1. Introduction
Multiple sclerosis (MS) is a neurodegenerative disease associated with inflammation in the central nervous system [
1]. A total of 2.3 million people worldwide suffer from this disease, and environmental and genetic factors contribute to the development of MS [
2]. Magnetic resonance imaging (MRI) can detect sites of neurological lesions in the brain, often correlated with the clinical symptoms seen in patients. In most cases of MS, initial clinical symptoms are intermittent, and then progress to a series of chronic events, typically diagnosed as relapsing–remitting MS (RRMS) [
1]. In MS, the immune system destroys the insulating sheath (myelin) enveloping nerve fibers, which impairs the efficiency of nerves in communicating and transmitting messages, usually at a greater energy expenditure [
3]. There are no standard symptoms exhibited across all patients with MS; rather, symptoms depend on the severity of damage to white matter, and the locations of the affected nerves. Most patients diagnosed with MS (85%) initially have RRMS and experience exacerbation of new symptoms followed by a period of disease remission that can be brief or can last for several years [
3]. Symptoms of MS commonly include limitation of movement with numbness or weakness in one side of the body at a time, diminished coordination, and vision problems. Approximately 50% of patients with relapsing–remitting MS endure a steady progression of intensifying symptoms with each relapse [
3]. Although most individuals experience periods of disease remission, others have no remission throughout the process of symptom development. Instead, over time, up to 15% of MS patients will suffer irreversible progression of disease, termed secondary progressive MS (SPMS).
A dysregulation of the blood–brain barrier (BBB) is one of the first indications of cerebrovascular abnormalities seen in people diagnosed with MS [
4]. The BBB is an organized complex, including cerebral endothelial cells, supporting pericytes, astrocytes, and glia, all of which govern the exchange of solutes and immune cells between the blood and the brain. MS progression disrupts the function of the BBB, resulting in abnormal inflammatory responses of the central nervous system and leading to vascular injury in the brain [
5]. Current therapeutic measures can treat flare-ups in MS, but once a patient’s diagnosis progresses to SPMS, there are fewer treatments which can arrest progressive neurodegeneration. Standard methods of MS diagnosis use MRI and spinal fluid analysis; however, these do not always provide accurate assessments of MS prognosis and do not distinguish between RRMS and SPMS. Early identification of easily measurable biomarkers in MS patients might allow for earlier diagnosis of MS before irreversible neurodegenerative deterioration occurs.
The purpose of this study was to evaluate changes in the sulfide-generating enzymes (CSE, CBS, and MST) in brain endothelial cells and in apical and basolateral microparticles (AMPs, BMPs) derived from these cells when exposed to inflammatory cytokines, as a model of MS inflammation. Evidence of proinflammatory responses initiated by the circulation of AMPs and BMPs has prompted the investigation of the presence, and the measurement of the levels, of sulfide-producing enzymes in the vascular space in order to determine whether there may be a correlation between increased levels of hydrogen sulfide and forms of neurodegeneration. This study was prompted by our findings in another form of neurodegeneration, Alzheimer’s disease, where levels of circulating sulfides were significantly elevated in comparison with controls. Sulfides can be generated by CSE, CBS, and MST. Therefore, we sought to further study this in MS by evaluating the extent to which the levels of these circulating sulfide-generating enzymes are changed in serum from MS patients. Our goal was to identify a novel MS biomarker for diagnosing and possibly staging disease progression in MS.
A key feature of MS is neuroinflammation [
6]. At sites of inflammation, e.g., in response to cytokines, brain endothelial cells may increase their release of MPs [
7]. Brain endothelial cells release AMPs into the vascular space and BMPs into the perivascular space. Endothelial AMPs are regarded as biomarkers for vascular inflammation in the brain [
7]. Furthermore, the circulation of endothelial adhesion molecules and other proteins in various MPs derived from the parent cells may play an active role in the progression of MS by enhancing the proinflammatory responses of this disease [
6]. Evidence of MP accumulation in the brain parenchyma suggests BMPs may also contribute to the progression of neurovascular inflammatory diseases. In support of this concept, we found that both AMPs and BMPs derived from cytokine-stimulated cells depress the contractility of brain vascular smooth muscle cells [
6]. In MS, evaluation of lymphatic biomarkers in serum samples of patients with RRMS and SPMS revealed that human brain endothelial cells release neurolymphatic biomarkers within MPs [
8]. These studies provide evidence that endothelial cells may intensify inflammation via MPs and support the development of these particles as important biomarkers and mediators of MS disease activity.
Hydrogen sulfide plays important roles in the regulation and vascular homeostasis and pathophysiological processes in the cerebral vasculature [
9,
10]. Recent research shows that sulfides may be dysregulated in dementia and could represent a possible biomarker for diagnosing neurovascular disturbances [
9]. Cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfur transferase (MST) are three enzymes known to produce H
2S [
11,
12]. All three enzymes are expressed in the brain, with MST being primarily responsible for H
2S generation in the central and peripheral nervous systems. CBS is also expressed in the brain parenchyma, while CSE is the primary enzyme found in the brain vasculature [
10,
13]. CSE and CBS are cytosolic enzymes, whereas MST is localized in the mitochondria. H
2S has been shown to reduce expression of many proinflammatory cytokines, chemokines, and enzymes in endothelial cells [
14]. In the central nervous system, H
2S increases the activity of NMDA receptors, which are critical for memory retention, and alleviates oxidative stress by modulating reactive oxidative species (ROS), commonly found at sites of inflammation in the brain [
15]. Contrary to these beneficial functions of H
2S in the vascular systems, evidence shows neurological stress and vascular dysfunction can be provoked by sulfide metabolites of H
2S [
9]. It is plausible that, like another gasotransmitter, nitric oxide, H
2S, and its metabolites may be beneficial at low concentrations, but at high levels they may accumulate and intensify the progression of neurodegenerative diseases [
16,
17,
18,
19]. In addition, sulfides exist in different forms, which may contribute to their protective/deleterious properties. Importantly, our group has reported that endothelial CSE appears to be a prominent source of polysulfides in tissues. In CSE-deficient mice, we found a
decrease in blood–brain barrier permeability, indicating that CSE, a primary H
2S-producing enzyme in the endothelium, may diminish barrier function [
18]. Furthermore, we showed that elevated sulfide in the circulation serves as a biomarker for disease activity (cognitive impairment) and vascular disturbances (lesion volume) seen in Alzheimer’s disease and related dementias (ADRD) [
9]. Lesion volume is also used to assess MS severity because it relates to “bradyphrenia”, which can slow thoughts and delay responses in neurodegenerative diseases and disorders [
20].
Although there are treatments to suppress the severity of MS symptoms, no methods completely arrest the degenerative processes seen in MS, and early intervention and treatment of RRMS prior to progression into secondary progressive MS is a keystone of MS therapy. MRI imaging and spinal fluid analysis can be inaccurate (and delayed) predictors of MS progression. Therefore, we sought to evaluate whether sulfide-generating enzymes can be used as biomarkers for MS, possibly representing early inflammatory changes in the brain vasculature. Our measurement of these enzymes released into AMPs or BMPs provide further insight into how they may serve as a circulating biomarker of what is delivered into the brain parenchyma by inflamed endothelial cells and could help to explain how endothelial inflammation provokes perivascular inflammatory phenomena. Once validated, biomarkers originating from brain endothelial cells themselves could provide a method for identifying sulfide-producing enzymes as they relate to MS. Further investigations may help to specify which enzyme or panel of enzymes in combinations may be present that contribute to the central sulfide burden of the brain. Establishment of a simple and standardized procedure for identifying these enzymes as biomarkers in relapsing–remitting and secondary progressive MS could reinforce imaging and clinical/behavioral studies.
2. Materials and Methods
2.1. Clinical Specimens
Serum samples were collected under an IRB (‘MS-Omics project RQ00328′, HSIRB 435893-2, 9/6/13). Patients diagnosed with RRMS and SPMS and healthy control (HC) sample patients were enrolled at the Department of Neurology in the University of Buffalo, Buffalo, NY [
8] (
Table 1). Inclusion criteria were ages between 18 and 80 years with a diagnosis of RRMS or SPMS. MS was diagnosed according to the McDonald criteria and an MRI examination was performed less than 30 days of the clinical examination with the standardized study protocol. Patients were also required to have scores within the range of 0–8.5 on the Expanded Disability Status Scale (EDSS). Exclusion criteria included individuals who experienced a relapse or exacerbation of symptoms, had steroid treatment within less than 30 days of the start of this study, and had preexisting conditions associated with non-MS brain pathology or pregnancy. Healthy control subjects were obtained through hospital recruitment and advertisements. Physical examinations were performed, and individuals screened for autoimmune diseases, environmental and vascular risks, and personal habits capable of confounding this investigation and future studies. Blood was collected from all subjects, and MS serum samples were derived from blood collected from patients diagnosed with RRMS and SPMS. Serum is the remaining fluid following the centrifugation of blood to remove clots and blood cells. Frozen MS serum samples were collected from −80 degrees Celsius.
2.2. Cell Culture
A polarized human cerebral microvascular endothelial cells line (hCMEC/D3) was cultured in flasks coated with rat tail collagen type I (0.1 mg/mL). The cell line was provided by Dr. Pierre-Oliver Couraud, Inserm, Paris, France. Growth medium consisted of an EndoGRO™—MV complete media kit from MiliporeSigma in Burlington, MA, USA. Cells in this experiment were cultured at 37 °C in 5% CO2, and passages between 28 and 34 were used for experiments.
2.3. Cytokine Treatment
To evaluate endothelial microparticles, hCMEC/D3 were placed in 6-well plates with 3 µm inserts in complete growth media. A measure of 2 mL of medium was added to the bottom (basolateral chamber) of each well, and 1.5 mL of medium was added to the top (apical chamber) of the insert where cells were placed. Following a period of 48 h, cells reached confluency and media in both compartments were replaced with media containing 1000 U/mL of interferon-gamma (IFN-γ,) and/or 20 ng/mL of tumor necrosis factor-alpha (TNF-α) (T/I) or control medium. TNF-α and IFN-γ are cytokines known to play a role in multiple sclerosis [
21]. Media were separately removed from apical and basolateral compartments to isolate MP at 72 h following treatment. All experiments were normalized to the surface area of D3 cells used to produce MPs.
2.4. Isolation of Endothelial Microparticles from D3 Cells
We isolated endothelial “microparticles” which are released from endothelial cells based on a previous approach that used calibrating microparticle flow cytometric analysis using 0.5, 1, and 2 μm FluorsbriteTM Yellow Green Microspheres (Polysciences, Warrington, PA, USA) [
6]. Logarithmic-scale side-scatter plots of sizing beads were used to determine appropriate gating for the samples and found that particles isolated in this method were in the range of 0.5–1 μm in size. Therefore, we refer to these as “microparticles” because apoptotic bodies are larger in size (up to 5 μm) and exosomes are much smaller (smaller than 150 nm). We have only evaluated these particles and cannot exclude possible contributions of these other particle types which may be present and active in vivo.
Following exposure of D3 cells to control medium or medium supplemented with IFN-γ/TNF-α, supernatants from the apical and basolateral chambers were centrifuged at 400× g for 10 min at 4 °C. Resulting supernatants were transferred to fresh micro centrifuge tubes and were centrifuged again at 20,800× g for 1 h at 4 °C to pellet AMPs or BMPs from apical and basolateral chambers, respectively. Supernatants were aspirated and MP pellets were washed twice by centrifugation using 4 °C PBS plus 1 mM phenylmethylsulfonyl fluoride (PMSF) at 20,800× g for a period of 15 min at 4 °C. Supernatants were then discarded and MP pellets were stored at −80 °C. Cells were lysed in 200 µL of Laemmli sample buffer plus 1 mM PMSF, sonicated, and stored at −80 °C until immunoblotted.
2.5. Blotting Analysis
Frozen MP, cell, and MS serum samples were thawed, and 0.5 μL of each of the samples was loaded onto nitrocellulose membranes and left to dry overnight. Ponceau S staining was used for protein loading standardization. A measure of 5% milk and TBST were used to block (2 h) and rinse the blots (3X) after staining. Membranes were immunoblotted for cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfur transferase (MST) and incubated in Clarity Western Peroxide Reagent and visualized using Clarity Western Luminol/Enhancer Reagent (Biorad, Hercules, CA, USA). All primary antibodies were used at 1:500 dilution and incubated overnight. Secondary antibodies were used at 1:2000 dilution and incubated for 1 h at room temperature before washing and ECL reactions.
ChemiDoc imaging system (Biorad, Hercules, CA, USA) was used to develop images of membranes. Densitometry was performed using ImageJ analysis software, v.153 (NIH, Bethesda, MD, USA). Data were normalized to total protein using Ponceau S Staining (Sigma biochemicals, St. Louis, MO, USA).
2.6. Antibodies
Rabbit anti-Human Cystathionine-gamma-lyase (CSE) Polyclonal Antibody (cat. No. MBS2014844, MyBioSource, San Diego, CA, USA), Cystathionine-beta-synthase (CBS) (N-Term) Antibody (cat. No. ABIN629598, Antibodies Online, Limerick, PA, USA), Recombinant Anti-MST3 Antibody [EP1468Y] (cat. No. Ab51137, Abcam, Cambridge, MA, USA), and Anti-Rabbit IgG (whole molecule)-Peroxidase antibody produced in goat (cat. No. 028M4755V, Sigma-Aldrich, St. Louis, MO, USA) were used in blotting analysis procedures.
2.7. Statistical Analysis
Statistical analyses were performed on all datasets using a basic Student’s unpaired, two-sided
t-test. This analysis compared control samples to the cytokine-treated microparticle and cell samples in
Figure 1,
Figure 2 and
Figure 3. In
Figure 4, the t-test compared HC to RRMS and SPMS condensed data (MS serum samples). The distribution of the enzymes within healthy control and RRMS and SPMS samples was characterized using violin plots (
Figure 4); these were generated based on the results of the corresponding statistical test using the vioplot package for the R statistical language [
22]. A Kruskal–Wallis one-way analysis of variance (ANOVA) performed on condensed (MS), and uncondensed (RRMS and SPMS) datasets allow for comparison of the respective datasets to the HC samples.
4. Discussion
Among multiple neurovascular and cardiovascular diseases, MPs have been investigated for their potential as biomarkers for disease states. MPs also influence disease progression through their ability to induce signaling in recipient or target cells. In previous research, endothelial MPs were shown to be produced more abundantly in response to inflammatory cytokine treatment [
6]. We have previously observed that these human brain endothelial cells are polarized in nature and release AMPs and BMPs which are formed by apparently different cellular processes, and which carry different types of “cargo” derived from the parent cell lines [
6]. In that study, exposure to inflammatory cytokines did not change AMP diameters; however, the production of BMPs was increased whereas the BMPs formed were smaller, which increased their total surface area to favor BMP binding to target cells. In the same study it was found that BMPs from cytokine-treated endothelial cells impaired contractility of brain vascular smooth muscle cells. Such effects could detrimentally influence neurovascular perfusion. Furthermore, AMPs from cytokine-treated cells disrupted brain endothelial barrier function. Thus, the release of microparticles into both the sub-endothelial or “perivascular” space and into the circulation under inflammatory conditions induce pathological changes (
Figure 6). As part of these responses, changes in expression of CSE, CBS, and MST in the AMPs, BMPs, and human endothelial cells may contribute to the pathophysiology of MS and potentially other neurovascular conditions. While intriguing, we cannot claim that this model system exactly recapitulates the in vivo blood–brain barrier, but it may be a valuable first step towards understanding how these particles are formed and distributed in the vasculature.
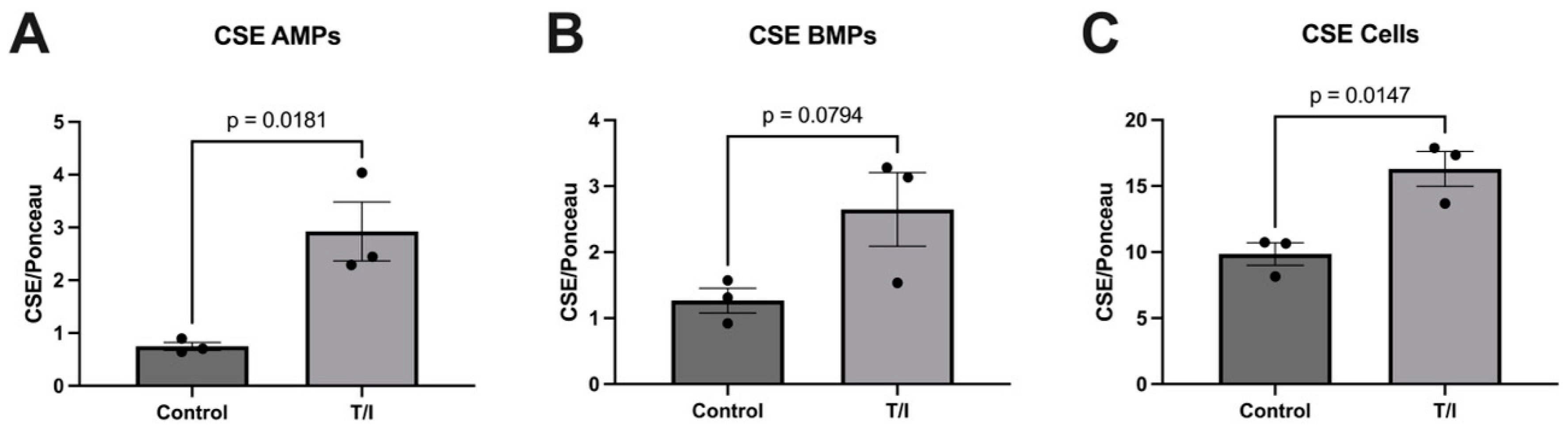
Based on prior investigations showing CSE, CBS, and MST in the vascular space, we explored whether inflammatory mediators increase expression of sulfide-generating enzymes in brain endothelial cells, as well as in their apically and basolaterally released MPs. Our blotting analysis results showed significant elevations in the expression of CSE in response to inflammatory cytokine stress (combined TNF/IFN—‘T/I’) compared with non-treated control samples. This increase in CSE expression in inflamed endothelial cells could help explain the elevation in circulating sulfides seen in neurodegenerative conditions, such as Alzheimer’s disease, which has also been ascribed to CSE activity [
9]. Importantly, the elevated sulfides in AD have been linked to the associated vascular stress and mediate half of the relationship between the microvascular abnormalities and cognitive impairment in this condition. These results suggest that in response to inflammatory stress, CSE is expressed by endothelial cells and is released into microparticles which may help drive elevated sulfides in AD and perhaps other neurodegenerative conditions. However, this is not reflected in MS, where the CSE levels were not elevated in the serum, thus revealing heterogeneity in sulfide metabolism between neurodegenerative diseases.
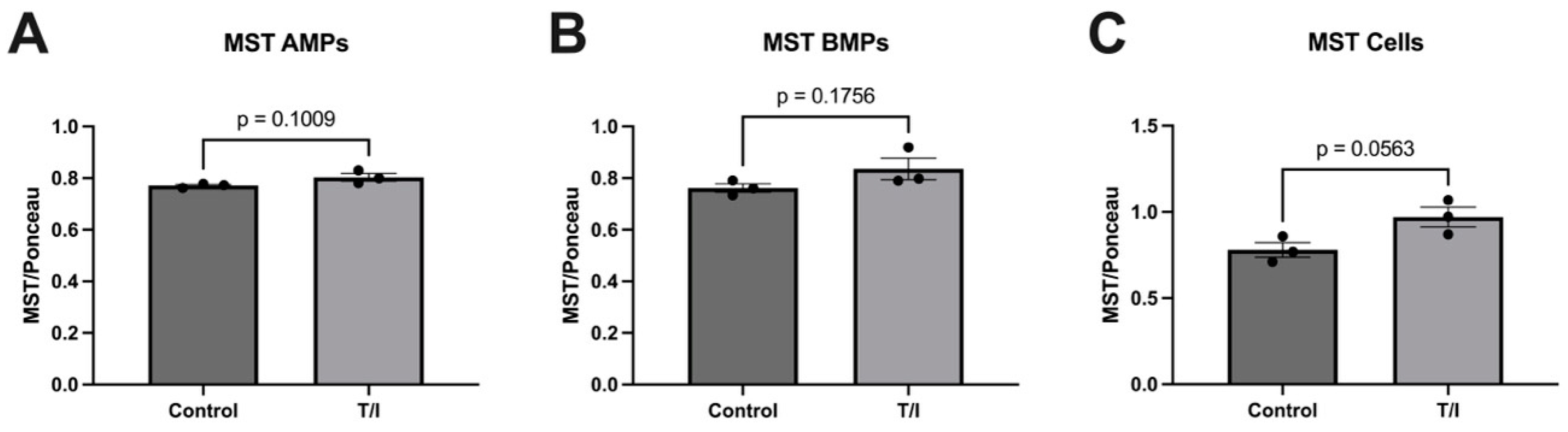
Our results also show that CBS may also be released from cells into both apical and basolateral MPs in response to cytokine treatment. This does not result in a loss of CBS from the endothelial cells, but rather the cells maintained a steady state of this enzyme. In contrast, the MST levels remained largely unchanged, with just minor increases in all samples. The fact that both CSE and CBS are released into MPs on both sides of the endothelial cells suggests that the expression of both enzymes in the blood may reflect release of CSE and CBS towards the brain, into the perivascular space. Together, our in vitro data highlight the differential regulation of expression and packaging into MPs of the three sulfide-generating enzymes in brain endothelial cells. We suggest further analysis should be performed to better understand their contributions to neurovascular/neurodegenerative diseases that have inflammatory components.
Once we determined that cytokines can regulate the release of sulfide-generating enzymes, in particular CBS and CSE, into the “vascular” space, i.e., AMPs, we next tested whether any of the enzymes may be suitable biomarkers for MS. Next, we sought to determine whether our findings from our brain endothelial cell study reflect what is seen in the serum of patients with MS. However, we saw only a modest increase in CSE expression (
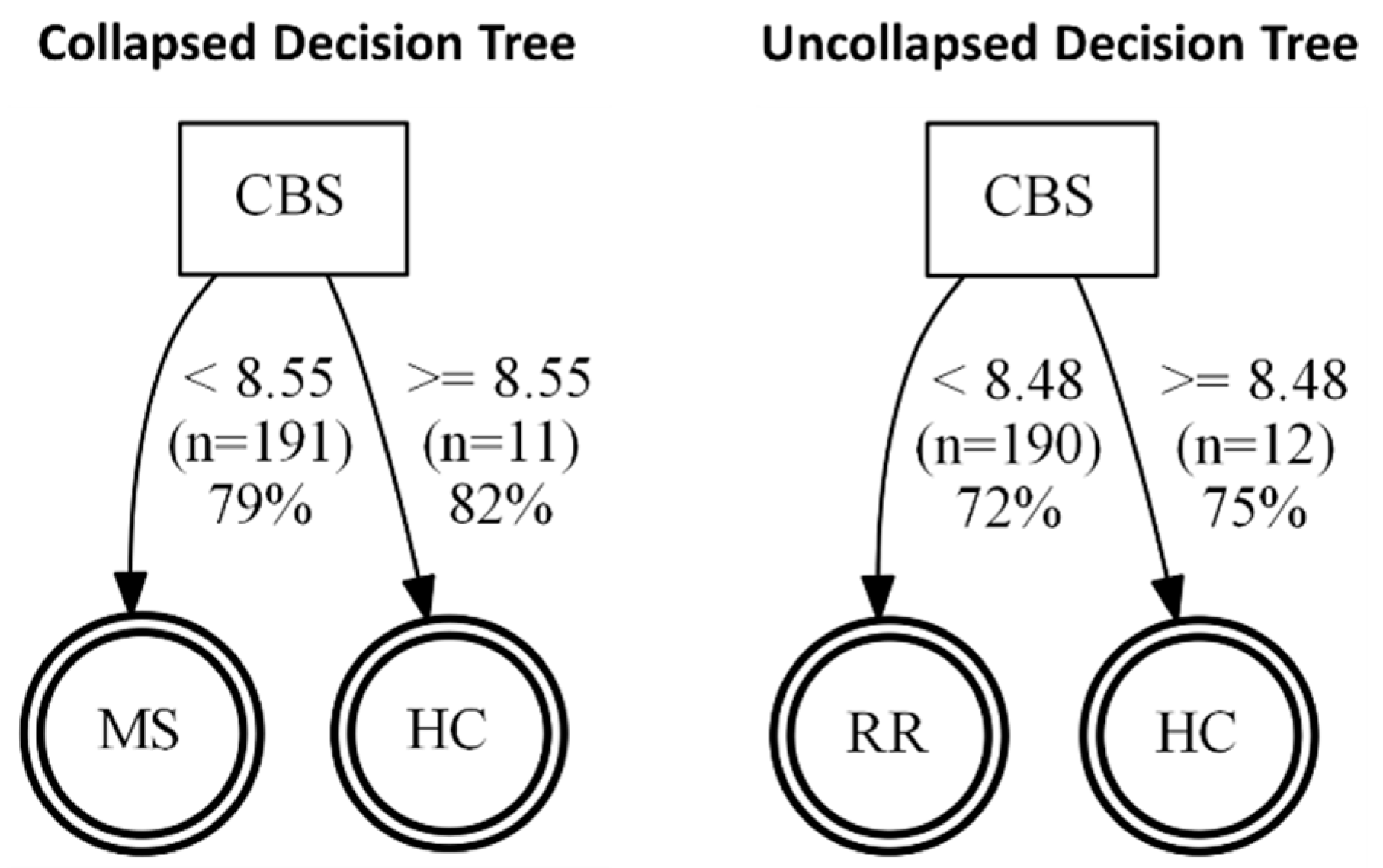
p = 0.0605) in MS (both RRMS and SPMS considered together as “collapsed”). When RRMS and SPMS were separated, this trend was not significant. We next considered changes in CBS expression and found remarkable reductions in CBS in the “collapsed” MS (RRMS+SPMS), which was still found to be significant when SPMS and RRMS were considered individually (“uncollapsed”). Using a decision tree analysis, this surprising reduction in serum CBS levels (
Figure 5) was found to predict MS disease activity, and specifically RRMS disease activity, with 82% and 75% predictive accuracy, respectively. While MST was also significantly reduced in “collapsed” and “uncollapsed” MS, we found that considering MST as a diagnostic “tool” did not improve the predictive power for anticipating MS over that of CBS alone. Taken together, our results support that modest mobilization of CSE, and “shedding” into serum, as well as a very significant reduction in circulating CBS (and MST), may indicate a signature pattern of sulfide dysregulation in MS. While there may be a role suggested for elevated CSE in AD, here we found that reductions in CBS, and to a lesser extent MST, better characterize, and may influence, MS disease activity.
We hypothesize that vascular dysfunction in MS appears to involve diminished levels of CBS and MST in the brain [
24] which may increase oxidative stress in the neurovasculature. In MS, increased brain oxidative stresses, particularly within the vascular and parenchymal spaces, could intensify disease progression through a reduction in protective sulfides generated in different brain “compartments” (neural/glial/vascular) which fail to limit oxidant clearance. Inflammatory stresses could therefore dysregulate brain sulfides in MS patients, who typically have a high burden of reactive oxidative species that may exacerbate cerebrovascular stress. Reductions in these enzymes could also diminish circulating sulfides to decrease cerebral blood flow as a mechanism of MS disease activity.
5. Conclusions
This study evaluated changes in protein levels of CSE, CBS, and MST in a polarized, immortalized human brain endothelial cell model and the apical and basolateral MPs generated by the cells [
25] with the goal of determining how sulfide-producing enzymes may govern the initiation and progression of RRMS and SPMS. MS is a neurodegenerative and neurovascular disease whose exacerbations are commonly associated with “cytokine storm” and inflammation in the central nervous system. The progression of MS disrupts brain vascular functions leading to inflammatory responses in the central nervous system, which results in vascular injury in the brain that can intensify neurodegeneration. There are limited treatments available to slow down or arrest the neurodegenerative processes in patients with RRMS and SPMS, with better efficacy when treatment is started in the early stages of the disease. Therefore, identification of early biomarkers is critical for therapeutic intervention. Prior evidence of proinflammatory cytokines initiating the release of endothelial AMPs and BMPs [
6] prompted us to investigate the presence of sulfide-producing enzymes in brain endothelial cells and their MPs. The discrepancies we see between the protein levels of CSE, CBS, and MST in serum seen in vivo versus those released by brain endothelial cells in vitro suggest that endothelial cells may contribute to the CSE elevations, but perhaps not to the reductions in CBS and MST. Therefore, endothelial responses may not be the only source influencing the results seen in MS patient serum. Alternatively, the differences may be due to the acute nature of the cell culture model versus the chronic timeline of the disease. Additionally, different cellular sources such as nerves, glia, and smooth muscle may represent alternative sources of these enzymes, which would affect serum levels.
Identification of CSE, CBS, and MST in serum, human brain endothelial cells, and their AMPs and BMPs now permits investigation of sulfide-producing enzymes in MS and other forms of neurodegeneration using inexpensive and simple serum testing. The dramatic reductions in CBS and MST suggest that, rather than endothelial cells, neuronal and mitochondrial sulfide hypo-expression may be important in MS pathophysiology. Conversely, the observation that CSE expression is also increased in serum and in endothelial cells could influence MS but to a lesser extent. By developing standardized tests for MS using these biomarkers, MS might be detected prior to advanced neurodegeneration, prompting treatment to arrest MS progression earlier.
While this study found remarkable changes in serum sulfide-producing enzymes, it is not yet clear whether the products of these enzymes, namely circulating sulfides, are in fact elevated in MS, as they have been reported to be increased in AD [
9]. Future studies comparing the serum levels of sulfides with their synthetic machinery may provide a clearer picture of these relationships. However, we have now demonstrated successfully for the first time that reductions in serum CBS (and MST) appear to represent useful markers of MS disease activity.