Advances in Cathepsin S Inhibition: Challenges and Breakthroughs in Drug Development
Abstract
1. Introduction
2. Methodology
3. Cathepsin S
4. Development of CatS Inhibitors
4.1. CatS Inhibitors from Proline Analogs with a Vinyl Sulfone Group
4.2. Pyrazole-Based Aryl Alkyne CatS Inhibitor
4.3. Central Cyclic Scaffold-Based CatS Inhibitors
4.4. LY3000328 CatS Inhibitor
4.5. Cathepsin S Inhibitor from Nuclear Magnetic Resonance Fragment Screening
4.6. RO5444101 as Cathepsin S Inhibitor
4.7. Substrate-Based CatS Inhibitor
4.8. Other CatS Inhibitors
5. Development of CatS Inhibitors for Various Diseases
5.1. Autoimmune Disease
5.2. Cancer
5.3. Cardiovascular Disease
5.4. Pain
6. Prospective Areas for Identifying CatS Inhibitors
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rossi, A.; Deveraux, Q.; Turk, B.; Sali, A. Comprehensive search for cysteine cathepsins in the human genome. Biol. Chem. 2004, 385, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Scarcella, M.; d’Angelo, D.; Ciampa, M.; Tafuri, S.; Avallone, L.; Pavone, L.M.; De Pasquale, V. The Key Role of Lysosomal Protease Cathepsins in Viral Infections. Int. J. Mol. Sci. 2022, 23, 9089. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Ahn, S.J.; Lee, A.R.; Seo, J.S.; Kim, M.-S.; Kim, J.K.; Chung, J.K.; Lee, H.H. Cloning, expression analysis and enzymatic characterization of cathepsin S from olive flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 157, 238–247. [Google Scholar] [CrossRef]
- Tu, N.H.; Inoue, K.; Chen, E.; Anderson, B.M.; Sawicki, C.M.; Scheff, N.N.; Tran, H.D.; Kim, D.H.; Alemu, R.G.; Yang, L.; et al. Cathepsin S Evokes PAR2-Dependent Pain in Oral Squamous Cell Carcinoma Patients and Preclinical Mouse Models. Cancers 2021, 13, 4697. [Google Scholar] [CrossRef]
- Fernandez, P.L. Expression of cathepsins B and S in the progression of prostate carcinoma. Int. J. Cancer 2001, 95, 51–55. [Google Scholar] [CrossRef]
- Kos, J. Cathepsin S in tumours, regional lymph nodes and sera of patients with lung cancer: Relation to prognosis. Br. J. Cancer 2001, 85, 1193–1200. [Google Scholar] [CrossRef]
- Karimkhanloo, H.; Keenan, S.N.; Sun, E.W.; Wattchow, D.A.; Keating, D.J.; Montgomery, M.K.; Watt, M.J. Circulating cathepsin S improves glycaemic control in mice. J. Endocrinol. 2021, 248, 167–179. [Google Scholar] [CrossRef]
- Wu, H.; Du, Q.; Dai, Q.; Ge, J.; Cheng, X. Cysteine Protease Cathepsins in Atherosclerotic Cardiovascular Diseases. J. Atheroscler. Thromb. 2018, 25, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Hsu, H.C.; Ho, W.J.; Chang, G.J.; Pang, J.H.S.; Chen, W.J.; Huang, C.C.; Lai, Y.J. Cathepsin S promotes the development of pulmonary arterial hypertension. Am. J. Physiol.—Lung Cell. Mol. Physiol. 2019, 317, L1–L13. [Google Scholar] [CrossRef]
- Shalia, K.K.; Mashru, M.R.; Shah, V.K.; Soneji, S.L.; Payannavar, S. Levels of cathepsins in acute myocardial infarction. Indian Heart J. 2012, 64, 290–294. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Lu, B.; Yang, Y.; Zhang, W.; Wang, X.; Zhou, H.; Wen, J.; Yang, Z.; Hu, R. Elevated circulating cathepsin S levels are associated with metabolic syndrome in overweight and obese individuals. Diabetes/Metab. Res. Rev. 2019, 35, e3117. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, K.; Ishimaru, N.; Yanagi, K.; Arakaki, R.; Ogawa, K.; Saito, I.; Katunuma, N.; Hayashi, Y. Cathepsin S inhibitor prevents autoantigen presentation and autoimmunity. J. Clin. Investig. 2002, 110, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Singh, R.K.; Dastidar, S.; Ray, A. Cysteine cathepsin S as an immunomodulatory target: Present and future trends. Expert Opin. Ther. Targets 2008, 12, 291–299. [Google Scholar] [CrossRef]
- Lai, C.H.; Chang, J.Y.; Wang, K.C.; Lee, F.T.; Wu, H.L.; Cheng, T.L. Pharmacological inhibition of cathepsin s suppresses abdominal aortic aneurysm in mice. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 990–999. [Google Scholar] [CrossRef]
- Vizovišek, M.; Vidak, E.; Javoršek, U.; Mikhaylov, G.; Bratovš, A.; Turk, B. Cysteine cathepsins as therapeutic targets in inflammatory diseases. Expert Opin. Ther. Targets 2020, 24, 573–588. [Google Scholar] [CrossRef]
- Samokhin, A.O.; Lythgo, P.A.; Gauthier, J.Y.; Percival, M.D.; Brömme, D. Pharmacological inhibition of cathepsin S decreases atherosclerotic lesions in apoe-/-mice. J. Cardiovasc. Pharmacol. 2010, 56, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, N.; Meta, M.; Schuppan, D.; Nuhn, L.; Schirmeister, T. Novel opportunities for cathepsin S inhibitors in cancer immunotherapy by nanocarrier-mediated delivery. Cells 2020, 9, 2021. [Google Scholar] [CrossRef]
- Peng, K.; Liu, H.; Yan, B.; Meng, X.W.; Song, S.Y.; Ji, F.H.; Xia, Z. Inhibition of cathepsin S attenuates myocardial ischemia/reperfusion injury by suppressing inflammation and apoptosis. J. Cell. Physiol. 2021, 236, 1309–1320. [Google Scholar] [CrossRef]
- Kawato, Y.; Fukahori, H.; Nakamura, K.; Kanno, A.; Kubo, K.; Hiramitsu, M.; Matsuda, T.; Hanada, Y.; Furukawa, T.; Nakajima, Y. Potential benefit of the cathepsin S inhibitor, ASP1617, as a treatment for systemic lupus erythematosus. Eur. J. Pharmacol. 2022, 919, 174826. [Google Scholar] [CrossRef]
- Leroy, V.; Thurairatnam, S. Cathepsin S inhibitors. Expert Opin. Ther. Pat. 2004, 14, 301–311. [Google Scholar] [CrossRef]
- Lee-Dutra, A.; Wiener, D.K.; Sun, S. Cathepsin S inhibitors: 2004–2010. Expert Opin. Ther. Pat. 2011, 21, 311–337. [Google Scholar] [CrossRef]
- Brown, R.; Nath, S.; Lora, A.; Samaha, G.; Elgamal, Z.; Kaiser, R.; Taggart, C.; Weldon, S.; Geraghty, P. Cathepsin S: Investigating an old player in lung disease pathogenesis, comorbidities, and potential therapeutics. Respir. Res. 2020, 21, 111. [Google Scholar] [CrossRef]
- Paraoan, L.; Gray, D.; Hiscott, P.; Garcia-Finana, M.; Lane, B.; Damato, B.; Grierson, I. Cathepsin S and its inhibitor cystatin C: Imbalance in uveal melanoma. FBL 2009, 14, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Guay, J.; Falgueyret, J.P.; Ducret, A.; Percival, M.D.; Mancini, J.A. Potency and selectivity of inhibition of cathepsin K, L and S by their respective propeptides. Eur. J. Biochem. 2000, 267, 6311–6318. [Google Scholar] [CrossRef] [PubMed]
- Smyth, P.; Sasiwachirangkul, J.; Williams, R.; Scott, C.J. Cathepsin S (CTSS) activity in health and disease—A treasure trove of untapped clinical potential. Mol. Asp. Med. 2022, 88, 101106. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.E.; Palmer, J.T.; Brömme, D.; Somoza, J.R. Crystal structure of human cathepsin S. Protein Sci. 1998, 7, 1294–1302. [Google Scholar] [CrossRef]
- Zhao, B.; Janson, C.A.; Amegadzie, B.Y.; D’Alessio, K.; Griffin, C.; Hanning, C.R.; Jones, C.; Kurdyla, J.; McQueney, M.; Qiu, X.; et al. Crystal structure of human osteoclast cathepsin K complex with E-64. Nat. Struct. Biol. 1997, 4, 109–111. [Google Scholar] [CrossRef]
- Sosnowski, P.; Turk, D. Caught in the act: The crystal structure of cleaved cathepsin L bound to the active site of Cathepsin L. FEBS Lett. 2016, 590, 1253–1261. [Google Scholar] [CrossRef]
- Fujishima, A.; Imai, Y.; Nomura, T.; Fujisawa, Y.; Yamamoto, Y.; Sugawara, T. The crystal structure of human cathepsin L complexed with E-64. FEBS Lett. 1997, 407, 47–50. [Google Scholar] [CrossRef]
- McGrath, M.E.; Klaus, J.L.; Barnes, M.G.; Bromme, D. Crystal structure of human cathepsin K complexed with a potent inhibitor. Nat. Struct. Biol. 1997, 4, 105–109. [Google Scholar] [CrossRef]
- Patterson, A.W.; Wood, W.J.; Hornsby, M.; Lesley, S.; Spraggon, G.; Ellman, J.A. Identification of selective, nonpeptidic nitrile inhibitors of cathepsin S using the substrate activity screening method. J. Med. Chem. 2006, 49, 6298–6307. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.; Turk, D.; Turk, B. The future of cysteine cathepsins in disease management. Trends Pharmacol. Sci. 2017, 38, 873–898. [Google Scholar] [CrossRef] [PubMed]
- Pauly, T.A.; Sulea, T.; Ammirati, M.; Sivaraman, J.; Danley, D.E.; Griffor, M.C.; Kamath, A.V.; Wang, I.-K.; Laird, E.R.; Seddon, A.P. Specificity determinants of human cathepsin S revealed by crystal structures of complexes. Biochemistry 2003, 42, 3203–3213. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, J.; Song, J.; Suh, K.H.; Kim, Y.H.; Min, K.H.; Lee, K.-O. Synthesis of proline analogues as potent and selective cathepsin S inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 3140–3144. [Google Scholar] [CrossRef]
- Kim, M.; Jeon, J.; Baek, J.; Choi, J.; Park, E.J.; Song, J.; Bang, H.; Suh, K.H.; Kim, Y.H.; Kim, J. Synthesis of vinyl sulfone-tethered proline derivatives as highly selective cathepsin S inhibitors. Bull. Korean Chem. Soc. 2014, 35, 345–346. [Google Scholar] [CrossRef][Green Version]
- Palmer, J.T.; Rasnick, D.; Klaus, J.L.; Bromme, D. Vinyl sulfones as mechanism-based cysteine protease inhibitors. J. Med. Chem. 1995, 38, 3193–3196. [Google Scholar] [CrossRef]
- Ameriks, M.K.; Cai, H.; Edwards, J.P.; Gebauer, D.; Gleason, E.; Gu, Y.; Karlsson, L.; Nguyen, S.; Sun, S.; Thurmond, R.L. Pyrazole-based arylalkyne cathepsin S inhibitors. Part II: Optimization of cellular potency. Bioorg. Med. Chem. Lett. 2009, 19, 6135–6139. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.J.; Wickboldt, A.T.; Nguyen, S.; Sun, S.; Rynberg, R.; Rizzolio, M.; Karlsson, L.; Edwards, J.P.; Grice, C.A. Pyrazole-based arylalkyne cathepsin S inhibitors. Part III: Modification of P4 region. Bioorg. Med. Chem. Lett. 2013, 23, 1070–1074. [Google Scholar] [CrossRef]
- Wei, J.; Pio, B.A.; Cai, H.; Meduna, S.P.; Sun, S.; Gu, Y.; Jiang, W.; Thurmond, R.L.; Karlsson, L.; Edwards, J.P. Pyrazole-based cathepsin S inhibitors with improved cellular potency. Bioorg. Med. Chem. Lett. 2007, 17, 5525–5528. [Google Scholar] [CrossRef]
- Hilpert, H.; Mauser, H.; Humm, R.; Anselm, L.; Kuehne, H.; Hartmann, G.; Gruener, S.; Banner, D.W.; Benz, J.; Gsell, B.; et al. Identification of potent and selective cathepsin S inhibitors containing different central cyclic scaffolds. J. Med. Chem. 2013, 56, 9789–9801. [Google Scholar] [CrossRef]
- Białas, A.; Kafarski, P. Proteases as anti-cancer targets-Molecular and biological basis for development of inhibitor-like drugs against cancer. Anti-Cancer Agents Med. Chem. 2009, 9, 728–762. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.; Schiffler, M.; Gavardinas, K.; Kim, E.; Matthews, D.; Staszak, M.; Coffey, D.; Shaw, B.; Cassidy, K.; Brier, R.; et al. Discovery of cathepsin s inhibitor LY3000328 for the treatment of abdominal aortic aneurysm. ACS Med. Chem. Lett. 2014, 5, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Brömme, D.; Li, Z.; Barnes, M.; Mehler, E. Human cathepsin V functional expression, tissue distribution, electrostatic surface potential, enzymatic characterization, and chromosomal localization. Biochemistry 1999, 38, 2377–2385. [Google Scholar] [CrossRef]
- Schade, M.; Merla, B.; Lesch, B.; Wagener, M.; Timmermanns, S.; Pletinckx, K.; Hertrampf, T. Highly selective sub-nanomolar cathepsin S inhibitors by merging fragment binders with nitrile inhibitors. J. Med. Chem. 2020, 63, 11801–11808. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.-L.; Aikawa, M.; Zheng, C.; Aaron, J.; Lax, L.; Libby, P.; de Lima Filho, J.L.; Gruener, S.; Fingerle, J.; Haap, W.; et al. Selective cathepsin s inhibition attenuates atherosclerosis in apolipoprotein e–deficient mice with chronic renal disease. Am. J. Pathol. 2015, 185, 1156–1166. [Google Scholar] [CrossRef]
- Ahmad, S.; Bhagwati, S.; Kumar, S.; Banerjee, D.; Siddiqi, M.I. Molecular modeling assisted identification and biological evaluation of potent cathepsin S inhibitors. J. Mol. Graph. Model. 2020, 96, 107512. [Google Scholar] [CrossRef]
- Hewitt, E.; Pitcher, T.; Rizoska, B.; Tunblad, K.; Henderson, I.; Sahlberg, B.L.; Grabowska, U.; Classon, B.; Edenius, C.; Malcangio, M.; et al. Selective cathepsin s inhibition with MIV-247 attenuates mechanical allodynia and enhances the antiallodynic effects of gabapentin and pregabalin in a mouse model of neuropathic pain. J. Pharmacol. Exp. Ther. 2016, 358, 387–396. [Google Scholar] [CrossRef]
- Tato, M.; Kumar, S.V.; Liu, Y.; Mulay, S.R.; Moll, S.; Popper, B.; Eberhard, J.N.; Thomasova, D.; Rufer, A.C.; Gruner, S.; et al. Cathepsin S inhibition combines control of systemic and peripheral pathomechanisms of autoimmune tissue injury. Sci. Rep. 2017, 7, 2775. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Y.; Xu, X.; Liu, Y.; Zhao, J.; Ma, L.; Lei, J.; Ge, W.; Li, N.; Ma, E.; et al. Design and synthesis of dual cathepsin L and S inhibitors and antimetastatic activity evaluation in pancreatic cancer cells. Bioorg. Med. Chem. Lett. 2023, 80, 129087. [Google Scholar] [CrossRef]
- Singh, M.; Tam, B.; Akabayov, B. NMR-fragment based virtual screening: A brief overview. Molecules 2018, 23, 233. [Google Scholar] [CrossRef]
- Kirsch, P.; Hartman, A.M.; Hirsch, A.K.H.; Empting, M. Concepts and core principles of fragment-based drug design. Molecules 2019, 24, 4309. [Google Scholar] [CrossRef]
- Mureddu, L.G.; Vuister, G.W. Fragment-based drug discovery by NMR. Where are the successes and where can it be improved? Front. Mol. Biosci. 2022, 9, 834453. [Google Scholar] [CrossRef]
- Olivier, T.; Haslam, A.; Prasad, V. Sotorasib in KRASG12C mutated lung cancer: Can we rule out cracking KRAS led to worse overall survival? Transl. Oncol. 2023, 28, 101591. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Cathepsin S in Clinical Trials. Available online: https://clinicaltrials.gov/search?term=Cathepsin%20S&city= (accessed on 10 June 2024).
- Galibert, M.; Wartenberg, M.; Lecaille, F.; Saidi, A.; Mavel, S.; Joulin-Giet, A.; Korkmaz, B.; Brömme, D.; Aucagne, V.; Delmas, A.F.; et al. Substrate-derived triazolo- and azapeptides as inhibitors of cathepsins K and S. Eur. J. Med. Chem. 2018, 144, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Medivir AB. Medivir to Present Data from Its Cathepsin S Inhibitor Program Including MIV-247 for Neuropathic Pain, at the 15th World Congress on Pain. Available online: https://www.medivir.com/investors/press-releases/2014/medivir-to-present-data-from-its-cathepsin-s-inhibitor-program-including-miv-247-for-neuropathic-pain-at-the-15th-world-congress-on-pain (accessed on 10 June 2024).
- Yen, T.-H.; Ho, W.-J.; Yeh, Y.-H.; Lai, Y.-J. Cathepsin s inhibition suppresses experimental systemic lupus erythematosus-associated pulmonary arterial remodeling. Int. J. Mol. Sci. 2022, 23, 12316. [Google Scholar] [CrossRef]
- Hargreaves, P.; Daoudlarian, D.; Theron, M.; Kolb, F.A.; Manchester Young, M.; Reis, B.; Tiaden, A.; Bannert, B.; Kyburz, D.; Manigold, T. Differential effects of specific cathepsin S inhibition in biocompartments from patients with primary Sjögren syndrome. Arthritis Res. Ther. 2019, 21, 175. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Imada, S.; Yamamoto, E.; Tsuchiya, K.; Harayama, Y.; Matsumoto, S. Phenyldifluoromethyl-Substituted Prolineamide Compound. US10532979B2, 14 January 2020. [Google Scholar]
- Zhang, L.; Wang, H.; Xu, J.; Zhu, J.; Ding, K. Inhibition of cathepsin S induces autophagy and apoptosis in human glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K and JNK signaling pathways. Toxicol. Lett. 2014, 228, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.U.; Kim, T.H.; Kim, D.E.; Min, K.J.; Kwon, T.K. NOX4-mediated ROS production induces apoptotic cell death via down-regulation of c-FLIP and Mcl-1 expression in combined treatment with thioridazine and curcumin. Redox Biol. 2017, 13, 608–622. [Google Scholar] [CrossRef]
- Fei, M.; Zhang, L.; Wang, H.; Zhu, Y.; Niu, W.; Tang, T.; Han, Y. Inhibition of cathepsin s induces mitochondrial apoptosis in glioblastoma cell lines through mitochondrial stress and autophagosome accumulation. Front. Oncol. 2020, 10, 516746. [Google Scholar] [CrossRef]
- Yan, L.; Ding, S.; Gu, B.; Ma, P. Clinical application of simultaneous detection of cystatin C, cathepsin S, and IL-1 in classification of coronary artery disease. J. Biomed. Res. 2017, 31, 315–320. [Google Scholar] [CrossRef]
- Flannery, T. The clinical significance of cathepsin S expression in human astrocytomas. Am. J. Pathol. 2003, 163, 175–182. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flannery, T.; McQuaid, S.; McGoohan, C.; McConnell, R.S.; McGregor, G.; Mirakhur, M.; Hamilton, P.; Diamond, J.; Cran, G.; Walker, B.; et al. Cathepsin S expression: An independent prognostic factor in glioblastoma tumours—A pilot study. Int. J. Cancer 2006, 119, 854–860. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Lin, C.-W.; Chen, M.-K.; Chien, S.-Y.; Lo, Y.-S.; Chuang, Y.-C.; Hsi, Y.-T.; Lin, C.-C.; Chen, J.-C.; Yang, S.-F. Inhibition of cathepsin S confers sensitivity to methyl protodioscin in oral cancer cells via activation of p38 MAPK/JNK signaling pathways. Sci. Rep. 2017, 7, 45039. [Google Scholar] [CrossRef]
- da Costa, A.C.; Santa-Cruz, F.; Mattos, L.A.R.; Aquino, M.A.R.; Martins, C.R.; Ferraz, Á.A.B.; Figueiredo, J.L. Cathepsin S as a target in gastric cancer. Mol. Clin. Oncol. 2020, 12, 99–103. [Google Scholar] [CrossRef]
- Burden, R.E. Inhibition of Cathepsin S by Fsn0503 enhances the efficacy of chemotherapy in colorectal carcinomas. Biochimie 2012, 94, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Sevenich, L.; Bowman, R.L.; Mason, S.D.; Quail, D.F.; Rapaport, F.; Elie, B.T.; Brogi, E.; Brastianos, P.K.; Hahn, W.C.; Holsinger, L.J.; et al. Analysis of tumour-and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 2014, 16, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Shao, J.; Qin, Y.; Ji, Q.; Zhang, X.; Du, J. Cathepsin S-mediated autophagic flux in tumor-associated macrophages accelerate tumor development by promoting M2 polarization. Mol. Cancer 2014, 13, 43. [Google Scholar] [CrossRef]
- Bararia, D.; Hildebrand, J.A.; Stolz, S.; Haebe, S.; Alig, S.; Trevisani, C.P.; Osorio-Barrios, F.; Bartoschek, M.D.; Mentz, M.; Pastore, A.; et al. Cathepsin S alterations induce a tumor-promoting immune microenvironment in follicular lymphoma. Cell Rep. 2020, 31, 107522. [Google Scholar] [CrossRef]
- Dheilly, E.; Battistello, E.; Katanayeva, N.; Sungalee, S.; Michaux, J.; Duns, G.; Wehrle, S.; Sordet-Dessimoz, J.; Mina, M.; Racle, J.; et al. Cathepsin s regulates antigen processing and T cell activity in non-hodgkin lymphoma. Cancer Cell 2020, 37, 674–689.e12. [Google Scholar] [CrossRef]
- Seo, S.U.; Woo, S.M.; Min, K.-j.; Kwon, T.K. Z-FL-COCHO, a cathepsin S inhibitor, enhances oxaliplatin-induced apoptosis through upregulation of Bim expression. Biochem. Biophys. Res. Commun. 2018, 498, 849–854. [Google Scholar] [CrossRef]
- Zheng, J.; Zhuang, H.; Zhang, T.; Wang, Y.; Ran, T.; He, J.; Han, N.; Duan, J. Cathepsin S inhibitor reduces high-fat-induced adipogenesis, inflammatory infiltration, and hepatic lipid accumulation in obese mice. Ann. Transl. Med. 2022, 10, 1172. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Ding, K.; Lu, X.; Li, T.; Wang, J.; Wang, C.; Wang, J. Inhibition of cathepsin s produces neuroprotective effects after traumatic brain injury in mice. Mediat. Inflamm. 2013, 2013, 187873. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.; Yassa, N.; Mazaheri, Z.; Rajaei, M.; Pourabouk, M.; Ghorghanlu, S.; Basiri, S.; Khori, V. Cardioprotective and anti-apoptotic effects of Potentilla reptans L. root via Nrf2 pathway in an isolated rat heart ischemia/reperfusion model. Life Sci. 2018, 215, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Ajani, T.A.; Obikeze, K.; Magwebu, Z.E.; Egieyeh, S.; Chauke, C.G. In-silico and in-vitro screening of Asiatic acid and Asiaticoside A against Cathepsin S enzyme. BMC Pharmacol. Toxicol. 2023, 24, 67. [Google Scholar] [CrossRef] [PubMed]
- Obikeze, K.; Gainsford, G.; Kamaar, M. Crataegus monogyna and Centella asiatica extracts as inhibitors of cathepsin s. Adv. Pharmacol. Pharm. 2022, 10, 247–252. [Google Scholar] [CrossRef]
- Vidal-Albalat, A.; González, F.V. Natural products as cathepsin inhibitors. Stud. Nat. Prod. Chem. 2016, 50, 179–213. [Google Scholar]
- Li, Y.; Ai, X.; Zou, C.; Liu, Y.; Ma, L.; Men, J.; Liu, D.; Sheng, L.; Ruan, X.; Liu, H. Discovery of a novel and selective cathepsin L inhibitor with anti-metastatic ability in vitro and in vivo against breast cancer cells. Bioorg. Chem. 2021, 115, 105256. [Google Scholar] [CrossRef] [PubMed]
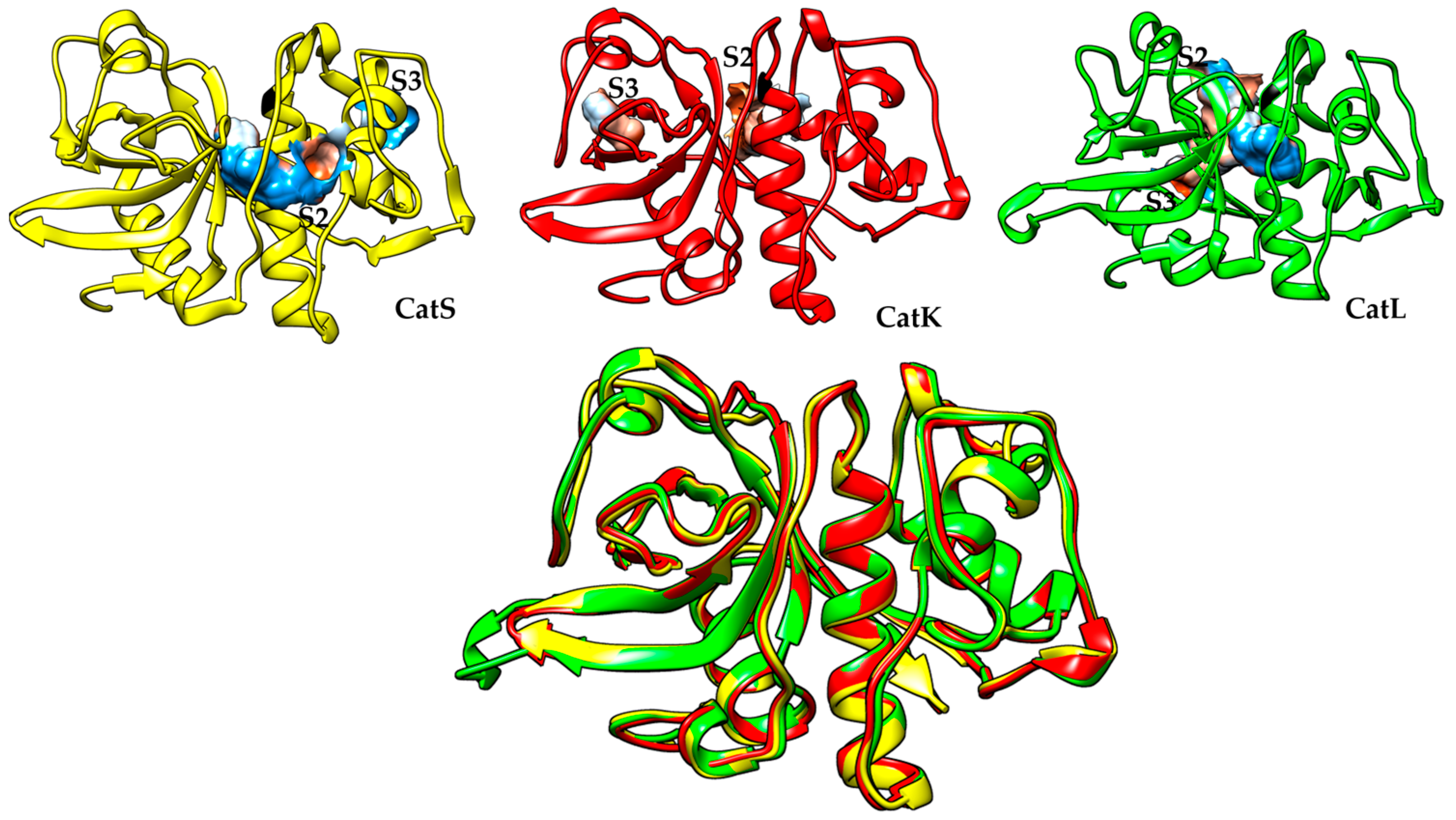
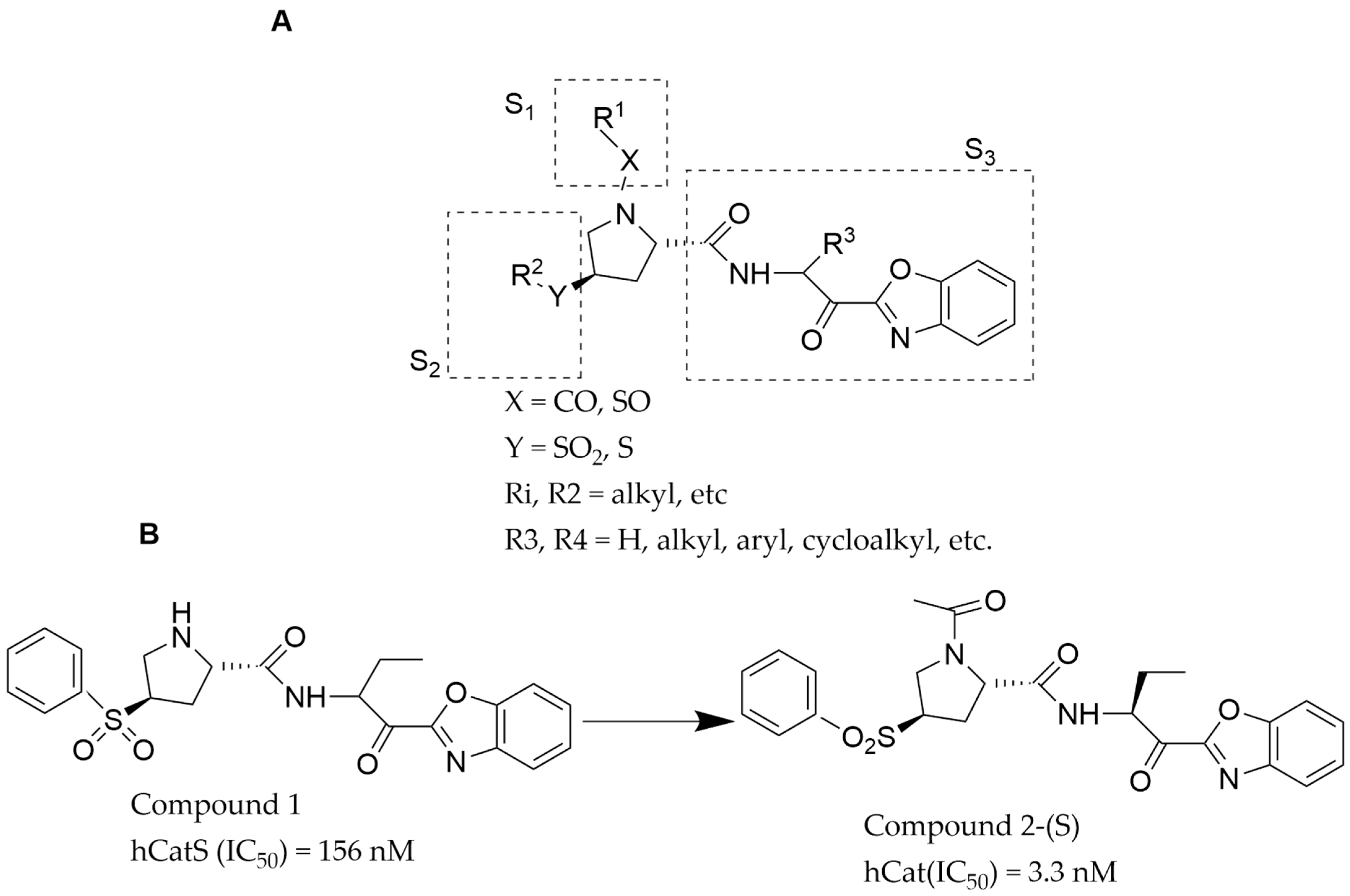
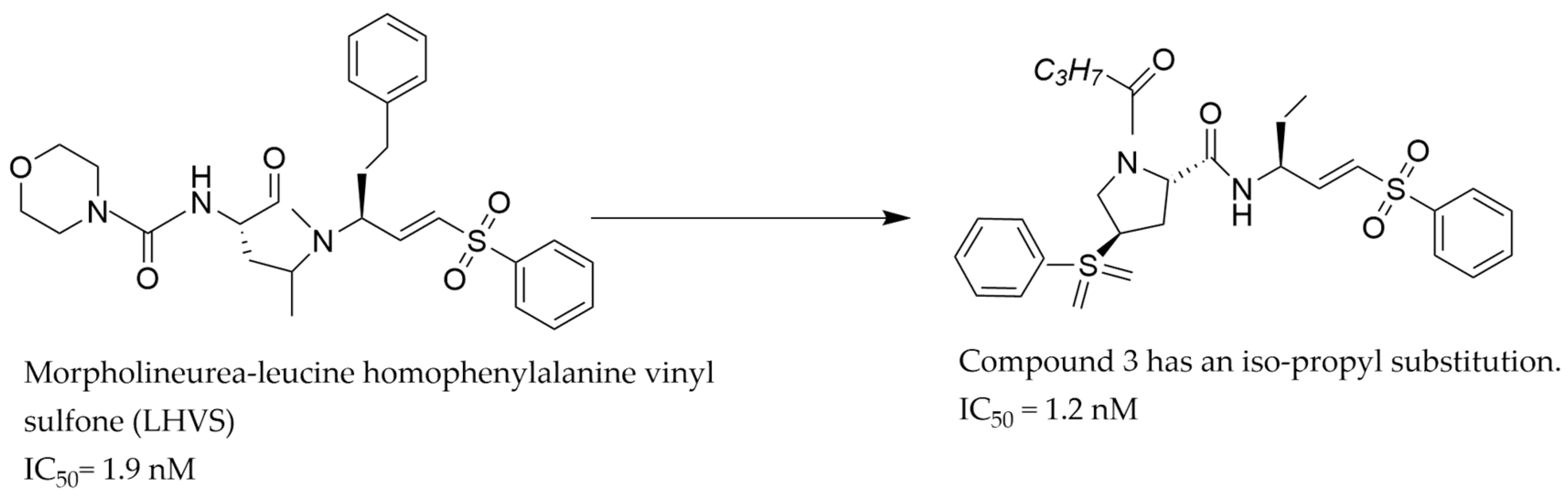
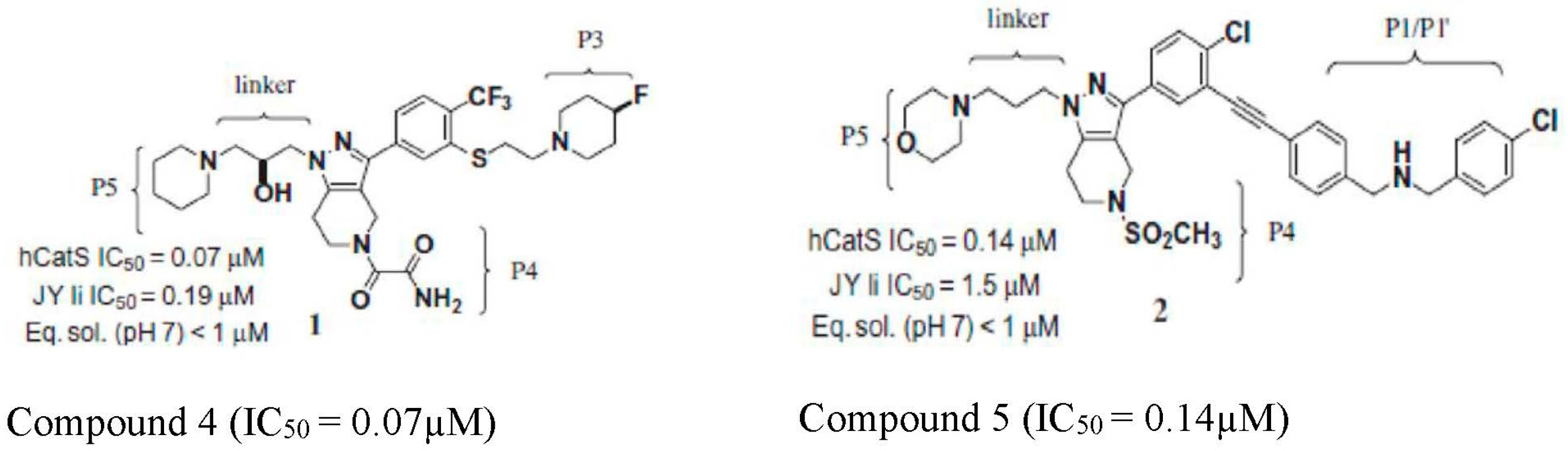
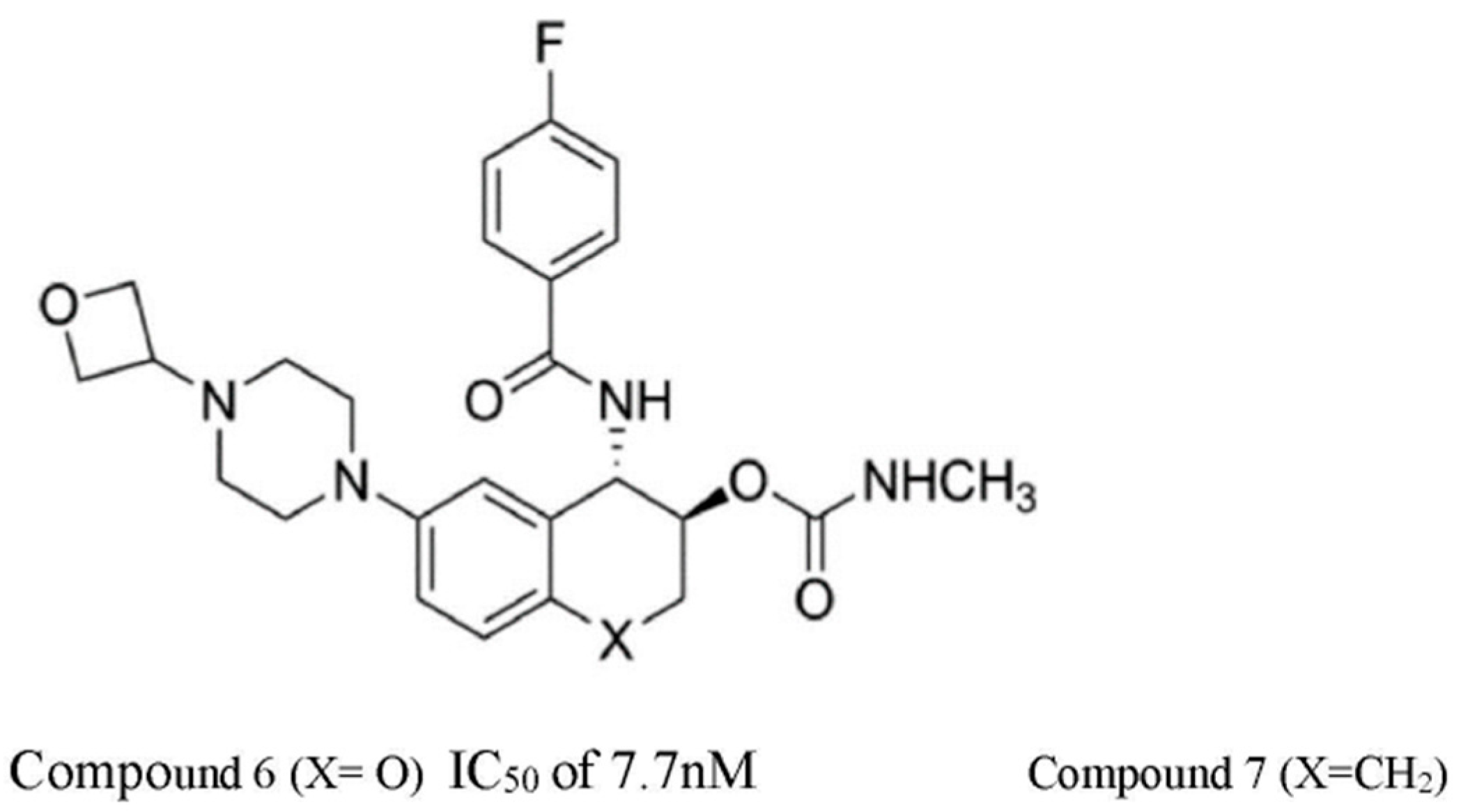
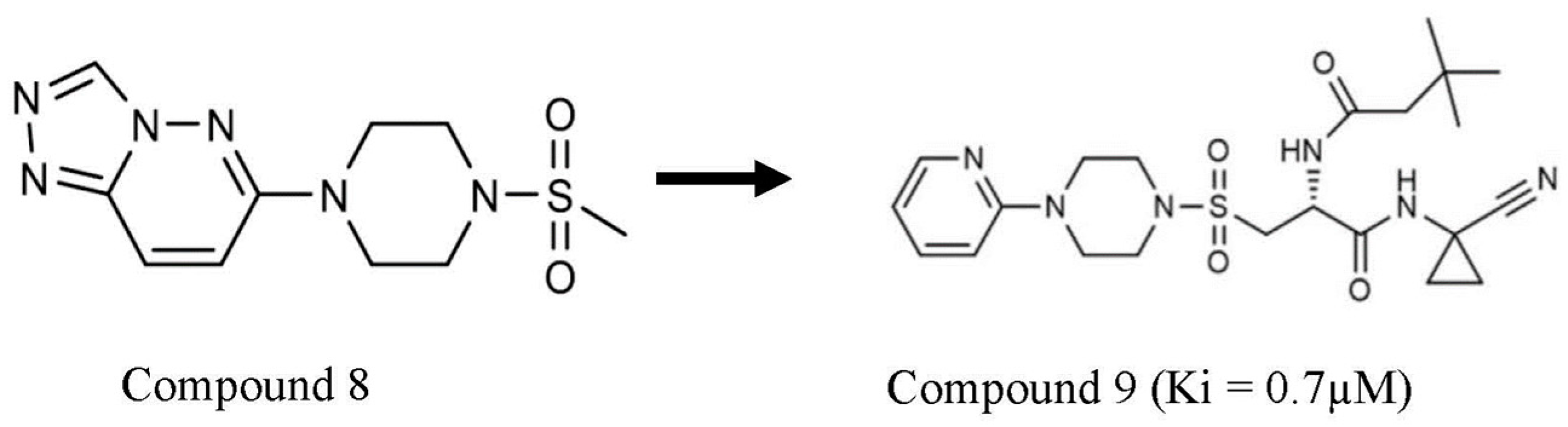
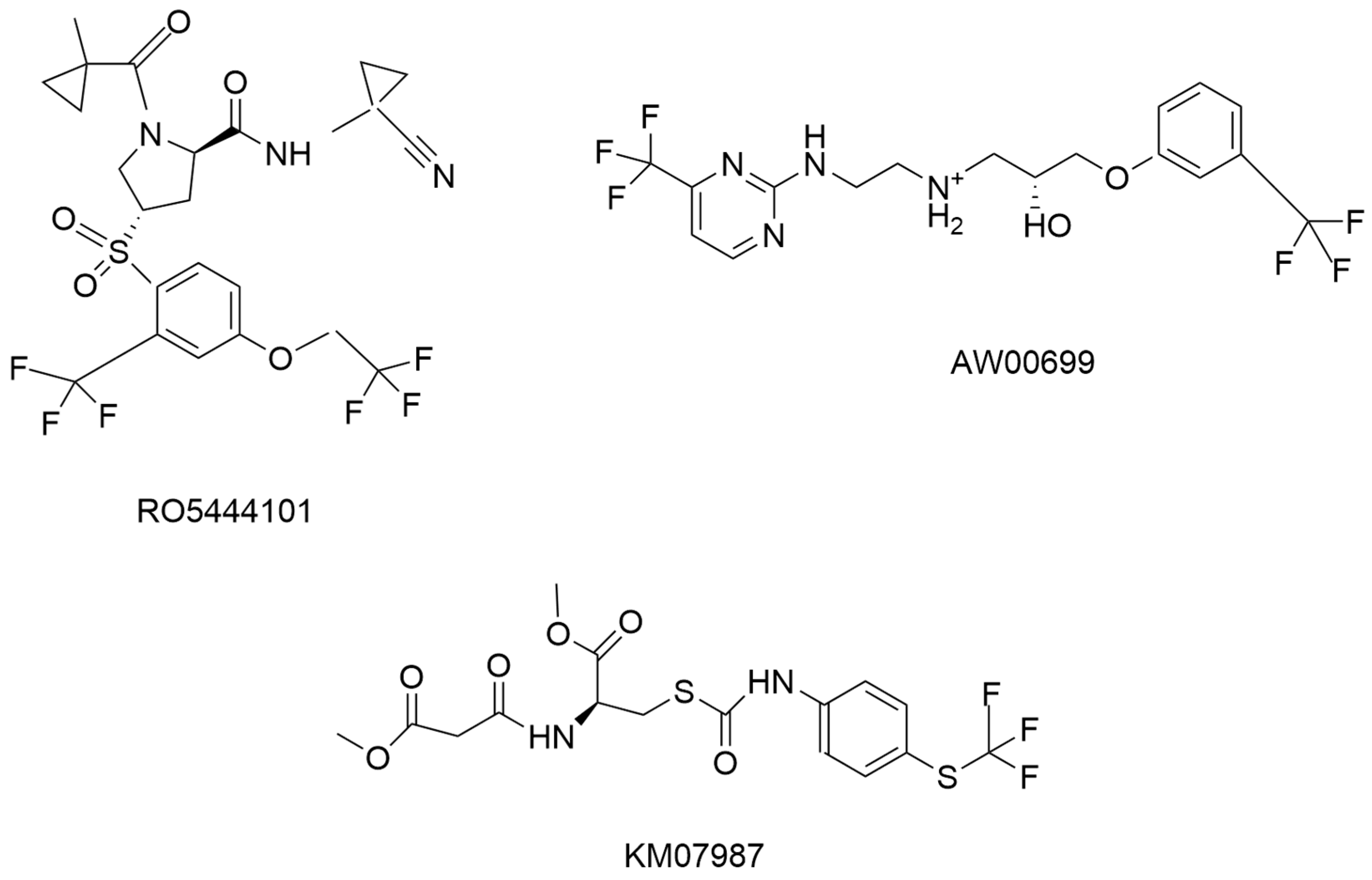
| S/N | Compound Name | IC50 nM | CatS Selectivity | References |
|---|---|---|---|---|
| 1 | Compound 2 | 3.3 | Yes | [34] |
| 2 | Compound 3 | 1.2 | Yes | [35] |
| 3 | Compound 5 | 60 | Yes | [38] |
| 4 | compound 9 | 45 | Yes | [44] |
| 5 | Compound 10 | 0.7 | Yes | [40] |
| 6 | LY3000328 | 7.7 | Yes | [42] |
| 7 | RO5444101 | 0.2 | Yes | [45] |
| 8 | KM0787 | 5000 | No report | [46] |
| 9 | MIV-247 | 1 | No report | [47] |
| 10 | Millipore-219393 | not available | not available | [9] |
| 11 | RO5459072 | not available | not available | [48] |
| 12 | ASP1617 disuccinate | 4.6 | Yes | |
| 13 | ASPER-29 (Asperphenamate derivative) | 1790 | No | [49] |
| S/N | Drug Name | Company | Indication | Phase | Clinical Trial Identifier |
|---|---|---|---|---|---|
| 1 | VBY-036 | Virobay Inc., Menlo Park, CA, USA | Nerve pain | Phase I | NCT01911637 |
| 2 | RWJ-445380 | Johnson & Johnson Pharmaceutical Research and Development, LLC, Raritan, NJ, USA | Rheumatoid Arthritis | Phase II | NCT00425321 |
| 3 | VBY-891 | Virobay Inc., Menlo Park, CA, USA and LEO Pharma Ballerup, Ballerup, Denmark | Psoriasis (inflammatory autoimmune disease) | Phase I | NCT01947738 |
| 4 | Not Provided | Children’s Hospital of Orange County, Orange, CA, USA and Ultragenyx Pharmaceutical Inc., Novato, CA, USA | Mucopolysaccharidoses | Recruiting for Phase I | NCT05063435 |
| 5 | IPHAAB | Medical University of Vienna and Austrian Federal Ministry of Defense and Sports, Vienna, Austria | Not indicated | Not indicated | NCT02097199 |
| 6 | RO5459072 | Hoffmann-La Roche, Basel, Switzerland | Celiac Disease | Phase I | NCT02679014 |
| 7 | LY3000328 | Eli Lilly and Company, Indianapolis, IN, USA | Not indicated (abdominal aortic aneurysm) | Phase I | NCT01515358 |
| 8 | LIPOGAIN | Uppsala University, Uppsala, Sweden | Weight Gain | Not Applicable | NCT01427140 |
| 9 | Vorinostat | Boston University and Merck Sharp & Dohme LLC, Rahway, NJ, USA | Mycosis Fungoides | Withdrawn | NCT01801670 |
| 10 | ASP1617disuccinate | Astellas Pharma Global Development, Inc., Northbrook, IL, USA | Autoimmune diseases (systemic lupus erythematosus) | Phase I | NCT04077879 |
| 11 | LIPOGAIN | Uppsala University, Uppsala, Sweden | Weight Gain | Not Applicable | NCT01427140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajani, T.A.; Magwebu, Z.E.; Chauke, C.G.; Obikeze, K. Advances in Cathepsin S Inhibition: Challenges and Breakthroughs in Drug Development. Pathophysiology 2024, 31, 471-487. https://doi.org/10.3390/pathophysiology31030035
Ajani TA, Magwebu ZE, Chauke CG, Obikeze K. Advances in Cathepsin S Inhibition: Challenges and Breakthroughs in Drug Development. Pathophysiology. 2024; 31(3):471-487. https://doi.org/10.3390/pathophysiology31030035
Chicago/Turabian StyleAjani, Temitope A., Zandisiwe E. Magwebu, Chesa G. Chauke, and Kenechukwu Obikeze. 2024. "Advances in Cathepsin S Inhibition: Challenges and Breakthroughs in Drug Development" Pathophysiology 31, no. 3: 471-487. https://doi.org/10.3390/pathophysiology31030035
APA StyleAjani, T. A., Magwebu, Z. E., Chauke, C. G., & Obikeze, K. (2024). Advances in Cathepsin S Inhibition: Challenges and Breakthroughs in Drug Development. Pathophysiology, 31(3), 471-487. https://doi.org/10.3390/pathophysiology31030035






