajab, A.; El-Quessny, M.; Seman, A.; Coury, S.N.; Barkovich, A.J.; Yang, E.; Walsh, C.A.; Mochida, G.H.; et al. Novel loss-of-function variants in DIAPH1 associated with syndromic microcephaly, blindness, and early onset seizures. Am. J. Med. Genet. A 2016, 170A, 435–440. [Google Scholar] [CrossRef]
- Esmaeilzadeh, E.; Biglari, S.; Mosallaei, M.; Khorshid, H.R.K.; Vahidnezhad, H.; Tabatabaiefar, M.A. A Novel Homozygote Pathogenic Variant in the DIAPH1 Gene Associated With Seizures, Cortical Blindness, and Microcephaly Syndrome (SCBMS): Report of a Family and Literature Review. Mol. Genet. Genom. Med. 2024, 12, e70031. [Google Scholar] [CrossRef]
- McFarlane, R.; Opie-Martin, S.; Caravaca Puchades, A.; Chiò, A.; Corcia, P.; Galvin, M.; Heverin, M.; Hobin, F.; Holmdahl, O.; Ingre, C.; et al. Clinical trajectories of genetic variants in ALS: A European observational study within PRECISION-ALS. Amyotroph. Lateral Scler. Front. Degener. 2025, 26, 41–49. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, X.; Tang, W.; Li, J.; Yang, S.; Chen, Y.; Zhao, X.; Zong, H.; Liu, C.; Shen, C. Association of DIAPH1 gene polymorphisms with ischemic stroke. Aging 2020, 12, 416–435. [Google Scholar] [CrossRef]
- Zhou, S.L.; Tan, C.C.; Hou, X.H.; Cao, X.P.; Tan, L.; Yu, J.T. TREM2 Variants and Neurodegenerative Diseases: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2019, 68, 1171–1184. [Google Scholar] [CrossRef]
- Mutti, V.; Carini, G.; Filippini, A.; Castrezzati, S.; Giugno, L.; Gennarelli, M.; Russo, I. LRRK2 Kinase Inhibition Attenuates Neuroinflammation and Cytotoxicity in Animal Models of Alzheimer’s and Parkinson’s Disease-Related Neuroinflammation. Cells 2023, 12, 1799. [Google Scholar] [CrossRef]
- Soraci, L.; Corsonello, A.; Paparazzo, E.; Montesanto, A.; Piacenza, F.; Olivieri, F.; Gambuzza, M.E.; Savedra, E.V.; Marino, S.; Lattanzio, F.; et al. Neuroinflammaging: A Tight Line Between Normal Aging and Age-Related Neurodegenerative Disorders. Aging Dis. 2024, 15, 1726–1747. [Google Scholar] [CrossRef]
- Giallongo, S.; Longhitano, L.; Denaro, S.; D’Aprile, S.; Torrisi, F.; La Spina, E.; Giallongo, C.; Mannino, G.; Lo Furno, D.; Zappalà, A.; et al. The Role of Epigenetics in Neuroinflammatory-Driven Diseases. Int. J. Mol. Sci. 2022, 23, 15218. [Google Scholar] [CrossRef]
- Słowikowski, B.; Owecki, W.; Jeske, J.; Jezierski, M.; Draguła, M.; Goutor, U.; Jagodziński, P.P.; Kozubski, W.; Dorszewska, J. Epigenetics and the neurodegenerative process. Epigenomics 2024, 16, 473–491. [Google Scholar] [CrossRef]
- Lossi, L.; Castagna, C.; Merighi, A. An Overview of the Epigenetic Modifications in the Brain under Normal and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 3881. [Google Scholar] [CrossRef] [PubMed]
- Juranek, J.K.; Daffu, G.K.; Geddis, M.S.; Li, H.; Rosario, R.; Kaplan, B.J.; Kelly, L.; Schmidt, A.M. Soluble RAGE Treatment Delays Progression of Amyotrophic Lateral Sclerosis in SOD1 Mice. Front. Cell. Neurosci. 2016, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Juranek, J.; Osowski, A.; Wojtkiewicz, J.; Banach, M. Plasma levels of soluble RAGE, AGEs and AOPPs at the early stage of amyotrophic lateral sclerosis: A preliminary study. Polim. Med. 2023, 53, 105–110. [Google Scholar] [CrossRef]
- Liu, L.; Killoy, K.M.; Vargas, M.R.; Yamamoto, Y.; Pehar, M. Effects of RAGE inhibition on the progression of the disease in hSOD1(G93A) ALS mice. Pharmacol. Res. Perspect. 2020, 8, e00636. [Google Scholar] [CrossRef]
- Liliensiek, B.; Weigand, M.A.; Bierhaus, A.; Nicklas, W.; Kasper, M.; Hofer, S.; Plachky, J.; Gröne, H.J.; Kurschus, F.C.; Schmidt, A.M.; et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J. Clin. Investig. 2004, 113, 1641–1650. [Google Scholar] [CrossRef]
- Vodopivec, I.; Galichet, A.; Knobloch, M.; Bierhaus, A.; Heizmann, C.W.; Nitsch, R.M. RAGE does not affect amyloid pathology in transgenic ArcAbeta mice. Neurodegener. Dis. 2009, 6, 270–280. [Google Scholar] [CrossRef]
- Burstein, A.H.; Grimes, I.; Galasko, D.R.; Aisen, P.S.; Sabbagh, M.; Mjalli, A.M. Effect of TTP488 in patients with mild to moderate Alzheimer’s disease. BMC Neurol. 2014, 14, 12. [Google Scholar] [CrossRef]
- vTv Therapeutics. Evaluation of the Efficacy and Safety of Azeliragon (TTP488) in Patients With Mild Alzheimer’s Disease (STEADFAST). ClinicalTrials.gov NCT02080364. Available online: https://clinicaltrials.gov/study/NCT02080364 (accessed on 29 June 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juranek, J.K.; Kordas, B.; Podlasz, P.; Bossowska, A.; Banach, M. Current Evidence on the Involvement of RAGE–Diaph1 Signaling in the Pathology and Treatment of Neurodegenerative Diseases—An Overview. Pathophysiology 2025, 32, 43. https://doi.org/10.3390/pathophysiology32030043
Juranek JK, Kordas B, Podlasz P, Bossowska A, Banach M. Current Evidence on the Involvement of RAGE–Diaph1 Signaling in the Pathology and Treatment of Neurodegenerative Diseases—An Overview. Pathophysiology. 2025; 32(3):43. https://doi.org/10.3390/pathophysiology32030043
Chicago/Turabian StyleJuranek, Judyta K., Bernard Kordas, Piotr Podlasz, Agnieszka Bossowska, and Marta Banach. 2025. "Current Evidence on the Involvement of RAGE–Diaph1 Signaling in the Pathology and Treatment of Neurodegenerative Diseases—An Overview" Pathophysiology 32, no. 3: 43. https://doi.org/10.3390/pathophysiology32030043
APA StyleJuranek, J. K., Kordas, B., Podlasz, P., Bossowska, A., & Banach, M. (2025). Current Evidence on the Involvement of RAGE–Diaph1 Signaling in the Pathology and Treatment of Neurodegenerative Diseases—An Overview. Pathophysiology, 32(3), 43. https://doi.org/10.3390/pathophysiology32030043





