Sepia pharaonis Ink Mitigates Dehydroepiandrosterone-Induced Insulin Resistance in Mouse Model of Polycystic Ovarian Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Kits
2.2. Sepia pharaonis Ink (SI) Preparation
2.3. Experimental Design
2.4. Measurement of Body and Ovary Weights
2.5. Determination of Estrous Cycle Regularity
2.6. IPGTT (Intraperitoneal Glucose Tolerance Test)
2.7. IPITT (Intraperitoneal Insulin Tolerance Test)
2.8. Determination of Hormone Levels
2.9. Determination of Insulin Resistance Biomarkers IGF-1, IRS-1, and AR-1
2.10. Histopathology
2.11. Statistical Analysis
3. Results
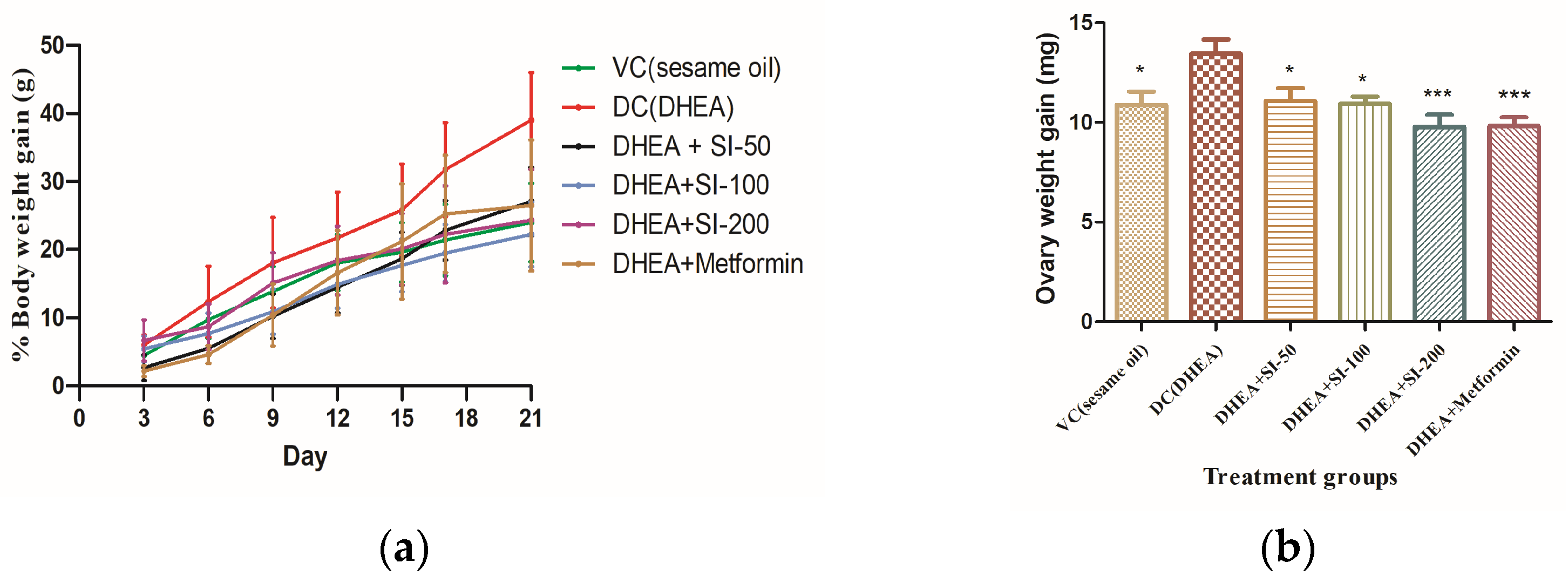
3.1. Determination of Body and Ovary Weights
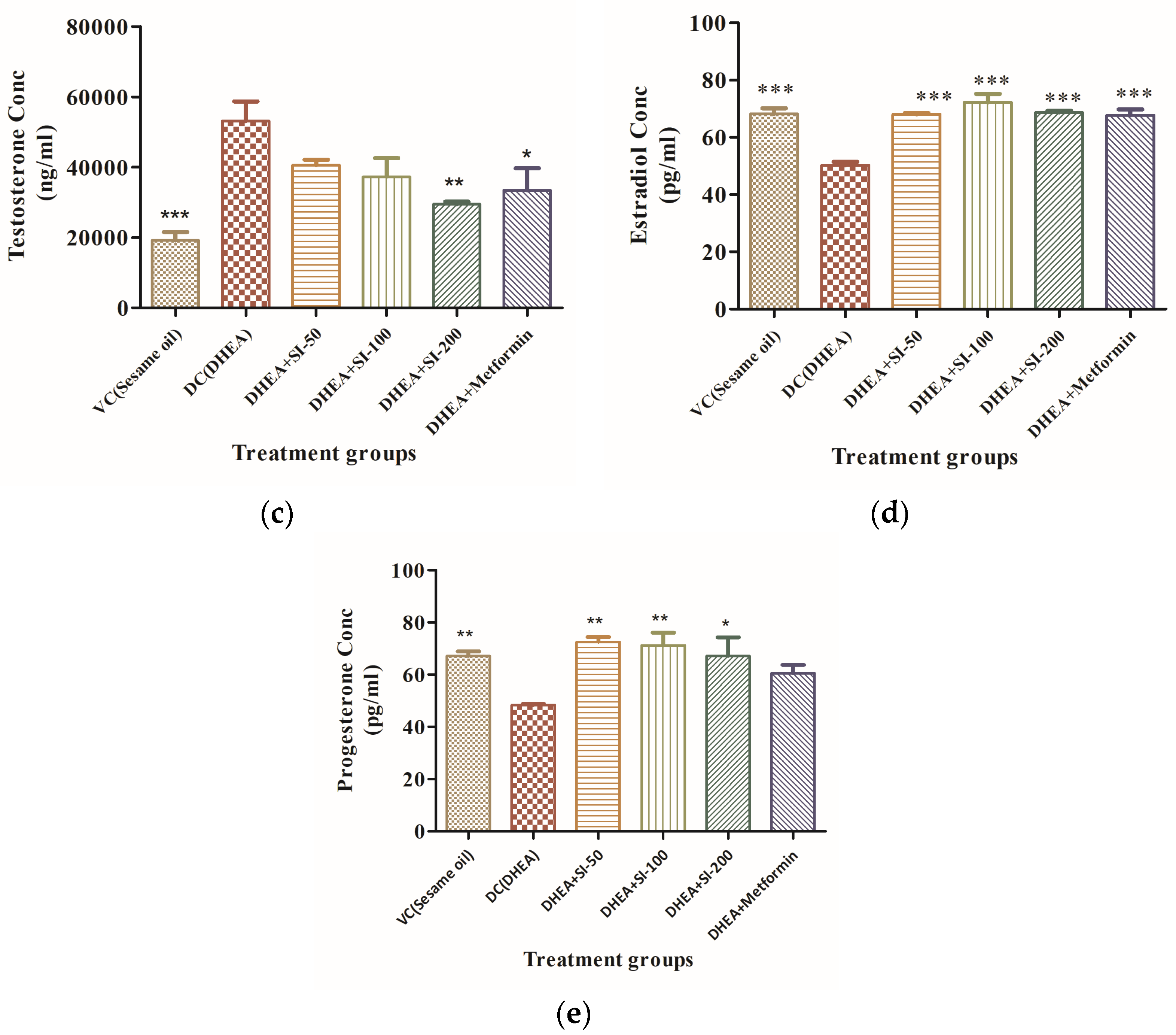
3.2. Effect of SI on Anovulation and Major Reproductive Hormones
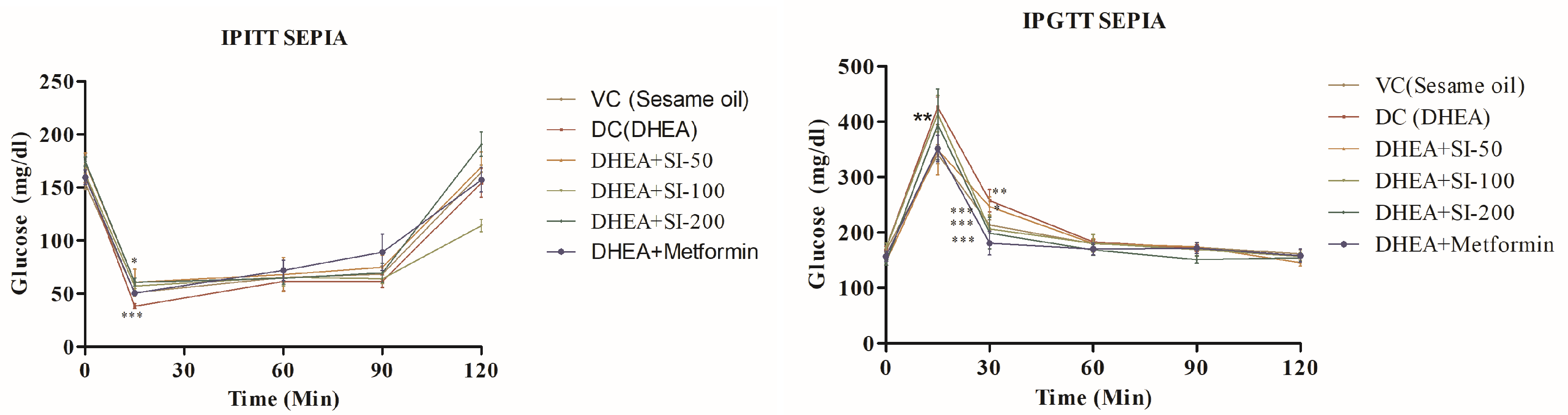
3.3. Effect of SI on Glucose Tolerance
3.4. Effect of SI on Ovary and Liver Histopathology
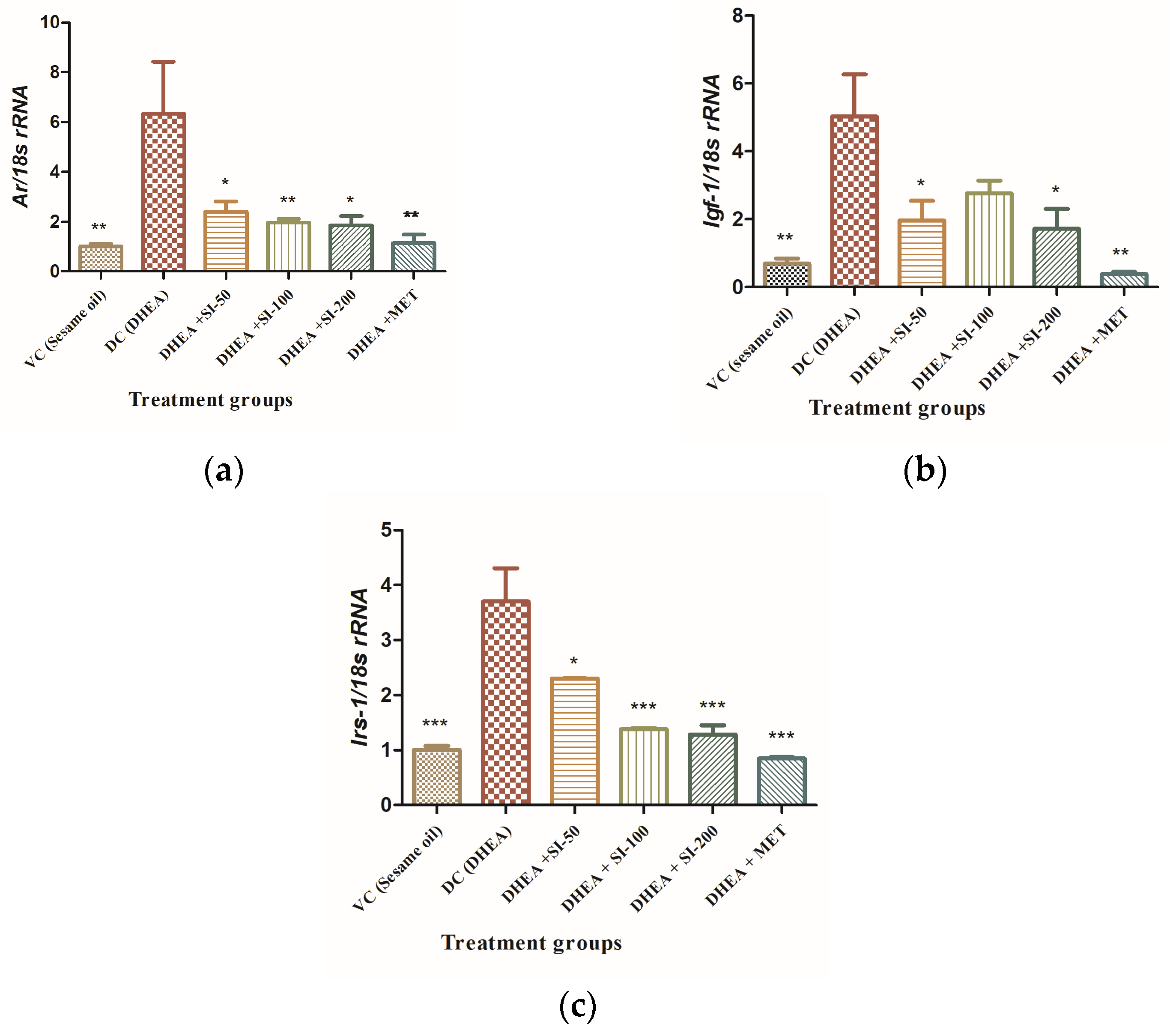
3.5. Effect of SI on Expression of Genes Related to Insulin Resistance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reaven, G.M. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Chan, L.; Zhou, S.W. Omentin: Linking metabolic syndrome and cardiovascular disease. Curr. Vasc. Pharmacol. 2014, 12, 136–143. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.W.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.L.; Ehrmann, D.A. Clinical Manifestations of Polycystic Ovary Syndrome in Adults; UpToDate: Waltham, MA, USA, 2014; p. 17. [Google Scholar]
- Speroff, L.; Fritz, M.A. Clinical Gynecologic Endocrinology and Infertility, 8th ed.; Lipppincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Norman, R.J.; Dewailly, D.; Legro, R.S.; Hickey, T.E. Polycystic ovary syndrome. Lancet 2007, 370, 685–697. [Google Scholar] [CrossRef]
- Mirghafourvand, M.; Charandabi, S.M.-A.; Aliasghari, F. Predictors of depression in iranian women with polycystic ovarian syndrome. Community Ment. Health J. 2018, 54, 1274–1283. [Google Scholar] [CrossRef]
- Gholizadeh Shamasbi, S.; Dehgan, P.; Mohammad-Alizadeh Charandabi, S.; Aliasgarzadeh, A.; Mirghafourvand, M. The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: A randomized, triple-blind, controlled, clinical trial. Eur. J. Nutr. 2019, 58, 629–640. [Google Scholar] [CrossRef]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Shafabakhsh, R.; Hoseini, A.; Asemi, Z. Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 2020, 36, 128–133. [Google Scholar] [CrossRef]
- Heshmati, J.; Moini, A.; Sepidarkish, M.; Morvaridzadeh, M.; Salehi, M.; Palmowski, A.; Mojtahedi, M.F.; Shidfar, F. Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: A randomized double-blind placebo-controlled clinical trial. Phytomed 2021, 80, 15. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.J.; Chang, C.Y.; Wu, M.Y.; Chen, C.H.; Horng, Y.S.; Wu, H.C. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: Systematic review with meta-analysis and trial sequential analysis. Nutrients 2021, 13, 684. [Google Scholar] [CrossRef]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular mechanisms underlying curcumin-mediated therapeutic effects in type 2 diabetes and cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef]
- Gupta, J.; Kulshreshtha, D.; Lamba, C.D.; Gupta, P.; Shinde, V.; Wadhwa, B.; Soren, A.; Arya, J.; Koley, M.; Pramanik, A.; et al. Homoeopathic medicine—Sepia for the management of menopausal symptoms: A multicentric, randomised, double-blind placebo-controlled clinical trial. Indian J. Res. Homoeopath. 2019, 13, 219–228. [Google Scholar] [CrossRef]
- Shinde, V. Polycystic ovarian syndrome with endometrial hyperplasia and uterine fibroids treated with homoeopathy: A case report. Indian J. Res. Homoeopath. 2022, 16, 231–238. [Google Scholar] [CrossRef]
- Gupta, G.; Gupta, N.; Singh, S.; Roja, V.; Dewan, D. Homoeopathic treatment of women with polycystic ovarian syndrome: A prospective observational study. Indian J. Res. Homoeopath. 2021, 15, 12–23. [Google Scholar] [CrossRef]
- Madaras, F.; Gerber, J.P.; Peddie, F.; Kokkinn, M.J. The effect of sampling methods on the apparent constituents of ink from the squid sepioteuthis australis. J. Chem. Ecol. 2010, 36, 1171–1179. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef] [PubMed]
- Sadek, S.A. Sepia officinalis ink mitigates gastric ulcer via modulation of antioxidant/anti-inflammatory pathways. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 63. [Google Scholar] [CrossRef]
- Liu, L.; Lu, K.; Xie, J.; Che, H.; Li, H.; Wancui, X. Melanin from Sepia pharaonis ink alleviates mucosal damage and reduces inflammation to prevent alcohol-induced gastric ulcers. Food Biosci. 2023, 51, 102266. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Ma, Y.; Xiao, J.; Luo, G.; Li, Y.; Wu, D. Multi-system reproductive metabolic disorder: Signifcance for the pathogenesis and therapy of polycystic ovary syndrome (PCOS). Life Sci. 2019, 228, 167–175. [Google Scholar] [CrossRef]
- Jeanes, Y.; Reeves, S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: Diagnostic and methodological challenges. Nutr. Res. Rev. 2017, 30, 97–105. [Google Scholar] [CrossRef]
- Wang, J.; Wu, D.; Guo, H.; Li, M. Hyperandrogenemia and insulin resistance: The chief culprit of polycystic ovary syndrome. Life Sci. 2019, 236, 116940. [Google Scholar] [CrossRef]
- Rice, S.; Christoforidis, N.; Gadd, C.; Nikolaou, D.; Seyani, L.; Donaldson, A.; Margara, R.; Hardy, K.; Franks, S. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum. Reprod. 2005, 20, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, Y.; Guo, X.; Jia, W.; Liu, G.; Zhang, J.; Li, J.; Cui, P.; Sferruzzi-Perri, A.N.; Han, Y.; et al. Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant ROS production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E794–E809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, M.; Yang, F.; Zhang, Y.; Ma, S.; Zhang, D.; Wang, X.; Sferruzzi-Perri, A.N.; Wu, X.; Brännström, M.; et al. Increased uterine androgen receptor protein abundance results in implantation and mitochondrial defects in pregnant rats with hyperandrogenism and insulin resistance. J. Mol. Med. 2021, 99, 1427. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.; Bhattacharya, K.; Basak, A.K.; Paul, N.; Bandyopadhyay, R.; Chaudhuri, G.R.; Purkait, M.P.; Bhattacharjee, A.; Bose, C.; Shukla, N.; et al. Inflammatory perspectives of polycystic ovary syndrome: Role of specific mediators and markers. Middle East Fertil. Soc. J. 2023, 28, 33. [Google Scholar] [CrossRef]
- Feng, Y.; Johansson, J.; Shao, R.; Mannerås, L.; Fernandez-Rodriguez, J.; Billig, H.; Stener-Victorin, E. Hypothalamic Neuroendocrine Functions in Rats with Dihydrotestosterone-Induced Polycystic Ovary Syndrome: Effects of Low-Frequency Electro-Acupuncture. PLoS ONE 2009, 4, e6638. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.; Shulman, G. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate fux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic ovary syndrome: A comprehensive review of pathogenesis, management, and drug repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef] [PubMed]
- Spremović Rađenović, S.; Pupovac, M.; Andjić, M.; Bila, J.; Srećković, S.; Gudović, A.; Dragaš, B.; Radunović, N. Prevalence, risk factors, and pathophysiology of non_alcoholic fatty liver disease (NAFLD) in women with Polycystic Ovary Syndrome (PCOS). Biomedicines 2022, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Vassilatou, E.; Lafoyianni, S.; Vryonidou, A.; Ioannidis, D.; Kosma, L.; Katsoulis, K.; Papavassiliou, E.; Tzavara, I. Increased androgen bioavailability is associated with non_alcoholic fatty liver disease in women with polycystic ovary syndrome. Hum. Reprod. 2010, 25, 212–220. [Google Scholar] [CrossRef]
- Baranova, A.; Tran, T.P.; Afendy, A.; Wang, L.; Shamsaddini, A.; Mehta, R.; Chandhoke, V.; Birerdinc, A.; Younossi, Z.M. Molecular signature of adipose tissue in patients with both Non-Alcoholic Fatty Liver Disease (NAFLD) and Polycystic Ovarian Syndrome (PCOS). J. Transl. Med. 2013, 11, 133. [Google Scholar] [CrossRef]
- Won, Y.B.; Seo, S.K.; Yun, B.H.; Lee, B.S. Non-alcoholic fatty liver disease in polycystic ovary syndrome women. Sci. Rep. 2021, 11, 7085. [Google Scholar] [CrossRef] [PubMed]
- Ayonrinde, O.T.; Adams, L.A.; Doherty, D.A.; Mori, T.A.; Beilin, L.J.; Oddy, W.H.; Hickey, M.; Sloboda, D.M.; Olynyk, J.K.; Hart, R. Adverse metabolic phenotype of adolescent girls with non-alcoholic fatty liver disease plus polycystic ovary syndrome compared with other girls and boys. J. Gastroenterol. Hepatol. 2016, 31, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Lerchbaum, E.; Gruber, H.J.; Schwetz, V.; Giuliani, A.; Möller, R.; Pieber, T.R.; Obermayer-Pietsch, B. Fatty liver index in polycystic ovary syndrome. Eur. J. Endocrinol. 2011, 165, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gao, H.; Fan, Y.; Chen, S.; Li, Y.; Liu, R.; Li, T.; Yin, C. Gut microbiota disorder induces liver dysfunction in polycystic ovary syndrome rats’ model by regulating metabolite rosmarinic acid. Life Sci. 2023, 330, 121912. [Google Scholar] [CrossRef]
- Kotipalli, R.S.S.; Patnaik, S.S.; Kumar, J.M.; Ramakrishna, S.; Muralidharan, K. Biochanin-A attenuates DHEA-induced polycystic ovary syndrome via upregulation of GDF9 and BMP15 signaling in vivo. Life Sci. 2023, 326, 121795. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, H.; Liu, W.; Liu, M.; Shi, M.; Wen, Z.; Li, C. Metformin inhibits growth of eutopic stromal cells from adenomyotic endometrium via AMPK activation and subsequent inhibition of AKT phosphorylation: A possible role in the treatment of adenomyosis. Reproduction 2013, 146, 397–406. [Google Scholar] [CrossRef]
- Sharpe, A.; Morley, L.; Tang, T.; Norman, R.; Balen, A. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2019, 12, CD013505. [Google Scholar] [CrossRef]


| Induction | Treatment | ||
|---|---|---|---|
| Group I | Vehicle control (VC) | Sesame oil | |
| Group II | DHEA group (DC) | DHEA in Sesame oil | |
| Group III | SI-50 | DHEA in Sesame oil | SI-50 mg/Kg body wt. |
| Group IV | SI-100 | DHEA in Sesame oil | SI-100 mg/Kg body wt. |
| Group V | SI-200 | DHEA in Sesame oil | SI-200 mg/Kg body wt. |
| Group VI | Metformin | DHEA in Sesame oil | MET-50 mg/Kg body wt. |
| Gene Name | Forward | Reverse |
|---|---|---|
| Igf1 | GTGGATGCTCTTCAGTTCGTGTG | TCCAGTCTCCTCAGATCACAGC |
| Irs1 | TGTCACCCAGTGGTAGTTGCTC | CTCTCAACAGGAGGTTTGGCATG |
| Ar | GGCGGTCCTTCACTAATGTCAACT | GAGACTTGTGCATGCGGTACTCAT |
| GAPDH | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamarthi, P.; Kotipalli, R.S.; Patnaik, S.; Veena, K.; Kathirvel, M.; Vutukuri, R.; Bhanoori, M. Sepia pharaonis Ink Mitigates Dehydroepiandrosterone-Induced Insulin Resistance in Mouse Model of Polycystic Ovarian Syndrome. Pathophysiology 2024, 31, 408-419. https://doi.org/10.3390/pathophysiology31030031
Yamarthi P, Kotipalli RS, Patnaik S, Veena K, Kathirvel M, Vutukuri R, Bhanoori M. Sepia pharaonis Ink Mitigates Dehydroepiandrosterone-Induced Insulin Resistance in Mouse Model of Polycystic Ovarian Syndrome. Pathophysiology. 2024; 31(3):408-419. https://doi.org/10.3390/pathophysiology31030031
Chicago/Turabian StyleYamarthi, Prathyusha, Rama Satyasri Kotipalli, Samatasai Patnaik, Kv Veena, Muralidharan Kathirvel, Rajkumar Vutukuri, and Manjula Bhanoori. 2024. "Sepia pharaonis Ink Mitigates Dehydroepiandrosterone-Induced Insulin Resistance in Mouse Model of Polycystic Ovarian Syndrome" Pathophysiology 31, no. 3: 408-419. https://doi.org/10.3390/pathophysiology31030031
APA StyleYamarthi, P., Kotipalli, R. S., Patnaik, S., Veena, K., Kathirvel, M., Vutukuri, R., & Bhanoori, M. (2024). Sepia pharaonis Ink Mitigates Dehydroepiandrosterone-Induced Insulin Resistance in Mouse Model of Polycystic Ovarian Syndrome. Pathophysiology, 31(3), 408-419. https://doi.org/10.3390/pathophysiology31030031






