Methamphetamine-Induced Loss of Syndecan-1 and Retinal Endothelial Integrity via the TAAR-1/MMP-9 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Blood Vessel Tissue Culture
2.2. Immunoblot Analysis
2.3. Total RNA Isolation, Reverse Transcription, and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
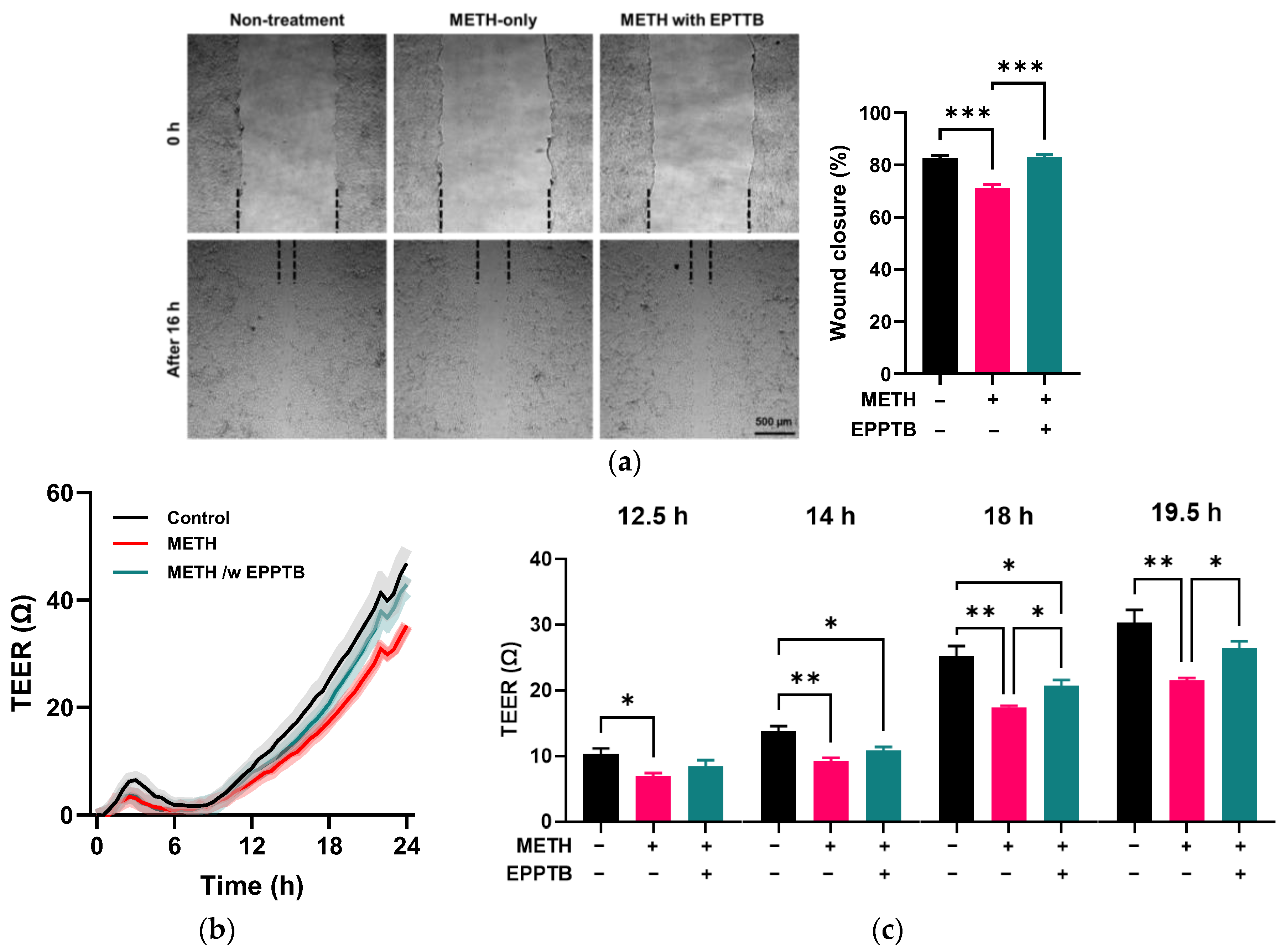
2.4. Scratch Cell Migration Assay
2.5. Transendothelial Electrical Resistance (TEER) Measurement
2.6. Statistics
3. Results
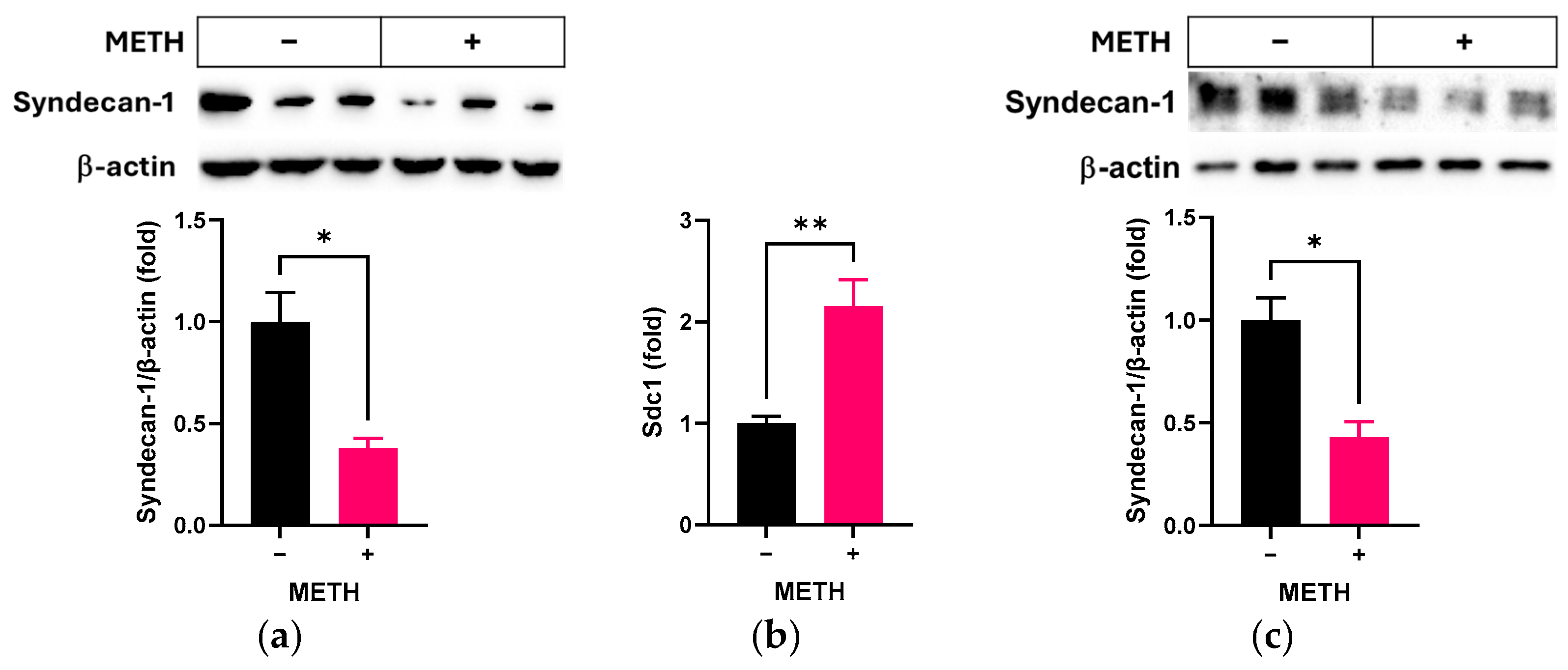
3.1. Effect of METH on Syndecan-1 in RRMECs and OA
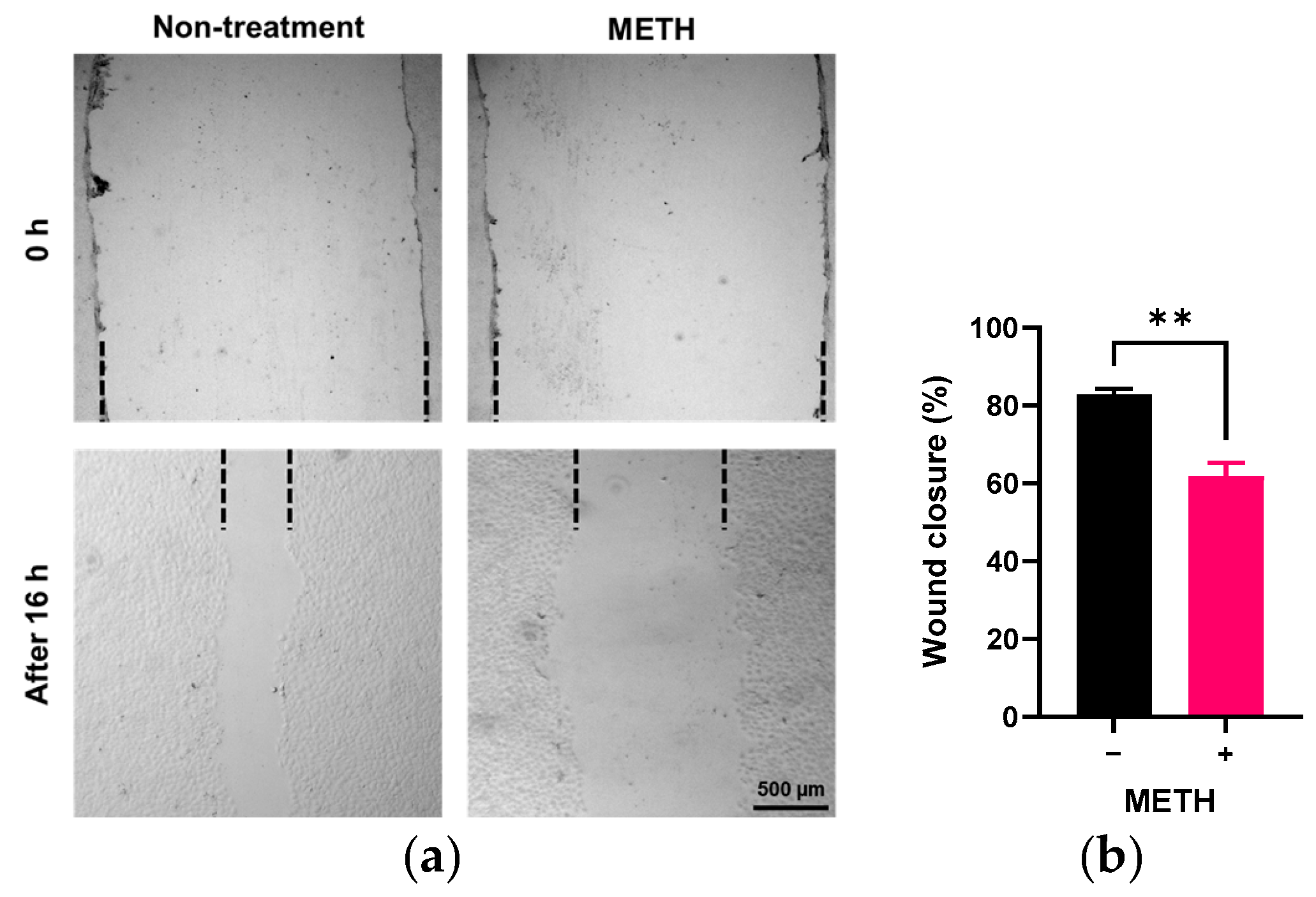
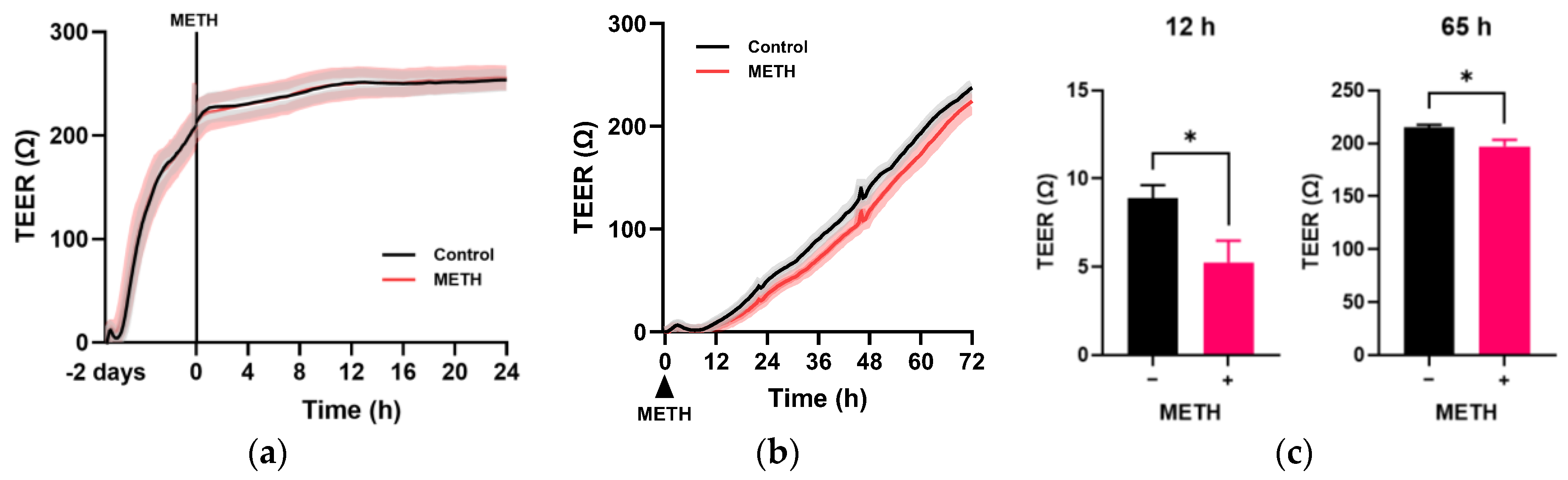
3.2. Effect of METH on the Endothelial Cell Migration and Permeability
3.3. Effect of METH on MMP Expression in RRMECs
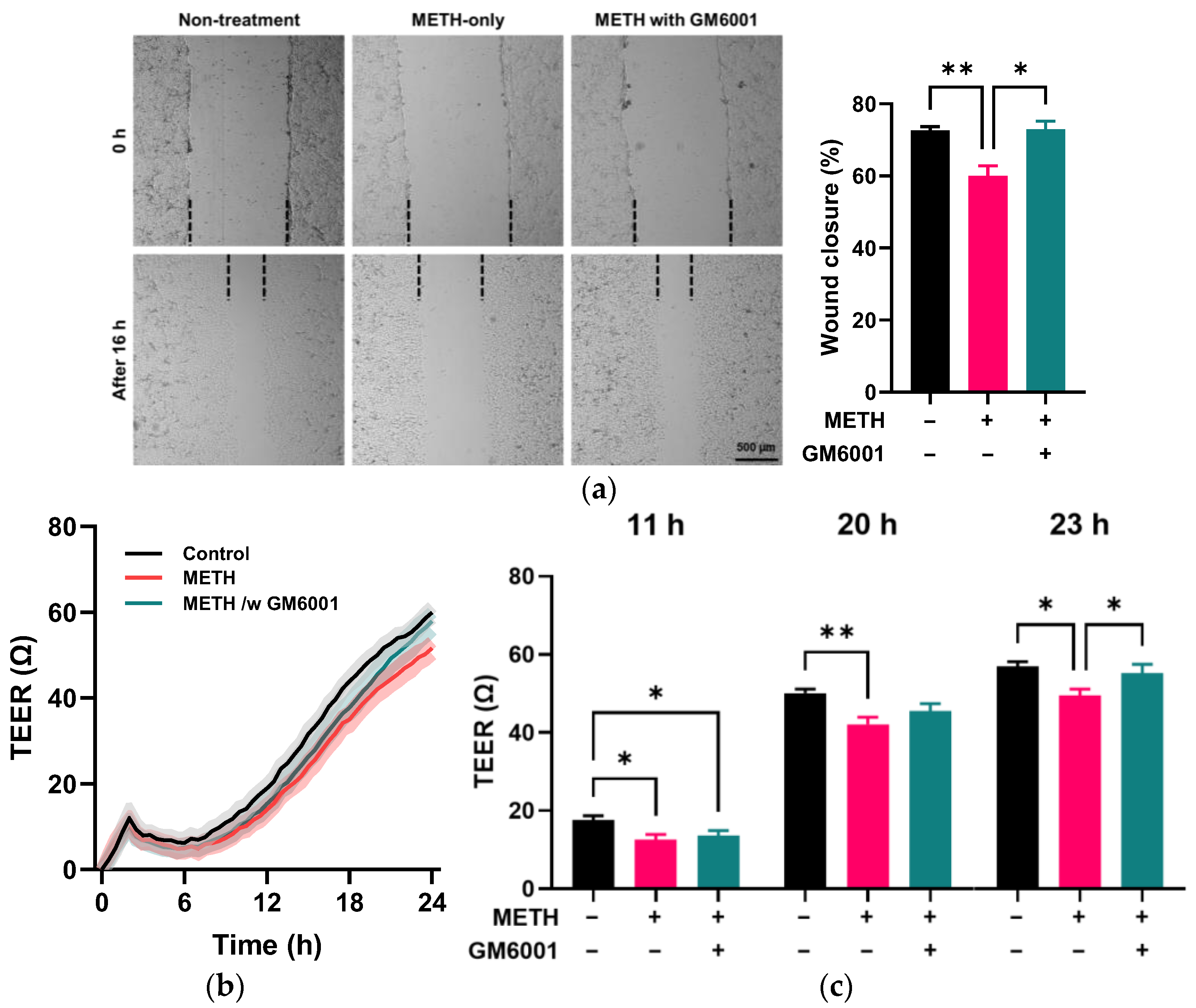
3.4. Role of MMP-9 in METH-Induced Disruption of Endothelial Integrity
3.5. Trace Amine-Associated Receptor-1 (TAAR-1)-Mediated Disruption of Endothelial Integrity by METH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM | A disintegrin and metalloproteinases |
| BBB | Blood–brain barrier |
| BRB | Blood–retina barrier |
| EPPTB | N-(3-ethoxyphenyl)-4-(1-pyrrolidinyl)-3-(trifluoromethyl)benzamide) |
| HIF-1α | hypoxia inducible factor-1α |
| Mapk1 | Mitogen activated protein kinase 1 |
| METH | Methamphetamine |
| MMP | Matrix metalloproteinase |
| OA | Ophthalmic artery |
| PBS | Phosphate-buffered saline |
| Ppia | Peptidylprolyl isomerase A |
| RRMEC | Primary rat retinal microvascular endothelial cell |
| Sdc1 | Syndecan 1 |
| TEER | Transendothelial Electrical Resistance |
| TAAR-1 | Trace amine-associated receptor-1 |
| VEGFa | Vascular endothelial growth factor a |
References
- Kevil, C.G.; Goeders, N.E.; Woolard, M.D.; Bhuiyan, M.S.; Dominic, P.; Kolluru, G.K.; Arnold, C.L.; Traylor, J.G.; Orr, A.W. Methamphetamine Use and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Leskova, W.; Eshaq, R.S.; Harris, N.R. Acute changes in the retina and central retinal artery with methamphetamine. Exp. Eye. Res. 2020, 193, 107964. [Google Scholar] [CrossRef]
- Martins, T.; Baptista, S.; Goncalves, J.; Leal, E.; Milhazes, N.; Borges, F.; Ribeiro, C.F.; Quintela, O.; Lendoiro, E.; Lopez-Rivadulla, M.; et al. Methamphetamine transiently increases the blood-brain barrier permeability in the hippocampus: Role of tight junction proteins and matrix metalloproteinase-9. Brain Res. 2011, 1411, 28–40. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Potula, R.; Fan, S.; Eidem, T.; Papugani, A.; Reichenbach, N.; Dykstra, H.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; et al. Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J. Cereb. Blood Flow Metab. 2009, 29, 1933–1945. [Google Scholar] [CrossRef]
- Sanches, E.S.; Boia, R.; Leitao, R.A.; Madeira, M.H.; Fontes-Ribeiro, C.A.; Ambrosio, A.F.; Fernandes, R.; Silva, A.P. Attention-Deficit/Hyperactivity Disorder Animal Model Presents Retinal Alterations and Methylphenidate Has a Differential Effect in ADHD versus Control Conditions. Antioxidants 2023, 12, 937. [Google Scholar] [CrossRef]
- Sanches, E.S.; Simoes, D.; Baptista, F.I.; Silva, A.P. Neurovascular dysfunction in psychiatric disorders: Underlying mechanisms and therapeutic approaches. Eur. J. Clin. Invest. 2025, 55, e14319. [Google Scholar] [CrossRef]
- Polesskaya, O.; Silva, J.; Sanfilippo, C.; Desrosiers, T.; Sun, A.; Shen, J.; Feng, C.; Polesskiy, A.; Deane, R.; Zlokovic, B.; et al. Methamphetamine causes sustained depression in cerebral blood flow. Brain Res. 2011, 1373, 91–100. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Di Giorgi, L.; Monastero, R. Focus of endothelial glycocalyx dysfunction in ischemic stroke and Alzheimer’s disease: Possible intervention strategies. Ageing Res. Rev. 2024, 99, 102362. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Suzuki, T.; Ishikawa, R.; Hattori, N.; Ito, R.; Umehara, K.; Furihata, T.; Dohmae, N.; Linhardt, R.J.; Igarashi, K.; et al. Ischemic stroke disrupts the endothelial glycocalyx through activation of proHPSE via acrolein exposure. J. Biol. Chem. 2020, 295, 18614–18624. [Google Scholar] [CrossRef] [PubMed]
- Lappin, J.M.; Darke, S.; Farrell, M. Stroke and methamphetamine use in young adults: A review. J. Neurol. Neurosurg. Psychiatry 2017, 88, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.H.; Aquino, R.S.; Park, P.W. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012, 31, 3–16. [Google Scholar] [CrossRef]
- Alvarez, I.A.; Lee, M.; Eshaq, R.S.; Leskova, W.; Harris, N.R. High Glucose Induces Oxidative Stress That Alters Glycocalyx Proteoglycan Levels in Primary Rat Retinal Microvascular Endothel. Cells and in Isolated Ophthalmic Arteries. Pathophysiology 2024, 31, 89–99. [Google Scholar] [CrossRef]
- Gotte, M.; Joussen, A.M.; Klein, C.; Andre, P.; Wagner, D.D.; Hinkes, M.T.; Kirchhof, B.; Adamis, A.P.; Bernfield, M. Role of syndecan-1 in leukocyte-endothelial interactions in the ocular vasculature. Invest. Ophthalmol. Vis. Sci. 2002, 43, 1135–1141. [Google Scholar]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Ramani, V.C.; Sanderson, R.D. Chemotherapy stimulates syndecan-1 shedding: A potentially negative effect of treatment that may promote tumor relapse. Matrix Biol. 2014, 35, 215–222. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.T.; Pan, Y.; Liu, X.F.; Xu, J.W.; Cui, W.J.; Qiao, X.R.; Dong, L. Syndecan-1 Shedding by Matrix Metalloproteinase-9 Signaling Regulates Alveolar Epithelial Tight Junction in Lipopolysaccharide-Induced Early Acute Lung Injury. J. Inflamm. Res. 2021, 14, 5801–5816. [Google Scholar] [CrossRef]
- Butler, M.J.; Down, C.J.; Foster, R.R.; Satchell, S.C. The Pathological Relevance of Increased Endothelial Glycocalyx Permeability. Am. J. Pathol. 2020, 190, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Leskova, W.; Pickett, H.; Eshaq, R.S.; Shrestha, B.; Pattillo, C.B.; Harris, N.R. Effect of diabetes and hyaluronidase on the retinal endothelial glycocalyx in mice. Exp. Eye. Res. 2019, 179, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Eshaq, R.S.; Lee, M.; Leskova, W.; Harris, N.R. Decreased retinal and choroidal endothelial surface molecules in spontaneously hypertensive rats. Exp. Eye. Res. 2023, 234, 109617. [Google Scholar] [CrossRef] [PubMed]
- Rapraeger, A.C.; Ell, B.J.; Roy, M.; Li, X.; Morrison, O.R.; Thomas, G.M.; Beauvais, D.M. Vascular endothelial-cadherin stimulates syndecan-1-coupled insulin-like growth factor-1 receptor and cross-talk between alphaVbeta3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis. FEBS J. 2013, 280, 2194–2206. [Google Scholar]
- Cotter, R.; Pei, Y.; Mus, L.; Harmeier, A.; Gainetdinov, R.R.; Hoener, M.C.; Canales, J.J. The trace amine-associated receptor 1 modulates methamphetamine’s neurochemical and behavioral effects. Front. Neurosci. 2015, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Buchy, D.; Chaboz, S.; Hoener, M.C.; Liechti, M.E. In Vitro Characterization of Psychoactive Substances at Rat, Mouse, and Human Trace Amine-Associated Receptor 1. J. Pharmacol. Exp. Ther. 2016, 357, 134–144. [Google Scholar] [CrossRef]
- Lee, M.; Wood, B.J.; Jeong, H.H.; Nam, H.W.; Keller, C.M.; Lee, B.; Kim, J.-I.; Murnane, K.S.; Goeders, N.E.; Harris, N.R. Retinal Angiogenesis in Methamphetamine Self-Administration Rats. Invest. Ophthalmol. Vis. Sci. 2025, 66, 8. [Google Scholar] [CrossRef]
- Lee, M.; Leskova, W.; Eshaq, R.S.; Harris, N.R. Retinal hypoxia and angiogenesis with methamphetamine. Exp. Eye. Res. 2021, 206, 108540. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Kwon, S.M. Angiogenesis and its therapeutic opportunities. Mediat. Inflamm. 2013, 2013, 127170. [Google Scholar] [CrossRef]
- Das, A.; McGuire, P.G. Retinal and choroidal angiogenesis: Pathophysiology and strategies for inhibition. Prog. Retin. Eye Res. 2003, 22, 721–748. [Google Scholar] [CrossRef] [PubMed]
- Melega, W.P.; Cho, A.K.; Harvey, D.; Lacan, G. Methamphetamine blood concentrations in human abusers: Application to pharmacokinetic modeling. Synapse 2007, 61, 216–220. [Google Scholar] [CrossRef]
- Manon-Jensen, T.; Multhaupt, H.A.; Couchman, J.R. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013, 280, 2320–2331. [Google Scholar] [CrossRef] [PubMed]



| Gene Symbol | Accession No. | Gene Name | Primer Pair (5′ to 3′) |
|---|---|---|---|
| Mapk1 | NM_053842.2 | Mitogen activated protein kinase 1 | ATCTCAAGATCTGTGACTTTGG |
| CTACATACTCTGTCAAGAACCC | |||
| Mmp2 | NM_031054.2 | Matrix metalloproteinase 2 | CTGCAAGCAAGACATTGTC |
| CGCCAAATAAACCGATCCT | |||
| Mmp9 | NM_031055.2 | Matrix metalloproteinase 9 | CCAACCTTTACCAGCTACTC |
| TTGTAGGGTCGGTTCTGA | |||
| Ppia | NM_017101.1 | Peptidylprolyl isomerase A | TGTGGCCCTCCTACATAAA |
| AGTAGGAGACTAACCACGTG | |||
| Sdc1 | NM_013026.2 | Syndecan 1 | CCAAATCCGGACACCAAA |
| GGGCACCAAACAGATAGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Ha, T.; Alvarez, I.A.; Leskova, W.; Park, C.; Harris, N.R. Methamphetamine-Induced Loss of Syndecan-1 and Retinal Endothelial Integrity via the TAAR-1/MMP-9 Pathway. Pathophysiology 2025, 32, 41. https://doi.org/10.3390/pathophysiology32030041
Lee M, Ha T, Alvarez IA, Leskova W, Park C, Harris NR. Methamphetamine-Induced Loss of Syndecan-1 and Retinal Endothelial Integrity via the TAAR-1/MMP-9 Pathway. Pathophysiology. 2025; 32(3):41. https://doi.org/10.3390/pathophysiology32030041
Chicago/Turabian StyleLee, Minsup, Taekyung Ha, Ivan A. Alvarez, Wendy Leskova, Changwon Park, and Norman R. Harris. 2025. "Methamphetamine-Induced Loss of Syndecan-1 and Retinal Endothelial Integrity via the TAAR-1/MMP-9 Pathway" Pathophysiology 32, no. 3: 41. https://doi.org/10.3390/pathophysiology32030041
APA StyleLee, M., Ha, T., Alvarez, I. A., Leskova, W., Park, C., & Harris, N. R. (2025). Methamphetamine-Induced Loss of Syndecan-1 and Retinal Endothelial Integrity via the TAAR-1/MMP-9 Pathway. Pathophysiology, 32(3), 41. https://doi.org/10.3390/pathophysiology32030041







