Distraction Enterogenesis in Rats: A Novel Approach for the Treatment of Short Bowel Syndrome
Abstract
1. Introduction
2. Materials and Methods
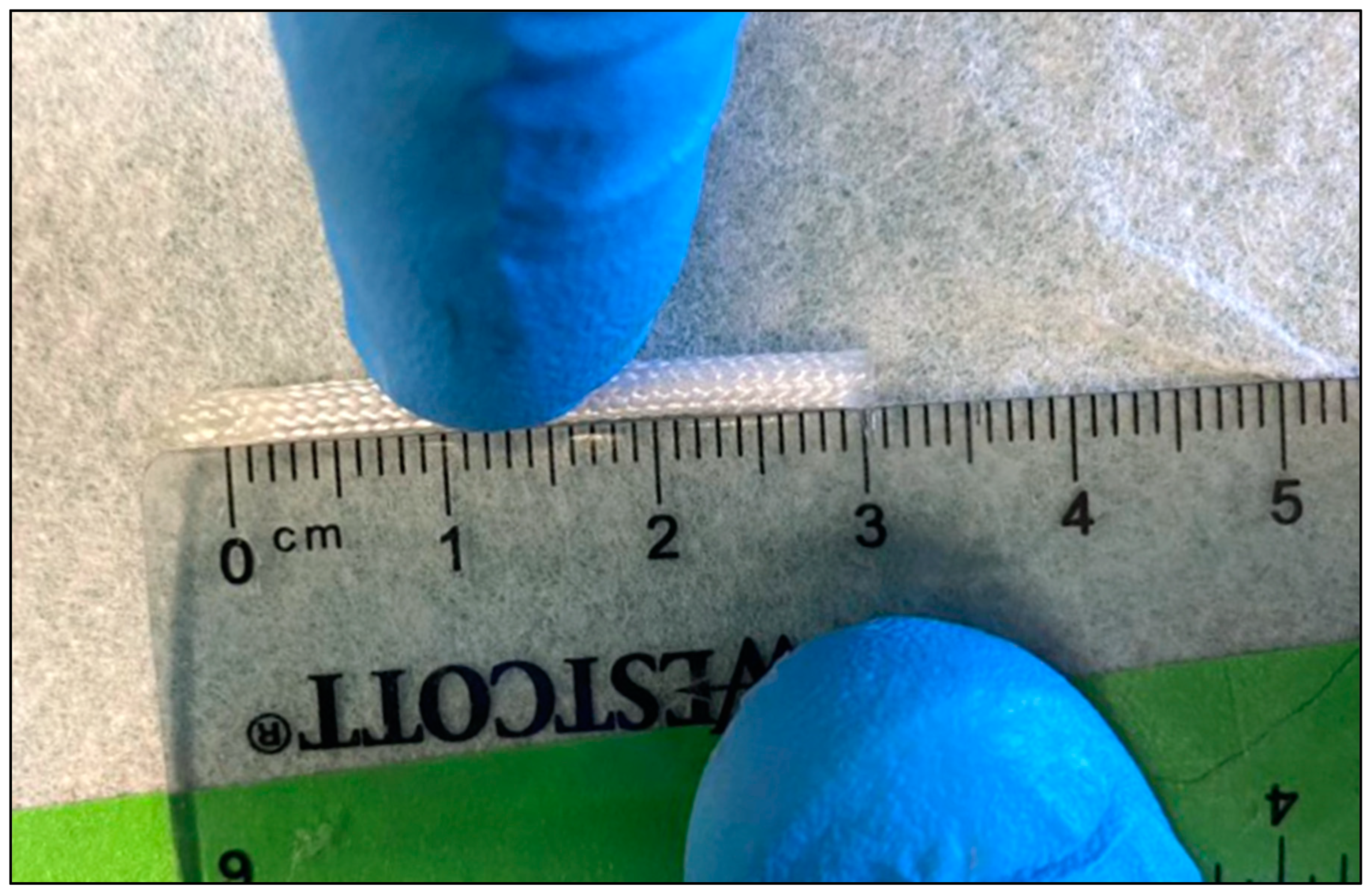
2.1. IES Distraction Force Characterization
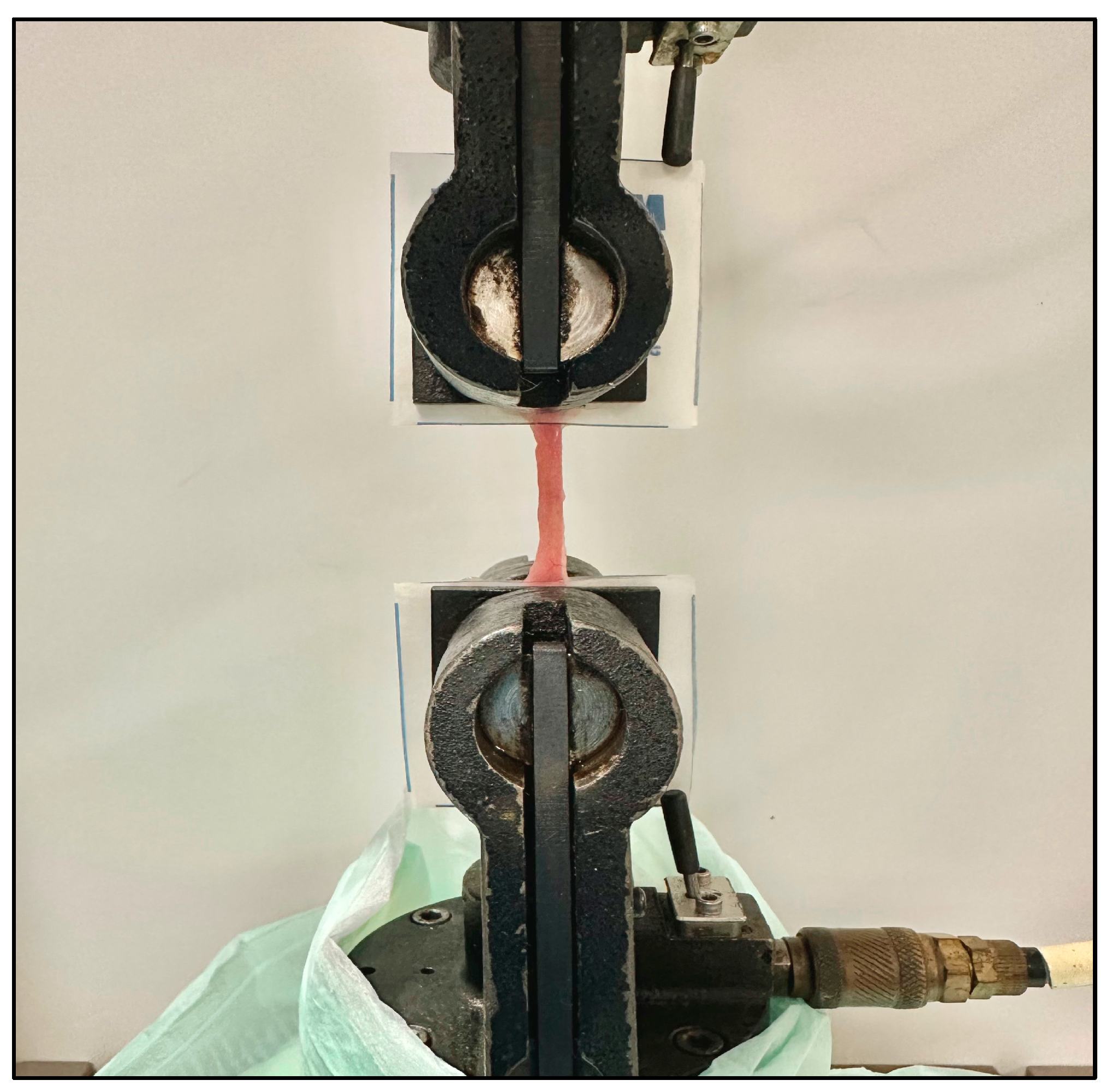
2.2. Small-Bowel Characterization
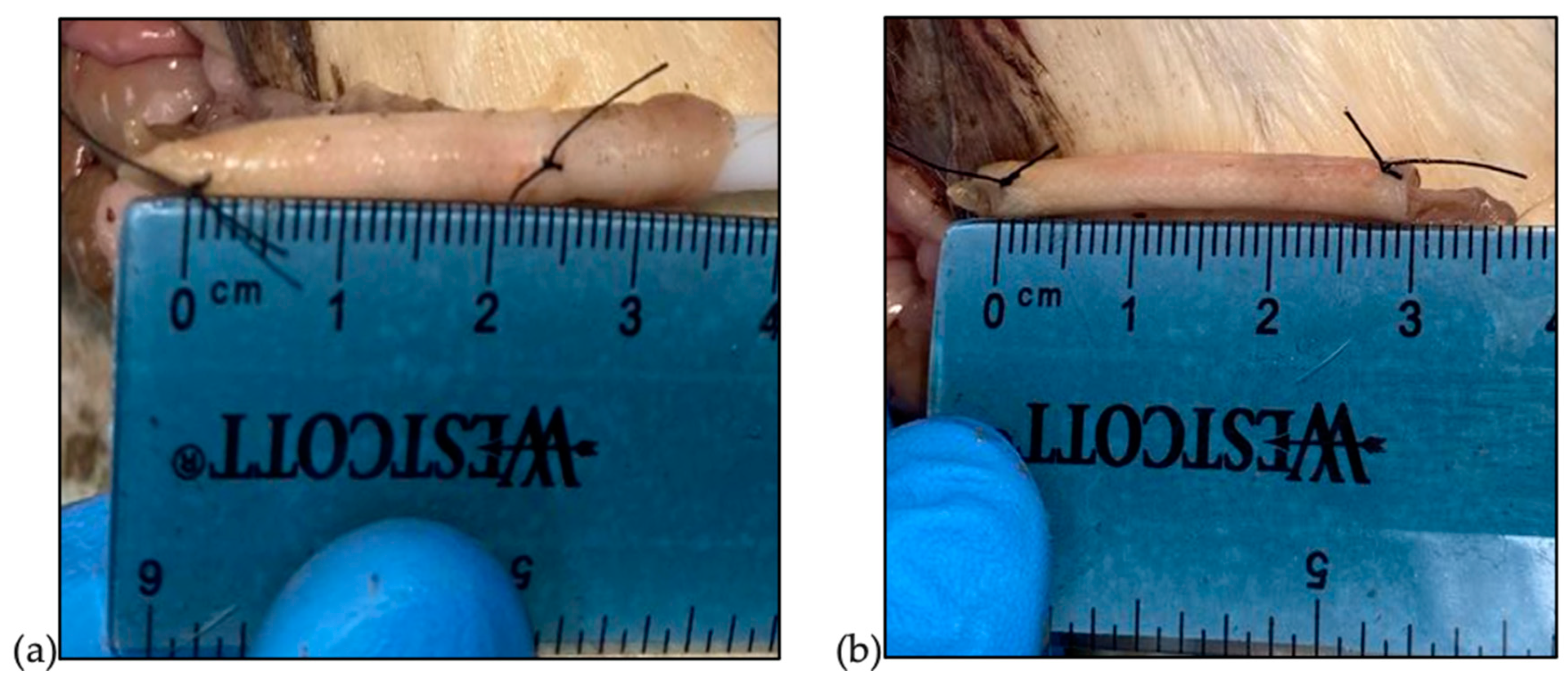
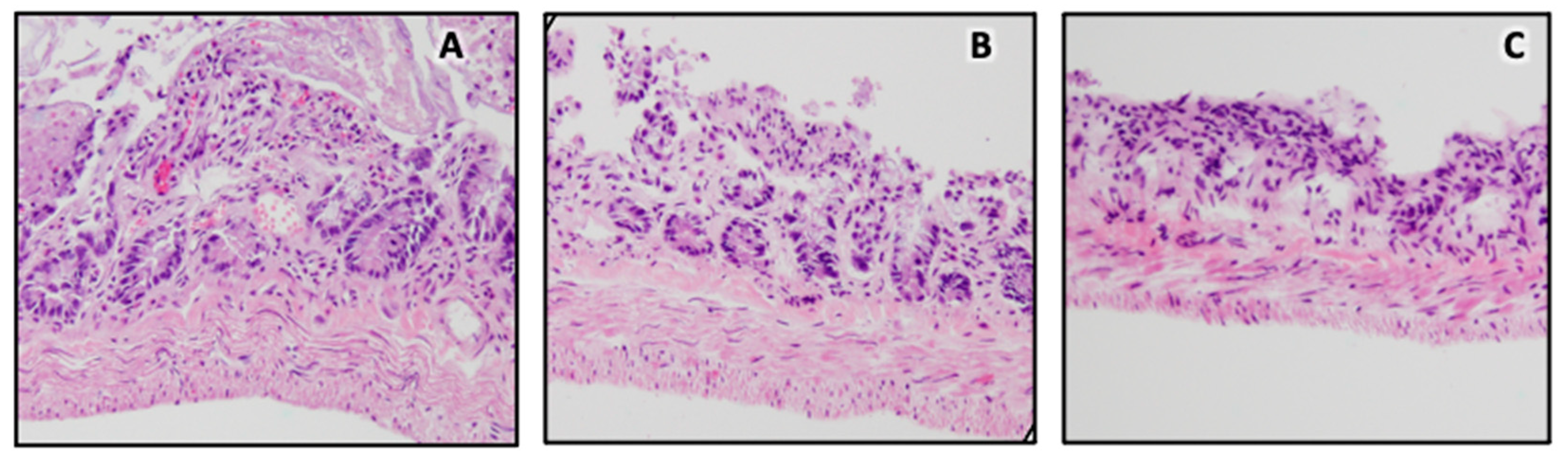
2.3. Ex Vivo Deployment and Histological Analysis
2.4. Statistical Analysis
3. Results
3.1. IES Distraction Force
3.2. Bowel Failure Load
3.3. Ex Vivo Deployment and Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guillen, B.; Atherton, N.S. Short Bowel Syndrome. [Updated 2022 July 26]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536935/ (accessed on 12 October 2023).
- Culbreath, K. Intestinal Failure; Pediatric Surgery NaT, American Pediatric Surgical Association: Terrace, IL, USA, 2022; APSA Webapp; Available online: www.pedsurglibrary.com/apsa/view/Pediatric-Surgery-NaT/829019/all/Intestinal_Failure (accessed on 10 January 2022).
- Spencer, A.U.; Neaga, A.; West, B.; Safran, J.; Brown, P.; Btaiche, I.; Kuzma-O’Reilly, B.; Teitelbaum, D.H. Pediatric short bowel syndrome: Redefining predictors of success. Ann. Surg. 2005, 242, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Negri, E.; Coletta, R.; Morabito, A. Congenital short bowel syndrome: Systematic review of a rare condition. J. Pediatr. Surg. 2020, 55, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Pironi, L. Definitions of intestinal failure and the short bowel syndrome. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, K.A. Intestinal adaptation following resection. J. Parenter. Enter. Nutr. 2014, 38, 23S–31S. [Google Scholar] [CrossRef] [PubMed]
- Calkins, K.L.; Venick, R.S.; Devaskar, S.U. Complications Associated with Parenteral Nutrition in the Neonate. Clin. Perinatol. 2014, 41, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Turpin, R.S.; Canada, T.; Liu, F.X.; Mercaldi, C.J.; Pontes-Arruda, A.; Wischmeyer, P. Nutrition therapy cost analysis in the US: Pre-mixed multi-chamber bag vs compounded parenteral nutrition. Appl. Health. Econ. Health Policy 2011, 9, 281–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rege, A. The Surgical Approach to Short Bowel Syndrome—Autologous Reconstruction versus Transplantation. Viszeralmedizin. 2014, 30, 179–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bianchi, A. Intestinal loop lengthening—A technique for increasing small intestinal length. J. Pediatr. Surg. 1980, 15, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Fauza, D.; Garza, J.; Oh, J.T.; Nurko, S.; Jaksic, T. Serial transverse enteroplasty (STEP): A novel bowel lengthening procedure. J. Pediatr. Surg. 2003, 38, 425–429. [Google Scholar] [CrossRef]
- King, B.; Carlson, G.; Khalil, B.A.; Morabito, A. Intestinal bowel lengthening in children with short bowel syndrome: Systematic review of the Bianchi and STEP procedures. World J. Surg. 2013, 37, 694–704. [Google Scholar] [CrossRef]
- Walker, S.R.; Nucci, A.; Yaworski, J.A.; Barksdale, E.M. The Bianchi procedure: A 20-year single institution experience. J. Pediatr. Surg. 2006, 41, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Lacaille, F.; Lambe, C. Intestinal Failure: Etiologies and Outcomes and Decision-Making Between Rehabilitation and Transplantation. In Solid Organ Transplantation in Infants and Children. Organ and Tissue Transplantation; Dunn, S., Horslen, S., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Ilizarov, G.A.; Devyatov, A.A.; Kamerin, V.K. Plastic reconstruction of longitudinal bone defects by means of compression and subsequent distraction. Acta Chir. Plast. 1980, 22, 32–41. [Google Scholar]
- Demehri, F.R.; Stephens, L.; Herrman, E.; West, B.; Mehringer, A.; Arnold, M.A.; Brown, P.I.; Teitelbaum, D.H. Enteral autonomy in pediatric short bowel syndrome: Predictive factors one year after diagnosis. J. Pediatr. Surg. 2015, 50, 131–135. [Google Scholar] [CrossRef]
- Billiauws, L.; Maggiori, L.; Joly, F.; Panis, Y. Medical and surgical management of short bowel syndrome. J. Visc. Surg. 2018, 155, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Printz, H.; Schlenzka, R.; Requadt, P.; Tscherny, M.; Wagner, A.C.C.; Eissele, R.; Rothmund, M.; Arnold, R.; Göke, B. Small bowel lengthening by mechanical distraction. Digestion 1997, 58, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Clayton, S.; Alexander, J.S.; Solitro, G.; White, L.; Villalba, S.; Winder, E.; Boudreaux, M.; Veerareddy, P.; Dong, E.; Minagar, A.; et al. Self-expanding intestinal expansion sleeves (IES) for short gut syndrome. Pediatr. Surg. Int. 2022, 38, 75–81. [Google Scholar] [CrossRef]
- Spencer, A.U.; Sun, X.; El-Sawaf, M.; Haxhija, E.Q.; Brei, D.; Luntz, J.; Yang, H. Enterogenesis in a clinically feasible model of mechanical small-bowel lengthening. Surgery 2006, 140, 212–220. [Google Scholar] [CrossRef]
- Fisher, J.G.; Sparks, E.A.; Khan, F.A.; Dionigi, B.; Wu, H.; Brazzo, J., III; Fauza, D.; Modi, B.; Safranski, D.L.; Jaksic, T. Extraluminal distraction enterogenesis using shape-memory polymer. J. Pediatr. Surg. 2015, 50, 938–942. [Google Scholar] [CrossRef]
- Huynh, N.; Rouch, J.D.; Scott, A.; Chiang, E.; Wu, B.M.; Shekherdimian, S.; Dunn, J.C. Spring-mediated distraction enterogenesis in-continuity. J. Pediatr. Surg. 2016, 51, 1983–1987. [Google Scholar] [CrossRef]
- Dubrovsky, G.; Huynh, N.; Thomas, A.L.; Shekherdimian, S.; Dunn, J.C. Intestinal lengthening via multiple in-continuity springs. J. Pediatr Surg. 2019, 54, 39–43. [Google Scholar] [CrossRef]
- Sullins, V.F.; Scott, A.; Wagner, J.P.; Steinberger, D.; Lee, S.L.; Wu, B.M.; Dunn, J.C.Y. Intestinal lengthening in an innovative rodent surgical model. J. Pediatr. Surg. 2014, 49, 1791–1794. [Google Scholar] [CrossRef] [PubMed]
- Diyaolu, M.; Thomas, A.L.; Wood, L.S.; Taylor, J.; Dunn, J.C. Mesenteric neovascularization during spring-mediated intestinal lengthening. J. Pediatr. Surg. 2021, 56, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Safford, S.D.; Freemerman, A.J.; Safford, K.M.; Bentley, R.; Skinner, M.A. Longitudinal mechanical tension induces growth in the small bowel of juvenile rats. Gut 2005, 54, 1085. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Sun, X.; Yang, H.; Nose, K.; Somara, S.; Bitar, K.N.; Owyang, C.; Okawada, M.; Teitelbaum, D.H. Distraction-induced intestinal enterogenesis: Preservation of intestinal function and lengthening after reimplantation into normal jejunum. Ann. Surg. 2012, 255, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Okawada, M.; Maria, H.M.; Teitelbaum, D.H. Distraction induced enterogenesis: A unique mouse model using polyethylene glycol. J. Surg. Res. 2011, 170, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, D.W.; Robinson, M.K. Short bowel syndrome. World J. Surg. 2000, 24, 1486–1492. [Google Scholar] [CrossRef]
- Ralls, M.W.; Sueyoshi, R.; Herman, R.S.; Utter, B.; Czarnocki, I.; Si, N.; Luntz, J.; Brei, D.; Teitelbaum, D.H. Mesenteric neovascularization with distraction-induced intestinal growth: Enterogenesis. Pediatr. Surg. Int. 2013, 29, 33–39. [Google Scholar] [CrossRef]


| Device Compression | Average Expansive Force [N] |
|---|---|
| 50% | 2.80 ± 0.36 |
| 40% | 0.36 ± 0.02 |
| 30% | 0.22 ± 0.01 |
| 20% | 0.13 ± 0.01 |
| 10% | 0.06 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Quin, C.; Clayton, S.D.; Trosclair, L.; Meyer, H.; Dao, N.H.; Minagar, A.; White, L.; Welch, V.; Solitro, G.; Alexander, J.S.; et al. Distraction Enterogenesis in Rats: A Novel Approach for the Treatment of Short Bowel Syndrome. Pathophysiology 2024, 31, 388-397. https://doi.org/10.3390/pathophysiology31030029
O’Quin C, Clayton SD, Trosclair L, Meyer H, Dao NH, Minagar A, White L, Welch V, Solitro G, Alexander JS, et al. Distraction Enterogenesis in Rats: A Novel Approach for the Treatment of Short Bowel Syndrome. Pathophysiology. 2024; 31(3):388-397. https://doi.org/10.3390/pathophysiology31030029
Chicago/Turabian StyleO’Quin, Collyn, Sean D. Clayton, Lexus Trosclair, Hannah Meyer, Nhi H. Dao, Andrew Minagar, Luke White, Valerie Welch, Giovanni Solitro, Jonathan Steven Alexander, and et al. 2024. "Distraction Enterogenesis in Rats: A Novel Approach for the Treatment of Short Bowel Syndrome" Pathophysiology 31, no. 3: 388-397. https://doi.org/10.3390/pathophysiology31030029
APA StyleO’Quin, C., Clayton, S. D., Trosclair, L., Meyer, H., Dao, N. H., Minagar, A., White, L., Welch, V., Solitro, G., Alexander, J. S., & Sorrells, D. (2024). Distraction Enterogenesis in Rats: A Novel Approach for the Treatment of Short Bowel Syndrome. Pathophysiology, 31(3), 388-397. https://doi.org/10.3390/pathophysiology31030029









