The Expression Level of ABCC6 Transporter in Colon Cancer Cells Correlates with the Activation of Different Intracellular Signaling Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Viability Assay
2.3. Migration Assay
2.4. Western Blot
2.5. Real-Time PCR
2.6. Statistical Analysis
3. Results
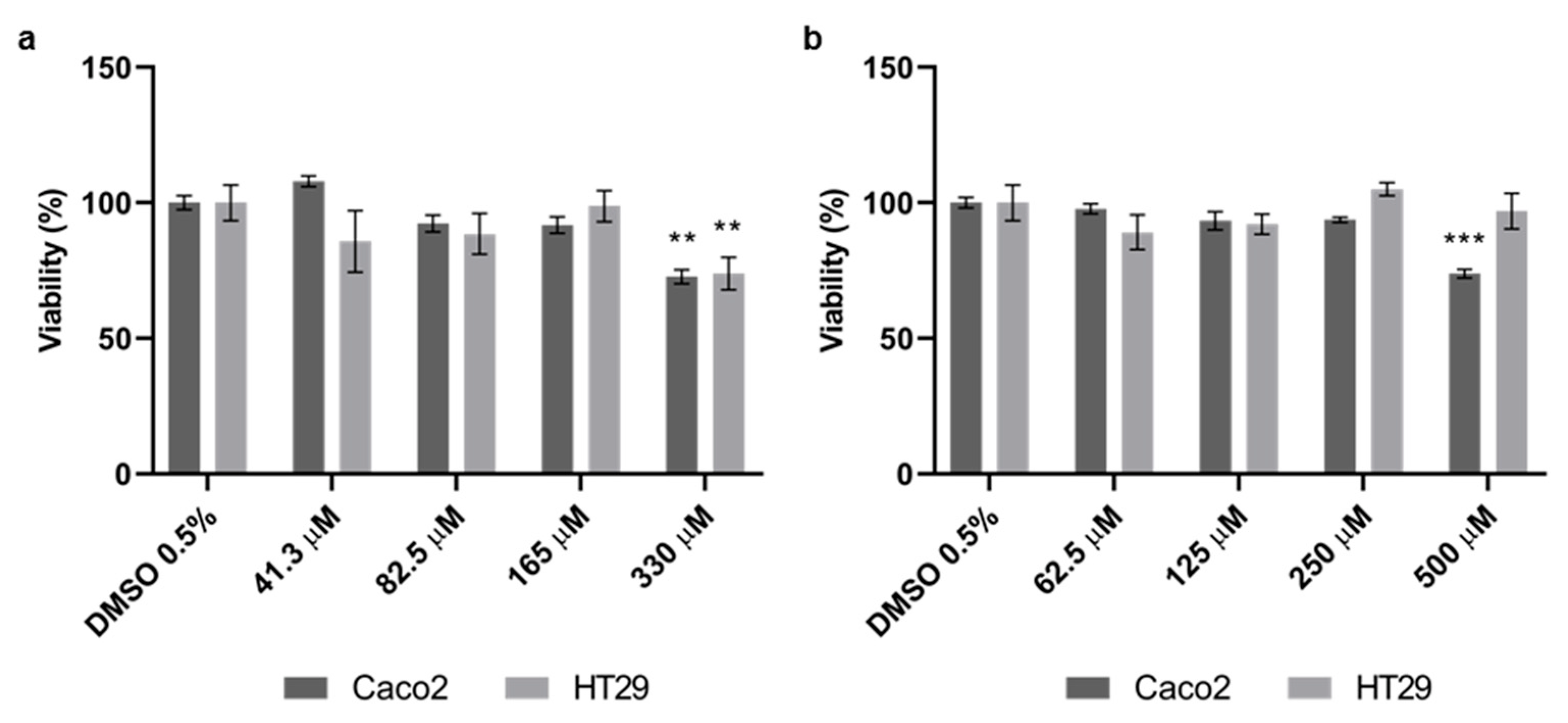
3.1. Effect of Quercetin and Probenecid on Cells Viability
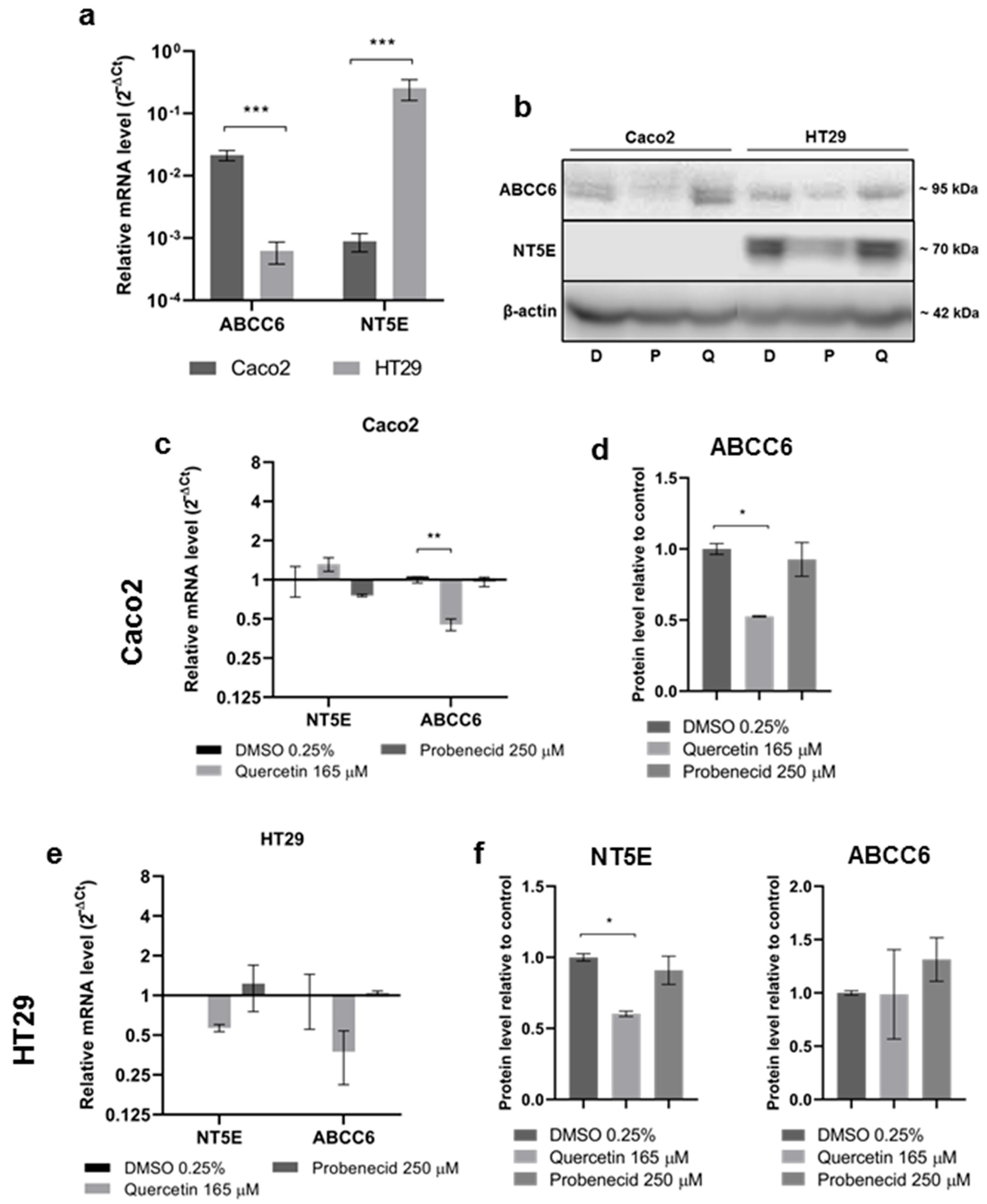
3.2. Effect of Quercetin and Probenecid on ABCC6 Expression
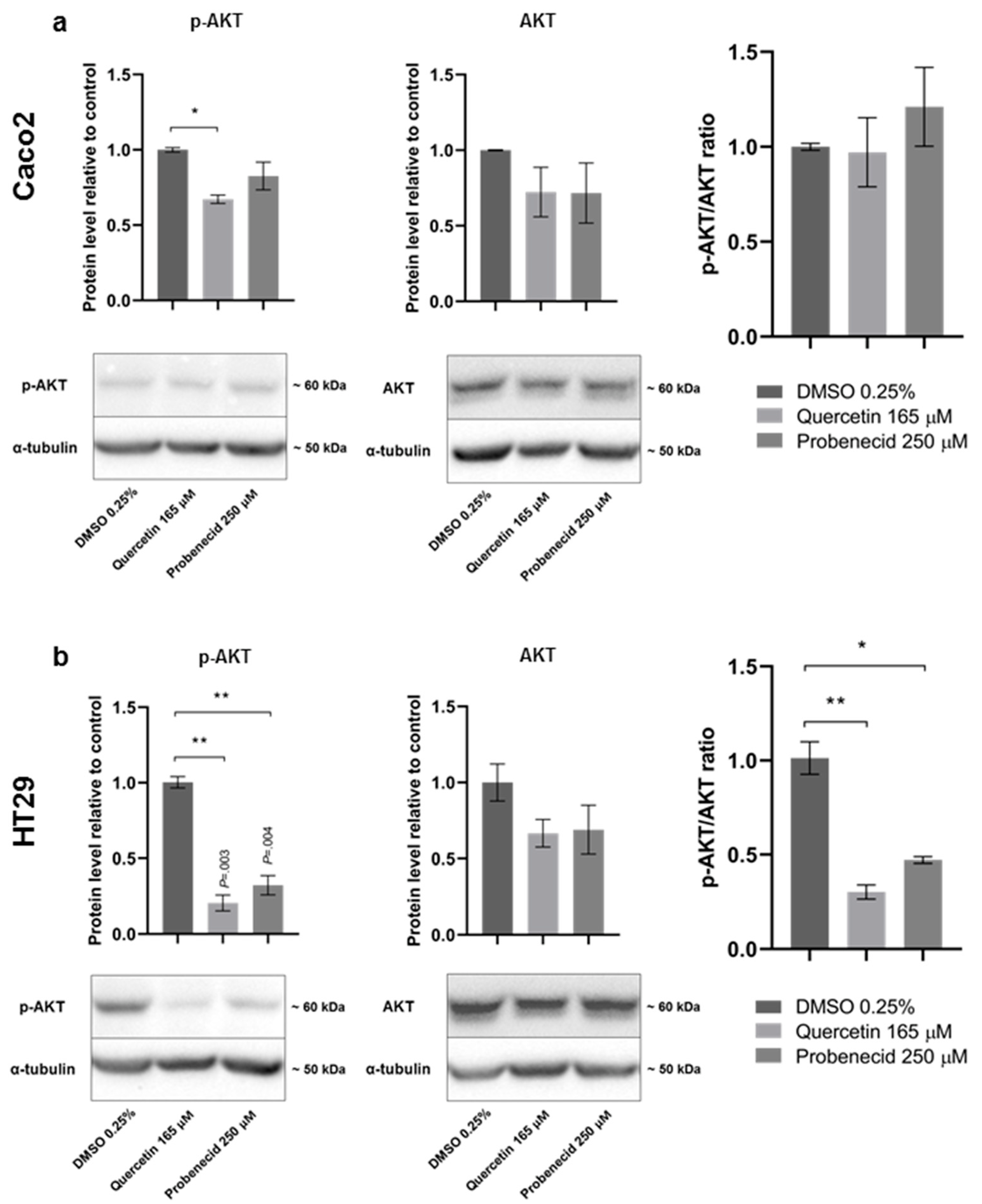
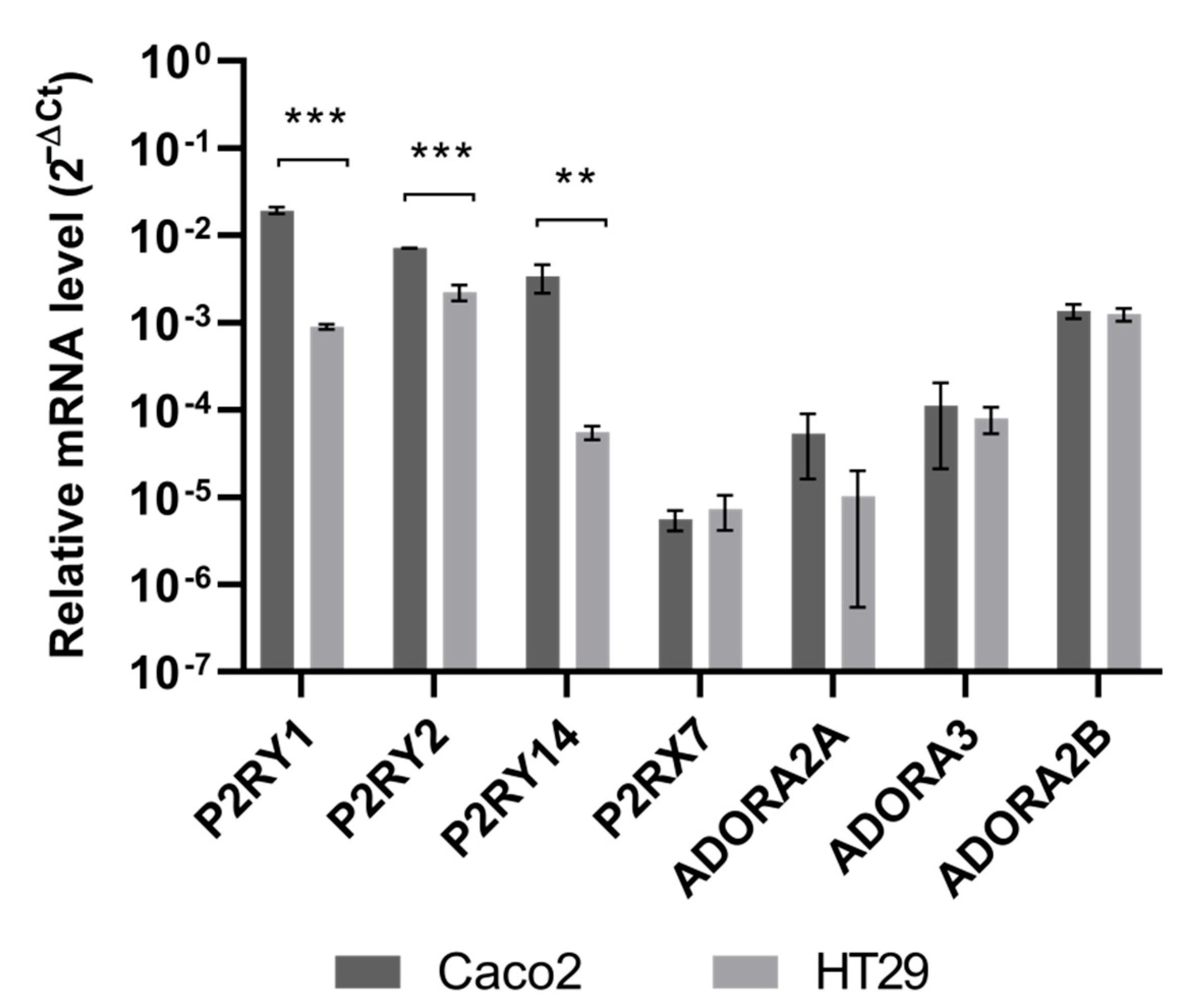
3.3. Quercetin and Probenecid Affect the Cell Migration Rate: Role of PI3K/Akt and Purinergic Pathways
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terry, S.F. The Human Face of ABCC6. FEBS Lett. 2020, 594, 4151–4157. [Google Scholar] [CrossRef] [PubMed]
- Verschuere, S.; Van Gils, M.; Nollet, L.; Vanakker, O.M. From membrane to mineralization: The curious case of the ABCC6 transporter. FEBS Lett. 2020, 594, 4109–4133. [Google Scholar] [CrossRef] [PubMed]
- Lofaro, F.D.; Mucciolo, D.P.; Murro, V.; Pavese, L.; Quaglino, D.; Boraldi, F. A Case Report of Pseudoxanthoma Elasticum with Rare Sequence Variants in Genes Related to Inherited Retinal Diseases. Diagnostics 2021, 11, 1800. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ralph, D.; Boraldi, F.; Quaglino, D.; Uitto, J.; Li, Q. Inhibition of the DNA Damage Response Attenuates Ectopic Calcification in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2022. [Google Scholar] [CrossRef]
- Miglionico, R.; Gerbino, A.; Ostuni, A.; Armentano, M.F.; Monné, M.; Carmosino, M.; Bisaccia, F. New insights into the roles of the N-terminal region of the ABCC6 transporter J. Bioenerg. Biomembr. 2016, 48, 259–267. [Google Scholar] [CrossRef]
- Cuviello, F.; Tellgren-Roth, Å.; Lara, P.; Selin, F.R.; Monné, M.; Bisaccia, F.; Nilsson, I.; Ostuni, A. Membrane insertion and topology of the amino-terminal domain TMD0 of multidrug-resistance associated protein 6 (MRP6). FEBS Lett. 2015, 589, 3921–3928. [Google Scholar] [CrossRef]
- Ostuni, A.; Miglionico, R.; Monné, M.; Castiglione Morelli, M.A.; Bisaccia, F. The nucleotide-binding domain 2 of the human transporter protein MRP6. J. Bioenerg. Biomembr. 2011, 43, 465–471. [Google Scholar] [CrossRef]
- Ostuni, A.; Miglionico, R.; Castiglione Morelli, M.A.; Bisaccia, F. Study of the nucleotide-binding domain 1 of the human transporter protein MRP6. Protein Pept. Lett. 2010, 17, 1553–1558. [Google Scholar] [CrossRef]
- Lee, H.; Lara, P.; Ostuni, A.; Presto, J.; Johansson, J.; Nilsson, I.; Kim, H. Live-cell topology assessment of URG7, MRP6102 and SP-C using glycosylatable green fluorescent protein in mammalian cells. Biochem. Biophys. Res. Commun. 2014, 450, 1587–1592. [Google Scholar] [CrossRef]
- Brampton, C.; Pomozi, V.; Chen, L.H.; Apana, A.; McCurdy, S.; Zoll, J.; Boisvert, W.A.; Lambert, G.; Henrion, D.; Blanchard, S.; et al. ABCC6 deficiency promotes dyslipidemia and atherosclerosis. Sci. Rep. 2021, 11, 3881. [Google Scholar] [CrossRef]
- Ibold, B.; Tiemann, J.; Faust, I.; Ceglarek, U.; Dittrich, J.; Gorgels, T.G.M.F.; Bergen, A.A.B.; Vanakker, O.; Van Gils, M.; Knabbe, C.; et al. Genetic deletion of Abcc6 disturbs cholesterol homeostasis in mice. Sci. Rep. 2021, 11, 2137. [Google Scholar] [CrossRef]
- Arányi, T.; Bacquet, C.; de Boussac, H.; Ratajewski, M.; Pomozi, V.; Fülöp, K.; Brampton, C.N.; Pulaski, L.; Le Saux, O.; Váradi, A. Transcriptional regulation of the ABCC6 gene and the background of impaired function of missense disease-causing mutations. Front. Genet. 2013, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Ratajewski, M.; Bartosz, G.; Pulaski, L. Expression of the human ABCC6 gene is induced by retinoids through the retinoid X receptor. Biochem. Biophys. Res. Commun. 2006, 350, 1082–1087. [Google Scholar] [CrossRef]
- Ratajewski, M.; de Ven, W.J.; Bartosz, G.; Pulaski, L. The human pseudoxanthoma elasticum gene ABCC6 is transcriptionally regulated by PLAG family transcription factors. Hum. Genet. 2008, 124, 451–463. [Google Scholar] [CrossRef]
- Jiang, Q.; Matsuzaki, Y.; Li, K.; Uitto, J. Transcriptional regulation and characterization of the promoter region of the human ABCC6 gene. J. Investig. Dermatol. 2006, 126, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Vető, B.; Bojcsuk, D.; Bacquet, C.; Kiss, J.; Sipeki, S.; Martin, L. The transcriptional activity of hepatocyte nuclear factor 4 alpha is inhibited via phosphorylation by ERK1/2. PLoS ONE 2017, 12, e0172020. [Google Scholar] [CrossRef] [Green Version]
- de Boussac, H.; Ratajewski, M.; Sachrajda, I.; Köblös, G.; Tordai, A.; Pulaski, L.; Buday, L.; Váradi, A.; Arányi, T. The ERK1/2-hepatocyte nuclear factor 4alpha axis regulates human ABCC6 gene expression in hepatocytes. J. Biol. Chem. 2010, 285, 22800–22808. [Google Scholar] [CrossRef] [Green Version]
- Ratajewski, M.; de Boussac, H.; Sachrajda, I.; Bacquet, C.; Kovács, T.; Váradi, A.; Pulaski, L.; Arányi, T. ABCC6 expression is regulated by CCAAT/enhancer-binding protein activating a primate-specific sequence located in the first intron of the gene. J. Investig. Dermatol. 2012, 132, 2709–2717. [Google Scholar] [CrossRef] [Green Version]
- Jansen, R.S.; Küçükosmanoglu, A.; de Haas, M.; Sapthu, S.; Otero, J.A.; Hegman, I.E.M.; Bergen, A.; Gorgels, T.; Borst, P.; Wetering, K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc. Natl. Acad. Sci. USA 2013, 110, 20206–20211. [Google Scholar] [CrossRef] [Green Version]
- Jansen, R.S.; Duijst, S.; Mahakena, S.; Sommer, D.; Szeri, F.; Váradi, A.; Plomp, A.; Bergen, A.A.; Oude Elferink, R.P.; Borst, P.; et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arter. Thromb. Vasc. Biol. 2014, 34, 1985–1989. [Google Scholar] [CrossRef] [Green Version]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Novitskiy, S.V.; Sachsenmeier, K.F.; Fornai, M.; Blandizzi, C.; Haskó, G. Switching off CD73: A way to boost the activity of conventional and targeted antineoplastic therapies. Drug Discov. Today 2017, 22, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. Immunity, inflammation and cancer: A leading role for adenosine. Nat. Rev. Cancer 2013, 13, 842–857. [Google Scholar] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Haskó, G. The Purinergic System as a Pharmacological Target for the Treatment of Immune-Mediated Inflammatory Diseases. Pharmacol. Rev. 2019, 71, 345–382. [Google Scholar] [CrossRef]
- Martinelli, F.; Cuviello, F.; Pace, M.C.; Armentano, M.F.; Miglionico, R.; Ostuni, A.; Bisaccia, F. Extracellular ATP regulates CD73 and ABCC6 expression in HepG2 Cells. Front. Mol. Biosci. 2018, 5, 75. [Google Scholar] [CrossRef] [Green Version]
- Ostuni, A.; Carmosino, M.; Miglionico, R.; Abruzzese, V.; Martinelli, F.; Russo, D.; Laurenzana, I.; Petillo, A.; Bisaccia, F. Inhibition of ABCC6 Transporter Modifies Cytoskeleton and Reduces Motility of HepG2 Cells via Purinergic Pathway. Cells 2020, 9, 1410. [Google Scholar] [CrossRef]
- Abruzzese, V.; Matera, I.; Martinelli, F.; Carmosino, M.; Koshal, P.; Milella, L.; Bisaccia, F.; Ostuni, A. Effect of Quercetin on ABCC6 Transporter: Implication in HepG2 Migration. Int. J. Mol. Sci. 2021, 22, 3871. [Google Scholar] [CrossRef]
- Ruiz-Pinto, S.; Pita, G.; Patiño-García, A.; García-Miguel, P.; Alonso, J.; Pérez-Martínez, A.; Sastre, A.; Gómez-Mariano, G.; Lissat, A.; Scotlandi, K.; et al. Identification of genetic variants in pharmacokinetic genes associated with Ewing Sarcoma treatment outcome. Ann. Oncol. 2016, 27, 1788–1793. [Google Scholar] [CrossRef]
- Salvia, A.M.; Cuviello, F.; Coluzzi, S.; Nuccorini, R.; Attolico, I.; Pascale, S.P.; Bisaccia, F.; Pizzuti, M.; Ostuni, A. Expression of Some ATP-Binding Cassette Transporters in Acute Myeloid Leukemia. Hematol. Rep. 2017, 9, 137–141. [Google Scholar] [CrossRef]
- Mao, X.; He, Z.; Zhou, F.; Huang, Y.; Zhu, G. Prognostic significance and molecular mechanisms of adenosine triphosphate-binding cassette subfamily C members in gastric cancer. Medicine 2019, 98, e18347. [Google Scholar] [CrossRef]
- Guo, R.Q.; Xiong, G.Y.; Yang, K.W.; Zhang, L.; He, S.M.; Gong, Y.Q.; He, Q.; Li, X.Y.; Wang, Z.C.; Bao, Z.Q.; et al. Detection of urothelial carcinoma, upper tract urothelial carcinoma, bladder carcinoma, and urothelial carcinoma with gross hematuria using selected urine-DNA methylation biomarkers: A prospective, single-center study. Urol. Oncol. 2018, 36, 342.e15–342.e23. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hlavata, I.; Mohelnikova-Duchonova, B.; Vaclavikova, R.; Liška, V.; Pitule, P.; Novák, P.; Bruha, J.; Vyčítal, O.; Holubec, L.; Třeška, V.; et al. The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis 2012, 27, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Maubon, N.; Le Vee, M.; Fossati, L.; Audry, M.; Le Ferrec, E.; Bolze, S.; Fardel, O. Analysis of drug transporter expression in human intestinal Caco-2 cells by real-time PCR. Fundam. Clin. Pharmacol. 2007, 21, 659–663. [Google Scholar] [CrossRef]
- Bu, Y.; Cai, G.; Shen, Y.; Huang, C.; Zeng, X.; Cao, Y.; Cai, C.; Wang, Y.; Huang, D.; Liao, D.F.; et al. Targeting NF-κB RelA/p65 phosphorylation overcomes RITA resistance. Cancer Lett. 2016, 383, 261–271. [Google Scholar] [CrossRef]
- Wang, H.; Chi, C.H.; Zhang, Y.; Shi, B.; Jia, R.; Wang, B.J. Effects of histone deacetylase inhibitors on ATP-binding cassette transporters in lung cancer A549 and colorectal cancer HCT116 cells. Oncol. Lett. 2019, 18, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Nylund, G.; Nordgren, S.; Delbro, D.S. Demonstration of functional receptors for noradrenaline and adenosine-5’-triphosphate, but not for prostaglandin E2, in HT-29 human colon cancer cell line. Auton. Autacoid Pharmacol. 2003, 23, 193–199. [Google Scholar] [CrossRef]
- Nylund, G.; Nordgren, S.; Delbro, D.S. Expression of P2Y2 purinoceptors in MCG 101 murine sarcoma cells, and HT-29 human colon carcinoma cells. Auton. Neurosci. 2004, 112, 69–79. [Google Scholar] [CrossRef]
- Nylund, G.; Hultman, L.; Delbro, D.S. P2Y2- and P2Y4 purinergic receptors are over-expressed in human colon cancer. Auton. Autacoid Pharmacol. 2007, 27, 79–84. [Google Scholar] [CrossRef]
- Zhang, W.J.; Hu, C.G.; Luo, H.L.; Zhu, Z.M. Activation of P2×7 Receptor Promotes the Invasion and Migration of Colon Cancer Cells via the STAT3 Signaling. Front. Cell Dev. Biol. 2020, 8, 586555. [Google Scholar] [CrossRef]
- Coutinho-Silva, R.; Stahl, L.; Cheung, K.K.; de Campos, N.E.; de Oliveira Souza, C.; Ojcius, D.M.; Burnstock, G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: Effects of extracellular nucleotides on apoptosis and cell proliferation. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, 1024–1035. [Google Scholar] [CrossRef] [Green Version]
- Souza, C.O.; Santoro, G.F.; Figliuolo, V.R.; Nanini, H.F.; de Souza, H.S.P.; Castelo-Branco, M.T.L.; Abalo, A.A.; Paiva, M.M.; Coutinho, C.M.L.M.; Coutinho-Silva, R. Extracellular ATP induces cell death in human intestinal epithelial cells. Biochim. Biophys. Acta 2012, 1820, 1867–1878. [Google Scholar] [CrossRef]
- Yaguchi, T.; Saito, M.; Yasuda, Y.; Kanno, T.; Nakano, T.; Nishizaki, T. Higher concentrations of extracellular ATP suppress proliferation of Caco-2 human colonic cancer cells via an unknown receptor involving PKC inhibition. Cell. Physiol. Biochem. 2010, 26, 125–134. [Google Scholar] [CrossRef]
- Schachter, J.; Alvarez, C.L.; Bazzi, Z.; Faillace, M.P.; Corradi, G.; Hattab, C.; Rinaldi, D.E.; Gonzalez-Lebrero, R.; Pucci Molineris, M.; Sévigny, J.; et al. Extracellular ATP hydrolysis in Caco-2 human intestinal cell line. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183679. [Google Scholar] [CrossRef]
- Bahrami, F.; Kukulski, F.; Lecka, J.; Tremblay, A.; Pelletier, J.; Rockenbach, L.; Sévigny, J. Purine-metabolizing ectoenzymes control IL-8 production in human colon HT-29 cells. Mediat. Inflamm. 2014, 2014, 879895. [Google Scholar] [CrossRef]
- Bisaccia, F.; Koshal, P.; Abruzzese, V.; Castiglione Morelli, M.A.; Ostuni, A. Structural and Functional Characterization of the ABCC6 Transporter in Hepatic Cells: Role on PXE, Cancer Therapy and Drug Resistance. Int. J. Mol. Sci. 2021, 22, 2858. [Google Scholar] [CrossRef]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef]
- Waizenegger, J.; Lenze, D.; Luckert, C.; Seidel, A.; Lampen, A.; Hessel, S. Dose-dependent induction of signaling pathways by the flavonoid quercetin in human primary hepatocytes: A transcriptomic study. Mol. Nutr. Food Res. 2015, 59, 1117–1129. [Google Scholar] [CrossRef]
- Shimizu, M.; Li, J.; Inoue, J.; Sato, R. Quercetin represses apolipoprotein B expression by inhibiting the transcriptional activity of C/EBPβ. PLoS ONE 2015, 10, e0121784. [Google Scholar] [CrossRef] [Green Version]
- Buzzi, N.; Bilbao, P.S.; Boland, R.; de Boland, A.R. Extracellular ATP activates MAP kinase cascades through a P2Y purinergic receptor in the human intestinal Caco-2 cell line. Biochim. Biophys. Acta. 2009, 1790, 1651–1659. [Google Scholar] [CrossRef]
- Buzzi, N.; Boland, R.; de Boland, A.R. Signal transduction pathways associated with ATP-induced proliferation of colon adenocarcinoma cells. Biochim. Biophys. Acta 2010, 1800, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Avizienyte, E.; Brunton, V.G.; Fincham, V.J.; Frame, M.C. The SRC-induced mesenchymal state in late-stage colon cancer cells. Cells Tissues Organs 2005, 179, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Shen, M.N.; Hu, B.; Wang, B.L.; Yang, W.J.; Lv, L.H.; Wang, H.; Zhou, Y.; Jin, A.L.; Sun, Y.F.; et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J. Hematol. Oncol. 2019, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Rychahou, P.G.; Jackson, L.N.; Silva, S.R.; Rajaraman, S.; Evers, B.M. Targeted molecular therapy of the PI3K pathway: Therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann. Surg. 2006, 243, 833–842. [Google Scholar] [CrossRef]
- Gulhati, P.; Bowen, K.A.; Liu, J.; Stevens, P.D.; Rychahou, P.G.; Chen, M.; Lee, E.Y.; Weiss, H.L.; O’Connor, K.L.; Gao, T.; et al. mTORC1 and mTORC2 regulate EMT, motility, and metastasis of colorectal cancer via RhoA and Rac1 signaling pathways. Cancer Res. 2011, 71, 3246–3256. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 2015, 12, 3446–3452. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.Q.; Ju, C.L.; Wang, B.J.; Wang, R.G. PABPC1L depletion inhibits proliferation and migration via blockage of AKT pathway in human colorectal cancer cells. Oncol. Lett. 2019, 17, 3439–3445. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, S.N.; Chen, J.; Hu, J.G.; Lü, H.Z. A transcriptomic study of probenecid on injured spinal cords in mice. PeerJ 2020, 8, e8367. [Google Scholar] [CrossRef] [Green Version]
- Lillo, M.A.; Gaete, P.S.; Puebla, M.; Burboa, P.C.; Poblete, I.; Figueroa, X.F. Novel Pannexin-1-Coupled Signaling Cascade Involved in the Control of Endothelial Cell Function and NO-Dependent Relaxation. Oxidative Med. Cell. Longev. 2021, 2021, 2678134. [Google Scholar] [CrossRef]


| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| ACTB | 5′-CCTGGCACCCAGCACAAT-3′ | 5′-GCCGATCCACACGGAGTACT-3′ |
| ABCC6 | 5′-ATCACTGATCCTTCCATCTTG-3′ | 5′-ACCAGCGACACAGAGAAGAGG-3′ |
| NT5E | 5′-GGGCGGAAGGTTCCTGTAG-3′ | 5′-GAGGAGCCATCCAGATAGACA-3′ |
| P2RY1 | 5′-TGTGGTGGTGGCGATCTCC-3′ | 5′-TCGCAGGTACTCGTCTGAGG-3′ |
| P2RY2 | 5′-TCAGCATTGTGTTCTTTGGG-3′ | 5′-CTGGGAAATCTCAAGGACTG-3′ |
| P2RY14 | 5′-TCAGCATTGTGTTCTTTGGG-3′ | 5′-TGCTGTAACTCACTGACTGG-3′ |
| ADORA2A | 5′-AACCTGCAGAACGTCACCAA-3′ | 5′- GTCACCAAGCCATTGTACCG -3′ |
| ADORA3 | 5′-GTCAGATACAAGAGGGTCAC-3′ | 5′-GTCAGTTTCATGTTCCAGCC-3′ |
| ADORA2B | 5′-GAGACACAGGACGCGCTGTACG-3′ | 5′-CGGGTCCCCGTGACCAAACT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abruzzese, V.; Sukowati, C.H.C.; Tiribelli, C.; Matera, I.; Ostuni, A.; Bisaccia, F. The Expression Level of ABCC6 Transporter in Colon Cancer Cells Correlates with the Activation of Different Intracellular Signaling Pathways. Pathophysiology 2022, 29, 173-186. https://doi.org/10.3390/pathophysiology29020015
Abruzzese V, Sukowati CHC, Tiribelli C, Matera I, Ostuni A, Bisaccia F. The Expression Level of ABCC6 Transporter in Colon Cancer Cells Correlates with the Activation of Different Intracellular Signaling Pathways. Pathophysiology. 2022; 29(2):173-186. https://doi.org/10.3390/pathophysiology29020015
Chicago/Turabian StyleAbruzzese, Vittorio, Caecilia H. C. Sukowati, Claudio Tiribelli, Ilenia Matera, Angela Ostuni, and Faustino Bisaccia. 2022. "The Expression Level of ABCC6 Transporter in Colon Cancer Cells Correlates with the Activation of Different Intracellular Signaling Pathways" Pathophysiology 29, no. 2: 173-186. https://doi.org/10.3390/pathophysiology29020015
APA StyleAbruzzese, V., Sukowati, C. H. C., Tiribelli, C., Matera, I., Ostuni, A., & Bisaccia, F. (2022). The Expression Level of ABCC6 Transporter in Colon Cancer Cells Correlates with the Activation of Different Intracellular Signaling Pathways. Pathophysiology, 29(2), 173-186. https://doi.org/10.3390/pathophysiology29020015






