Clinical Presentation of Hepatocellular Carcinoma in African Americans vs. Caucasians: A Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Presence of Comorbidities
3.3. AFP Values
3.4. Tumor Size at Presentation
3.5. Milan Score
3.6. Presence of Gallstones
3.7. Cholecystectomy
3.8. Sex
3.9. Marital Status
3.10. Serum Creatinine
3.11. Bilirubin
3.12. Hepatic Encephalopathy
3.13. Statin Use
3.14. Aspirin Use
3.15. US/CT/MRI Diagnostics
3.16. Discovery of Lesions by US
3.17. Univariate Analysis
3.18. Multivariate Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzmán, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, A.B.; Eheman, C.R.; Altekruse, S.F.; Ward, J.W.; Jemal, A.; Sherman, R.L.; Henley, S.J.; Holtzman, D.; Lake, A.; Noone, A.-M.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016, 122, 1312–1337. [Google Scholar] [CrossRef]
- Bialecki, E.S.; Di Bisceglie, A.M. Diagnosis of hepatocellular carcinoma. HPB 2005, 7, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, G.; Zhang, P.; Zhang, J.; Li, X.; Gan, D.; Cao, X.; Han, M.; Du, H.; Ye, Y. The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0228857. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; Henley, S.J.; Cucinelli, J.E.; McGlynn, K.A. Changing Hepatocellular Carcinoma Incidence and Liver Cancer Mortality Rates in the United States. Am. J. Gastroenterol. 2014, 109, 542–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Kim, Y.; Spolverato, G.; Gani, F.; Pawlik, T.M. Racial disparities in treatment and survival of patients with hepatocellular carcinoma in the United States. Hepatobiliary 2016, 5, 43–52. [Google Scholar]
- Ha, J.; Yan, M.; Aguilar, M.; Bhuket, T.; Tana, M.M.; Liu, B.; Gish, R.; Wong, R.J. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer 2016, 122, 2512–2523. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.; Fong, T.-L.; Zhang, J.; Liu, L. Striking Racial/Ethnic Disparities in Liver Cancer Incidence Rates and Temporal Trends in California, 1988–2012. J. Natl. Cancer Inst. 2018, 110, 1259–1269. [Google Scholar] [CrossRef]
- Wong, R.; Corley, D.A. Racial and Ethnic Variations in Hepatocellular Carcinoma Incidence within the United States. Am. J. Med. 2008, 121, 525–531. [Google Scholar] [CrossRef]
- O’Connor, S.; Ward, J.W.; Watson, M.; Momin, B.; Richardson, L.C. Hepatocellular carcinoma—United States, 2001–2006. Morb. Mortal. Wkly. Rep. 2010, 59, 517–520. [Google Scholar]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender Disparity in Liver Cancer Due to Sex Differences in MyD88-Dependent IL-6 Production. Science 2007, 317, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Beasley, R.; Lin, C.-C.; Hwang, L.-Y.; Chien, C.-S. Hepatocellular carcinoma and hepatitis B virus: A Prospective Study of 22,707 Men in Taiwan. Lancet 1981, 318, 1129–1133. [Google Scholar] [CrossRef]
- Colombo, M.; De Franchis, R.; Del Ninno, E.; Sangiovanni, A.; De Fazio, C.; Tommasini, M.; Donato, M.F.; Piva, A.; Di Carlo, V.; Dioguardi, N. Hepatocellular Carcinoma in Italian Patients with Cirrhosis. N. Engl. J. Med. 1991, 325, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Tsukuma, H.; Hiyama, T.; Tanaka, S.; Nakao, M.; Yabuuchi, T.; Kitamura, T.; Nakanishi, K.; Fujimoto, I.; Inoue, A.; Yamazaki, H.; et al. Risk Factors for Hepatocellular Carcinoma among Patients with Chronic Liver Disease. N. Engl. J. Med. 1993, 328, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Tran, T.; Everhart, J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology 2004, 126, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; Ohrr, H.; Sull, J.W.; Yun, J.E.; Ji, M.; Samet, J.M. Fasting serum glucose level and cancer risk in Korean men and women. JAMA 2005, 293, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Iwasaki, M.; Otani, T.; Sasazuki, S.; Noda, M.; Tsugane, S. Diabetes mellitus and the risk of cancer: Results from a large-scale population-based cohort study in Japan. Arch. Intern. Med. 2006, 166, 1871–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, S.-W.; Chen, P.-C.; Liao, K.-F.; Muo, C.-H.; Lin, C.-C.; Sung, F.-C. Risk of Hepatocellular Carcinoma in Diabetic Patients and Risk Reduction Associated with Anti-Diabetic Therapy: A Population-Based Cohort Study. Am. J. Gastroenterol. 2012, 107, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Salmon, D.; Bani-Sadr, F.; Loko, M.-A.; Stitou, H.; Gervais, A.; Durant, J.; Rosenthal, E.; Quertainmont, Y.; Barange, K.; Vittecoq, D.; et al. Insulin resistance is associated with a higher risk of hepatocellular carcinoma in cirrhotic HIV/HCV-co-infected patients: Results from ANRS CO13 HEPAVIH. J. Hepatol. 2012, 56, 862–868. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.-W.; Yang, X.; Fan, W.-B.; Yu, J.-H.; Xie, S.-S.; Liu, L.; Ma, L.-X.; Chen, S.-J.; Kato, N. Type 2 diabetes and hepatocellular carcinoma: A case-control study in patients with chronic hepatitis B. Int. J. Cancer 2011, 131, 1197–1202. [Google Scholar] [CrossRef]
- Arase, Y.; Kobayashi, M.; Suzuki, F.; Suzuki, Y.; Kawamura, Y.; Akuta, N.; Kobayashi, M.; Sezaki, H.; Saito, S.; Hosaka, T.; et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology 2013, 57, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-P.; Shieh, J.-J.; Chang, C.-C.; Chen, T.-T.; Lin, J.-T.; Wu, M.-S.; Lin, J.-H.; Wu, C.-Y. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: Population-based and in vitro studies. Gut 2013, 62, 606–615. [Google Scholar] [CrossRef]
- Wang, P.; Kang, D.; Cao, W.; Wang, Y.; Liu, Z. Diabetes mellitus and risk of hepatocellular carcinoma: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2012, 28, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-S.; Va, P.; Bray, F.; Gao, S.; Gao, J.; Li, H.-L.; Xiang, Y.-B. The Role of Pre-Existing Diabetes Mellitus on Hepatocellular Carcinoma Occurrence and Prognosis: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2011, 6, e27326. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Gong, G.; Ben, Q.; Qiu, W.; Chen, Y.; Li, G.; Wang, L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: A systematic review and meta-analysis of cohort studies. Int. J. Cancer 2012, 130, 1639–1648. [Google Scholar] [CrossRef]
- Welzel, T.M.; Graubard, B.I.; Zeuzem, S.; El-Serag, H.B.; Davila, J.A.; McGlynn, K.A. Metabolic syndrome increases the risk of primary liver cancer in the United States: A study in the SEER-medicare database. Hepatology 2011, 54, 463–471. [Google Scholar] [CrossRef] [Green Version]
- Bugianesi, E.; Leone, N.; Vanni, E.; Marchesini, G.; Brunello, F.; Carucci, P.; Musso, A.; De Paolis, P.; Capussotti, L.; Salizzoni, M.; et al. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002, 123, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, E.; Yatsuji, S.; Tobari, M.; Taniai, M.; Torii, N.; Tokushige, K.; Shiratori, K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J. Gastroenterol. 2009, 44, 89–95. [Google Scholar] [CrossRef]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Yasui, K.; Hashimoto, E.; Komorizono, Y.; Koike, K.; Arii, S.; Imai, Y.; Shima, T.; Kanbara, Y.; Saibara, T.; Mori, T.; et al. Characteristics of Patients with Nonalcoholic Steatohepatitis Who Develop Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 428–433. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131.e1. [Google Scholar] [CrossRef] [Green Version]
- Tsan, Y.-T.; Lee, C.-H.; Wang, J.-D.; Chen, P.-C. Statins and the Risk of Hepatocellular Carcinoma in Patients with Hepatitis B Virus Infection. J. Clin. Oncol. 2012, 30, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Tsan, Y.-T.; Lee, C.-H.; Ho, W.-C.; Lin, M.-H.; Wang, J.-D.; Chen, P.-C. Statins and the Risk of Hepatocellular Carcinoma in Patients with Hepatitis C Virus Infection. J. Clin. Oncol. 2013, 31, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Statins Are Associated with a Reduced Risk of Hepatocellular Cancer: A Systematic Review and Meta-analysis. Gastroenterology 2013, 144, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Friedman, G.D.; Achacoso, N.; Fireman, B.; Habel, L. Statins and Reduced Risk of Liver Cancer: Evidence for Confounding. J. Natl. Cancer Inst. 2016, 108, djw260. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; He, Y.; Li, T.; Xie, L.; Wang, J.; Qin, X.; Li, S. Risk of Primary Liver Cancer Associated with Gallstones and Cholecystectomy: A Meta-Analysis. PLoS ONE 2014, 9, e109733. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Yan, M.; Aguilar, M.; Tana, M.; Liu, B.; Frenette, C.T.; Bhuket, T.; Wong, R.J. Race/Ethnicity-specific Disparities in Hepatocellular Carcinoma Stage at Diagnosis and its Impact on Receipt of Curative Therapies. J. Clin. Gastroenterol. 2016, 50, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wilson, S.E.; Stewart, D.B.; Hollenbeak, C.S. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: Does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 2011, 35, 417–422. [Google Scholar] [CrossRef]
- Martínez, M.E.; Anderson, K.; Murphy, J.D.; Hurley, S.; Canchola, A.J.; Keegan, T.H.M.; Cheng, I.; Clarke, C.A.; Glaser, S.L.; Gomez, S.L. Differences in marital status and mortality by race/ethnicity and nativity among California cancer patients. Cancer 2016, 122, 1570–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez, S.L.; Hurley, S.; Canchola, A.J.; Keegan, T.H.M.; Cheng, I.; Murphy, J.D.; Clarke, C.A.; Glaser, S.L.; Martínez, M.E. Effects of marital status and economic resources on survival after cancer: A population-based study. Cancer 2016, 122, 1618–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-D.; Qian, J.-J.; Bai, D.-S.; Li, Z.-N.; Jiang, G.-Q.; Yao, J. Marital status independently predicts pancreatic cancer survival in patients treated with surgical resection: An analysis of the SEER database. Oncotarget 2016, 7, 24880–24887. [Google Scholar] [CrossRef] [Green Version]
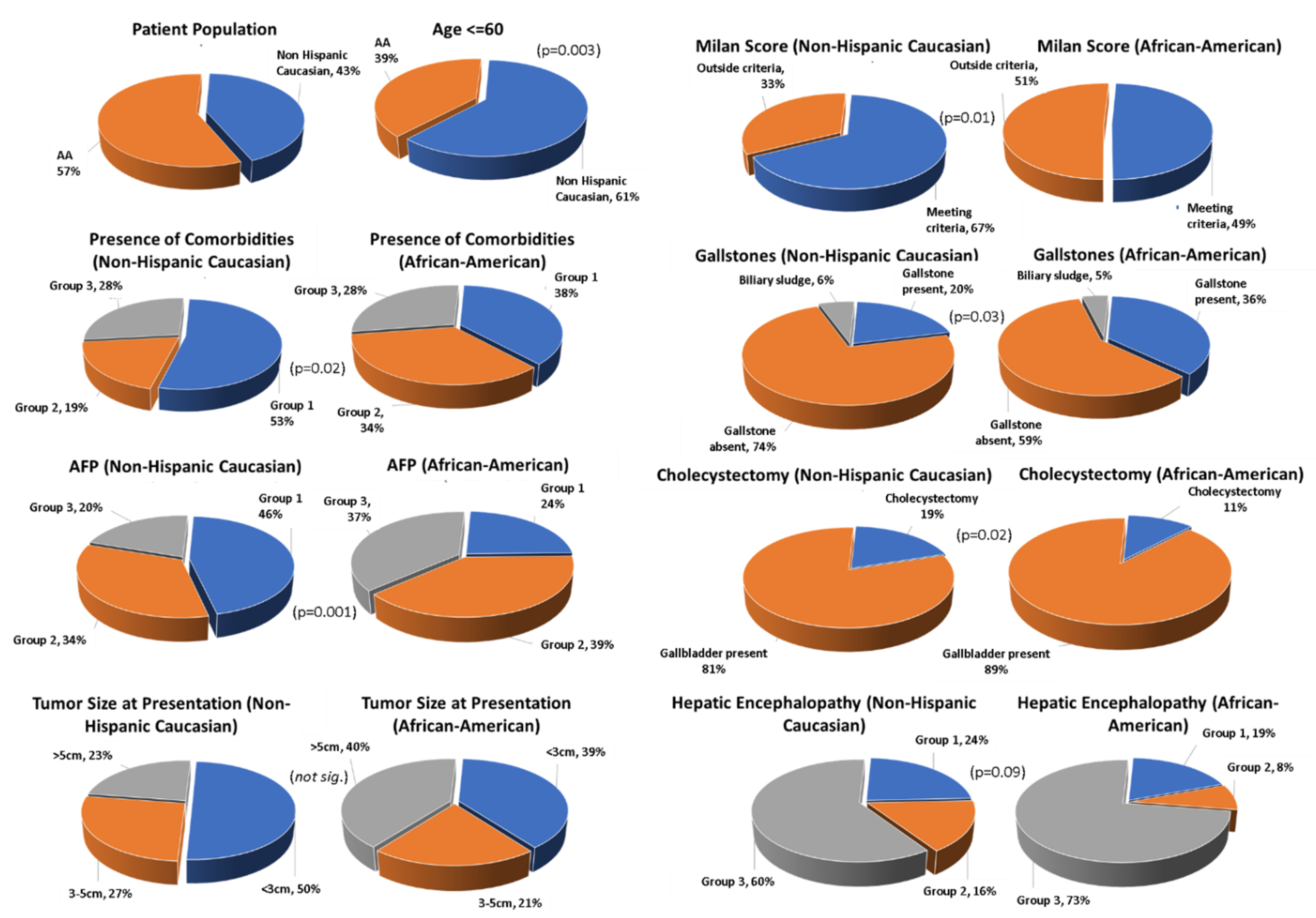
| Variable | Variable | Caucasian N (%) | African American N (%) | p-Value |
|---|---|---|---|---|
| Age (years) | 60 | 56 (57.14%) | 48 (36.64%) | 0.003 |
| Age (years) > 60 | 42 (42.86%) | 83 (63.36%) | ||
| Comorbidities: | Comorbidities: group 1 | 52 (53.06%) | 49 (37.40%) | 0.02 |
| Comorbidities: group 2 | 19 (19.30%) | 45 (34.35%) | ||
| Comorbidities: group 3 | 27 (27.55%) | 37 (28.24%) | ||
| AFP | AFP value 0–20 (group 1) | 42 (45.65%) | 30 (23.81%) | 0.001 |
| AFP value 21–500 (group 2) | 31 (33.70%) | 49 (38.89%) | ||
| AFP value > 500 (group 3) | 19 (20.65%) | 47 (37.30%) | ||
| HCC | HCC size < 3 cm | 49 (50%) | 51 (38.93%) | 0.02 |
| HCC size 3–5 cm | 26 (26.53%) | 27 (20.61%) | ||
| HCC size > 5 cm | 23 (23.47%) | 53 (40.46%) | ||
| Milan Score | Milan score (meeting criteria) | 60 (66.67%) | 60 (49.18%) | 0.01 |
| Milan score (outside the criteria) | 30 (33.33%) | 62 (50.82%) | ||
| Gallstone | Gallstone present | 19 (19.38%) | 46 (35.11%) | 0.03 |
| Gallstone absent | 69 (70.40%) | 75 (57.25%) | ||
| Presentation grouping | Biliary sludge | 6 (6.12%) | 6 (4.58%) | 0.02 |
| Cholecystectomy | 18 (19.14%) | 13 (10.23% | ||
| Gallbladder present | 76 (80.85%) | 114 (89.76%) | ||
| Sex | Male | 72 (73.47%) | 108 (82.44%) | 0.1 |
| Female | 26 (26.53%) | 23 (17.56%) | ||
| Marital Status | Married | 26 (26.53%) | 34 (25.95%) | 0.1 |
| Unmarried | 47 (47.96%) | 77 (58.78%) | ||
| Divorced | 25 (25.51%) | 20 (15.27%) | ||
| Serum Creatinine | Serum Creatinine < 1.3 (mg/dL) | 89 (90.82%) | 108 (83.08%) | 0.1 |
| Serum Creatinine > 1.3 (mg/dL) | 9 (9.18%) | 22 (16.92%) | ||
| Total bilirubin | Total bilirubin < 1 (mg/dL) | 38 (38.78%) | 64 (49.23%) | 0.1 |
| Total bilirubin > 1 (mg/dL) | 60 (61.22%) | 66 (50.77%) | ||
| Hepatic encephalopathy | Hepatic encephalopathy controlled on medications (group 1) | 23 (23.47%) | 25 (19.08%) | 0.09 |
| Hepatic encephalopathy uncontrolled on medications (group 2) | 16 (16.33%) | 11 (8.40%) | ||
| Hepatic encephalopathy absent (group 3) | 59 (60.20%) | 95 (75.52%) | ||
| Statin | Statin being used | 14 (14.29%) | 30 (22.90%) | 0.1 |
| Statin not being used | 84 (85.71%) | 101 (77.10%) | ||
| Aspirin | Aspirin being used | 22 (22.45%) | 41 (31.30%) | 0.1 ns |
| Aspirin not used | 76 (77.55%) | 90 (68.70%) | ||
| Diagnosed by | Diagnosed by ultrasound | 24 (25.53%) | 45 (35.71%) | 0.1 |
| Diagnosed by CT or MRI | 70 (74.47%) | 81 (64.29%) | ||
| ultrasound | Lesion on screening ultrasound | 19 (45.24%) | 44 (58.67%) | 0.1 |
| Lesion not seen on ultrasound | 23 (54.76%) | 31 (41.33%) | ||
| BCLC stage | BCLC stage A | 10 (11.24%) | 11 (9.02%) | 0.1 |
| BCLC stage B | 33 (37.08%) | 29 (23.77%) | ||
| BCLC stage C | 36 (40.45%) | 62 (50.82%) | ||
| BCLC stage D | 10 (11.24%) | 20 (16.39%) |
| Variable | Mean | SD |
|---|---|---|
| Age (years) | 61.07 | 7.32 |
| Platelet count (k/mm3) | 148.37 | 97.87 |
| Serum Sodium (Meq/L) | 137 | 3.83 |
| Total bilirubin (mg/dL) | 1.77 | 2.23 |
| Creatinine (mg/dL) | 1.08 | 1.06 |
| INR | 1.26 | 0.31 |
| Albumin (mg/dL) | 3.13 | 0.65 |
| MELD score | 12.34 | 5.19 |
| HCC size (largest in cm) | 5.04 | 4.10 |
| ALT (IU) | 91.13 | 97.57 |
| Variable | Sub-Category | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| ECOG | PS 0 | 1 | ||
| PS 1 | 2.29 | 0.83–6.28 | 0.1 | |
| PS 2/3/4 | 7.94 | 2.64–23.83 | 0.002 | |
| HCV infection | without alcohol use | 1 | ||
| with alcohol use | 2.28 | 1.02–5.12 | 0.04 | |
| Ascites | Absent | 1 | ||
| Controlled w/medications | 2.79 | 1.26–6.17 | 0.01 | |
| Uncontrolled w/medications | 3.50 | 1.47–8.34 | 0.004 | |
| Malignant portal vein thrombosis | Absent | 1 | ||
| Present | 5.63 | 1.41–22.47 | 0.01 | |
| Number of locally directed therapies | 0 | 1 | ||
| 1–2 | 0.41 | 0.22–0.78 | 0.006 | |
| 3–6 | 0.11 | 0.039–0.31 | 0.00004 | |
| Metastasis | Absent | 1 | ||
| Present | 2.37 | 1.15–4.90 | 0.01 | |
| HCC | Size 3–5 cm | 1 | ||
| Size > 5 cm | 2.32 | 0.76–7.05 | 0.1 | |
| BCLC | Stage D | 1 | ||
| Stage A | 0.16 | 0.01–1.52 | 0.1 | |
| Milan criteria | Outside | 1 | ||
| Within | 0.3 | 0.09–0.97 | 0.04 | |
| ALT | Normal level | 1 | ||
| High level | 1.6 | 0.8–3.07 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samant, H.; Kohli, K.; Patel, K.; Shi, R.; Jordan, P.; Morris, J.; Schwartz, A.; Alexander, J.S. Clinical Presentation of Hepatocellular Carcinoma in African Americans vs. Caucasians: A Retrospective Analysis. Pathophysiology 2021, 28, 387-399. https://doi.org/10.3390/pathophysiology28030026
Samant H, Kohli K, Patel K, Shi R, Jordan P, Morris J, Schwartz A, Alexander JS. Clinical Presentation of Hepatocellular Carcinoma in African Americans vs. Caucasians: A Retrospective Analysis. Pathophysiology. 2021; 28(3):387-399. https://doi.org/10.3390/pathophysiology28030026
Chicago/Turabian StyleSamant, Hrishikesh, Kapil Kohli, Krunal Patel, Runhua Shi, Paul Jordan, James Morris, Annie Schwartz, and Jonathan Steven Alexander. 2021. "Clinical Presentation of Hepatocellular Carcinoma in African Americans vs. Caucasians: A Retrospective Analysis" Pathophysiology 28, no. 3: 387-399. https://doi.org/10.3390/pathophysiology28030026
APA StyleSamant, H., Kohli, K., Patel, K., Shi, R., Jordan, P., Morris, J., Schwartz, A., & Alexander, J. S. (2021). Clinical Presentation of Hepatocellular Carcinoma in African Americans vs. Caucasians: A Retrospective Analysis. Pathophysiology, 28(3), 387-399. https://doi.org/10.3390/pathophysiology28030026







