Abstract
The canonical β-catenin-dependent wingless (Wnt) pathway is associated with endothelial function. We examined the effect of plasma dickkopf-1 (DKK-1), an inhibitor of the Wnt pathway, on the prediction of major adverse cardiac events (MACEs). We enrolled patients who had undergone selective coronary angiography for angina. DKK-1 levels were determined using plasma collected at the outpatient visit after fasting. MACEs served as the primary endpoint. All 470 enrolled patients were divided into four groups according to their median plasma DKK-1 levels and the presence of obstructive coronary artery disease (CAD). Forty-eight patients reached the primary endpoint during a median follow-up time of 4.8 years. Kaplan–Meier survival analysis indicated that the group with high DKK-1 and obstructive CAD had a significantly higher mortality rate than the other three groups (log-rank test p = 0.001). Compared with the low plasma DKK-1 without significant coronary obstruction group, the high DKK-1 with obstructive CAD group had a hazard ratio of 10.640 (95% confidence interval: 1.350–83.874) for MACEs, as determined by multivariable Cox proportional hazard regression analysis. In conclusion, we observed a synergistic effect between high plasma DKK-1 and obstructive CAD on the prediction of MACEs in patients with angina.
1. Introduction
The global number of patients with ischemic heart disease (IHD) is increasing, and IHD remains a major health burden worldwide [1,2]. According to a report from the Global Burden of Diseases, Injuries, and Risk Factors Study in 2017, the leading cause of death was IHD [3], and in 2019, the largest burden of disability was caused by IHD in people aged ≥ 50 years old [4]. However, traditional risk factors cannot predict the exact risk of cardiovascular events and mortality, and the identification of new biomarkers is warranted to improve upon this limitation [5].
Dickkopf-1 (DKK-1) is a secreted glycoprotein that acts as an inhibitor of the canonical β-catenin-dependent wingless (Wnt) pathway [6]. Wnt proteins regulate several physiological processes, including cell proliferation, differentiation, migration, and apoptosis [7]. Since the Wnt pathway is involved in cardiovascular development and endothelial function [8,9], increased expression of DKK-1 might be associated with the development of atherosclerosis [10].
Admission serum levels of DKK-1 in patients with acute coronary syndrome have been reported to be associated with major adverse cardiac events (MACEs), a composite of cardiovascular death, myocardial infarction (MI), or stroke [11]. DKK-1 is involved in the platelet-mediated endothelial cell activation induced by inflammation [12]. Notably, the coagulating process can induce the secretion of DKK-1, and serum DKK-1 levels have been reported to be higher in patients with angina than in controls. However, there was no significant difference in plasma DKK-1 levels between patients with angina and controls [12]. The association between plasma DKK-1 and cardiovascular outcomes remains unexplored. Therefore, we hypothesized that the DKK-1 concentration in plasma could predict MACEs.
2. Materials and Methods
2.1. Study Participants
This observational cohort study was conducted at Taichung Veterans General Hospital between January 2010 and March 2018. The study complied with the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Taichung Veterans General Hospital. Written informed consent was obtained from every participant before the start of any procedure. The inclusion criteria for patient enrollment were (a) a history of angina, (b) a diagnosis of IHD based on at least a noninvasive examination, and (c) a selective coronary angiography performed in our hospital. The exclusion criteria were as follows: (a) age < 20 years, (b) a history of known diabetes mellitus (DM), (c) a history of known cancer, (d) current medication for any autoimmune disease or any psychiatric disease, (e) current acute infection, (f) symptomatic congestive heart failure ≥ Class III based on the criteria of the New York Heart Association [13], and (g) pregnancy.
2.2. Procedures
After patients were discharged in a stable condition, the eligible patients were scheduled for an outpatient follow-up appointment. We enrolled 470 patients between January 2010 and December 2016. Following anthropometric measurements in the outpatient interview, fasting blood samples were collected. We recorded the coronary angiography and medications by reviewing electronic medical records. After the baseline assessment, all of the participants were followed up through March 2018. Death due to any cause or the first episode of nonfatal MI or nonfatal stroke was recorded as the primary endpoint of MACEs based on electronic medical records from our hospital. For patients without any endpoint recorded before February 2018, we arranged a telephone call interview in March 2018, and information on the primary endpoint was collected from the patients themselves or from their immediate family members. Among all of the enrolled participants, 431 (91.7%) completed follow-up, and the data accuracy was confirmed using death registration information obtained from the Ministry of Health and Welfare, Executive Yuan, Taiwan. In the 39 (8.3%) participants who were lost to follow-up, we confirmed death using death registration information. There was no significant difference in the mortality rate between the participants who were followed up and those who were lost to follow-up (p = 0.351).
2.3. Laboratory Assessments
The blood samples, which were drawn from the antecubital vein, were collected in tubes containing EDTA. After the samples were centrifugated at 3000 RPM for 10 minutes at 4 °C, the plasma was separated and stored at −80 °C. Plasma samples were prepared to detect glucose, insulin, N-terminal pro-brain natriuretic peptide (NT-proBNP), and DKK-1 levels. Human DKK-1 was measured using a commercial immunoassay kit (R&D Systems, Minneapolis, MN, USA). The precision of the DKK-1 measurements was indicated by an intra-assay coefficient of variation (CV) of 2.6% and an inter-assay CV of 8.1%. The minimum detectable level of the DKK-1 measurement was 0.419 pg/mL. Platelet counts were determined using an automated cell counter (Sysmex, Kobe, Hyogo, Japan). Human NT-proBNP was measured using a commercial immunoassay kit (Cusabio Biotech Co, Wuhan, China). Serum samples were prepared to measure creatinine levels, C-reactive protein (CRP), and the lipid profile. CRP was determined using an ELISA kit (R&D Systems, Minneapolis, MN, USA). A morning urine sample was collected for the measurement of albumin and creatinine to calculate the urine albumin to creatinine ratio (UACR).
The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated using the following formula: fasting insulin (µIU/mL) × fasting glucose (mmol/L)/22.5 for the quantitative evaluation of insulin resistance [14]. According to the Modification of Diet in Renal Diseases equation, the estimated glomerular filtration rate (eGFR) was calculated using the following formula: 186 × (serum creatinine [mg/dL])−1.154 × (age [years])−0.203 (× 0.742, if female) [15]. Chronic kidney disease (CKD) was defined as an eGFR < 60 mL/min/1.73 m2 [15]. UACR was calculated as the ratio of urine albumin (mg) to urine creatinine (g), and albuminuria was defined as UACR ≥ 300 mg/g [15]. Hypertension was defined at baseline when any of the following conditions were present: (a) a history of antihypertensive agent use, (b) systolic blood pressure ≥ 140 mmHg, or (c) diastolic blood pressure ≥ 90 mmHg on the day of the interview. The Framingham Risk Score (FRS) was calculated as a standard risk factor for MACEs [16]. CAD was defined as an obstructive lesion of the coronary artery at baseline when one or more of the following conditions were present: (a) a history of MI, (b) a history of coronary revascularization, or (c) a coronary lesion with lumen narrowing ≥ 50% according to angiography.
2.4. Statistical Analysis
Continuous variables are presented as the mean ± standard deviation, and we used the Kolmogorov–Smirnov test to examine the normality of the data. We used the t-test or the Mann–Whitney U test between two groups and analysis of variance (ANOVA) or the Kruskal–Wallis test among more than two groups to ascertain the significance of the difference in the continuous variables. Categorical variables are presented as numbers (with percentages). We used chi-squared tests to ascertain the significance of the difference in the categorical variables.
The risk of MACEs was examined by Kaplan–Meier survival analysis among the four groups, and statistically significant differences were tested by the log-rank test. The improvement in primary-endpoint prediction by adding the variable of DKK-1 was assessed by examining the increases in Harrell’s concordance index (C index) [17]. We used the integrated discrimination improvement (IDI) and continuous net reclassification improvement (NRI) to ascertain the significance of the difference in the performance of the model adding DKK-1 [18].
Multivariable Cox proportional hazards regressions were performed to evaluate the risk of MACEs according to the groups categorized by plasma DKK-1 level and CAD status. A two-sided p value < 0.05 was considered to be statistically significant. Statistical analysis was conducted using SPSS v22.0 (IBM, Armonk, NY, USA) and R software v3.4.
3. Results
Among the 470 patients enrolled in the present study, there were 187 patients without CAD and 283 patients with CAD. There was no significant difference in the plasma DKK-1 levels between patients without CAD and those with CAD (614 ± 192 vs. 606 ± 187 pg/mL, p = 0.622; Figure 1). We further divided all of the enrolled patients into four groups categorized according to their plasma DKK-1 level and CAD status. The cutoff point between low DKK-1 and high DKK-1 was the median value of 610 pg/mL (Figure 2). There was no significant difference in the duration between coronary angiography and outpatient interview: 13 days (interquartile range (IQR), 9–17 days) in the low DKK-1 without CAD group, 14 days (IQR, 10–20 days) in the high DKK-1 without CAD group, 11 days (IQR, 9–16 days) in the low DKK-1 with CAD group, and 13 days (IQR, 9–24 days) in the high DKK-1 with CAD group. There was no significant difference in the duration between coronary angiography and outpatient visits among groups (p = 0.146).
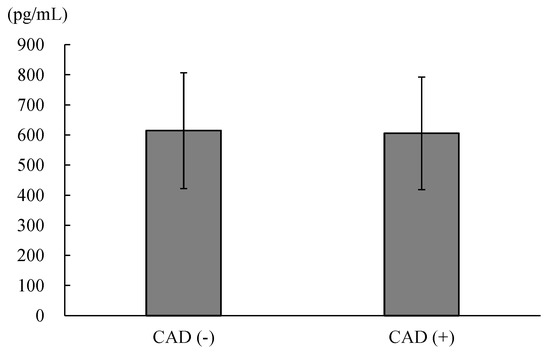
Figure 1.
The comparison of the plasma concentrations of dickkopf-1 between patients without coronary artery disease (CAD) and those with CAD (614 ± 192 vs. 606 ± 187, p = 0.622).
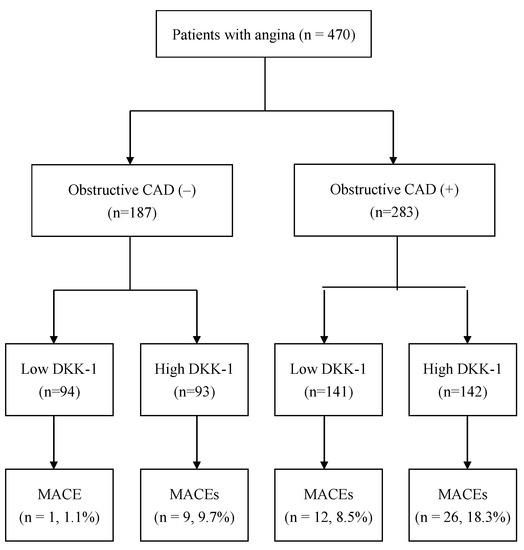
Figure 2.
Flow diagram of the enrollment of the study participants. Abbreviation: CAD = coronary artery disease, DKK-1 = dickkopf-1, and MACEs = major adverse cardiac events.
The baseline characteristics of these four groups are shown in Table 1. There were significant positive trends in age (p < 0.001 among the four groups), the proportion of current smokers (p = 0.044 among the four groups), and serum CRP (p = 0.003 among the four groups) from the low DKK-1 without CAD group to the high DKK-1 with CAD group. There were higher proportions of males (p < 0.001 among the four groups), hypertension (p = 0.033 among the four groups), and the use of statins (p < 0.001 among the four groups) in the patients with CAD than those without CAD. The eGFR (p < 0.001) and lipid profile, including total cholesterol (p < 0.001), high-density lipoprotein (HDL) cholesterol (p < 0.001), and triglycerides (p = 0.042), were significantly different among these four groups.

Table 1.
The baseline characteristics of enrolled patients categorized by CAD and median plasma DKK-1.
During the median follow-up time of 4.8 years, a total of 48 MACEs occurred. All the patients were divided into four groups (Figure 2): 94 patients were in the low DKK-1 without CAD group (1 MACE, 1.1%), 93 patients were in the high DKK-1 without CAD group (9 MACEs, 9.7%), 141 patients were in the low DKK-1 with CAD group (12 MACEs, 8.5%), and 142 patients were in the high DKK-1 with CAD group (26 MACEs, 18.3%, Figure 2). Figure 3 shows that the incidence of MACEs was significantly different among the four groups: MACE risk was the lowest in the low DKK-1 without CAD group and the highest in the high DKK-1 with CAD group according to Kaplan–Meier survival analysis (log-rank test: p = 0.001).
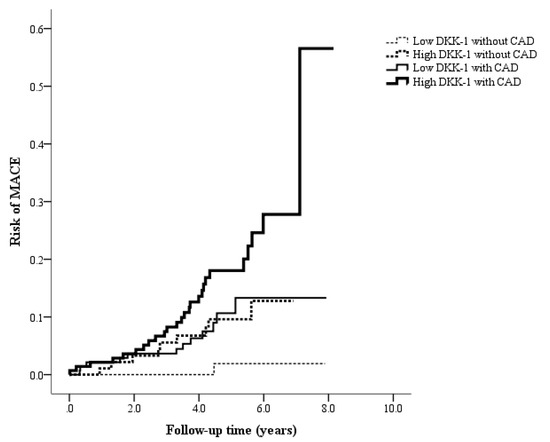
Figure 3.
Kaplan–Meier curves showing the risk of major adverse cardiac events (MACEs) across the four groups, categorized using the median dickkopf-1 (DKK-1) value of 610 pg/mL and coronary artery disease (CAD) status (log-rank test: p = 0.001).
We calculated the C index to compare the performance of MACE prediction among the different models. The C indices of the FRS-alone model, the FRS and CAD model, and the model adding DKK-1 to FRS and CAD were 0.64, 0.66, and 0.69, respectively (Figure 4). The model adding DKK-1 to FRS and CAD yielded a significant IDI of 0.012 (95% confidence interval (CI): 0.001–0.038, p = 0.027) and a significant NRI of 0.215 (95% CI: 0.009–0.370, p = 0.033) compared with the FRS and CAD model.
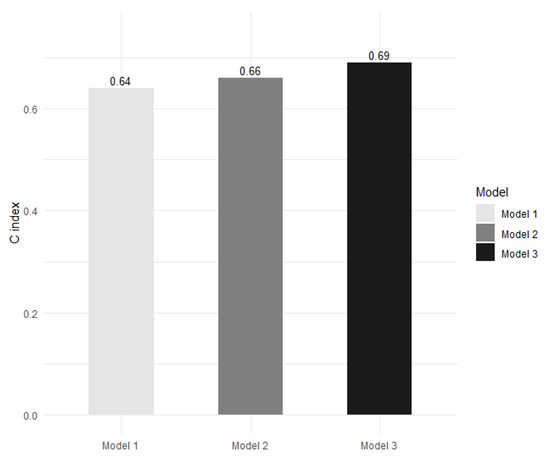
Figure 4.
The Harrell’s concordance index (C index) for the prediction of major adverse cardiac events in the Framingham Risk Score (FRS)-alone model (model 1), in the FRS and coronary artery disease (CAD) model (model 2), and in the model adding dickkopf-1 (DKK-1) to FRS and CAD (model 3). Model 3 presents a significant improvement in predictive ability compared with model 2 (p = 0.027 according to integrated discrimination improvement and p = 0.033 according to continuous net reclassification improvement).
Table 2 presents the DKK-1 levels between the paired groups categorized by the association of risk factors. Higher DKK-1 levels were observed in patients with older age, a current smoking habit, lower insulin resistance reflected by the HOMA-IR index, lower HDL cholesterol, lower eGFR, higher triglycerides, higher platelet count, higher CRP, and higher NT-proBNP. All of the other assessed risk factors did not exhibit a significant association with DKK-1 levels.

Table 2.
Plasma DKK-1 concentrations between the groups categorized by risk factors.
We conducted multivariable Cox regression, and the results are presented in Table 3. The risk of MACEs was significantly higher in the high DKK-1 with CAD group than in the low DKK-1 without CAD group (hazard ratio = 10.640, 95% CI: 1.350–83.874, p = 0.025) after adjusting for age, sex, smoking status, hypertension, HOMA-IR index, total cholesterol, HDL cholesterol, triglycerides, eGFR, platelet count, CRP, NT-proBNP, UACR, and the use of statins.

Table 3.
Cox proportional hazard regression models for the association between risk factors and major adverse cardiac events.
4. Discussion
The main finding of the present study is that a combination of plasma DKK-1 levels and obstructive CAD acted as a better predictor of MACEs than plasma DKK-1 alone or obstructive CAD alone for a median of 4.8 years of follow-up in these patients. In accordance with our findings, the DKK-1 levels in serum have been reported to be a significant predictor of MACEs in patients after acute coronary syndrome. In a population-based cohort study, Klingenschmid et al. [19] reported that higher serum DKK-1 levels were significantly associated with a higher risk of composite cardiovascular events. However, these endpoint events not only included myocardial infarction, stroke, and transient ischemic attack, but also included angina pectoris, peripheral vascular disease, and revascularization procedures during a median follow-up duration of 15.6 years. Notably, a baseline history of prior cardiovascular disease was reported to be a significant factor of interaction in this association [19]. A strength of the present study is that plasma DKK-1 levels have a synergistic effect with obstructive CAD on the prediction of long-term MACEs in patients who have experienced angina.
There have been discordant results regarding the association between plasma DKK-1 and CAD in previous studies: a higher plasma DKK-1 level was reported in patients with stroke than in healthy controls [20]; however, plasma DKK-1 levels were not significantly different between patients with acute coronary syndrome and healthy controls [12]. Plasma DKK-1 levels were inversely associated with coronary atherosclerotic calcified plaque [21]. It has been reported that circulating DKK-1 levels vary by time in patients after an acute coronary syndrome [11]. In the present study, we assessed the plasma DKK-1 levels in patients in a stable condition after discharge, but not the levels measured during acute coronary syndrome. Notably, we found that patients with a higher plasma DKK-1 level with significant coronary obstruction had a significantly higher hazard ratio of MACEs than those with a lower plasma DKK-1 level without significant coronary obstruction, as determined using Cox regression analysis in the present study. In line with our findings, Wang et al. [22] reported that patients with higher plasma DKK-1 levels had a higher odds ratio of MACEs among patients with acute coronary syndrome using binary logistic regression analysis.
The Wnt signaling pathway plays an important role in cardiovascular development and regeneration [23]. The aberrant regulation of Wnt signaling is involved in diverse cardiovascular diseases [8,24]. The activation of Wnt signaling pathway has been reported to improve left ventricular ejection fraction after MI in rats, and this benefit might result from a reduction in fibrosis after myocardial injury [25]. On the other hand, an overexpression of DKK-1 is associated with cardiovascular risk, including inflammation, endothelial dysfunction, and atherosclerotic development [26].
The endothelium is an important source of DKK-1 secretion. According to in vitro studies using human umbilical vein endothelial cells (HUVECs), the induced overexpression of DKK-1 in the endothelium could subsequently trigger monocyte adhesion to the endothelium, apoptosis of the endothelium, and a rupture risk for vulnerable plaques [10,27]. Moreover, DKK-1 is also released from activated platelets via thrombin receptor stimulation, and platelet-derived DKK-1 could induce the inflammatory pathway in HUVECs in vitro [12]. Platelet-derived DKK-1 also activated the intercellular adhesion of cultured cells to neutrophils [28]. Aspirin could inhibit the release of DKK-1 from activated platelets by thrombin receptor stimulation in an in vitro study [12]. Plasma DKK-1 levels were lower in heathy controls than in patients with diabetes, with increased platelet activity reflected by high urinary 11-dehydro-thromboxane B2, and aspirin was found to reduce plasma DKK-1 levels in patients with diabetes [29]. However, the plasma DKK-1 levels were not significantly different between the subjects who were using antiplatelet agents or not in a population-based cohort [19]. In the present study, the DKK-1 levels in plasma were not significantly associated with antihypertensive drug use or antiplatelet use.
Although angina is a clinical symptom of IHD [30,31], significant obstruction might not be detected on coronary angiography in some patients with angina [32]. In the present study, we enrolled patients with angina and suspected IHD based on noninvasive tests, including rest or exercise electrocardiography, stress nuclear imaging, and echocardiography. Only 29 (32.2%) of 90 female and 258 (66.8%) of 380 male patients had a significant obstruction detected on coronary angiography. In line with the Guidelines on Chronic Coronary Syndromes put forth by the European Society of Cardiology in 2019, the pretest probability of obstructive CAD is less than 27% in women and less than 52% in men [33]. Jespersen et al. [34] reported that no obstructive coronary lesion was detected in 65% of women and in 32% of men who had undergone coronary angiography for typical angina; however, these patients without obstructive CAD had an 85% increased risk of MACEs and a 52% increased risk of all-cause mortality compared with those without anginal symptoms. According to our results, a higher plasma DKK-1 level with obstructive CAD predicted a higher risk of MACEs compared with a lower plasma DKK-1 without obstructive coronary lesion.
There are several limitations in the present study. First, we did not directly assess the underlying mechanism involved in DKK-1 and MACEs in patients with angina. Second, we did not examine microvascular dysfunction using invasive stress tests in the enrolled patients who did not present with significant coronary obstruction [35]. Third, although the baseline plasma concentration of DKK-1 was not associated with CAD, it might have a synergistic effect with coronary obstruction on MACE occurrence. However, this was an observational study, and we did not investigate the effect of any specific intervention on lowering plasma DKK-1 levels. Therefore, we cannot speculate that MACEs will be prevented by decreasing DKK-1 levels. Finally, we excluded patients with known DM. Although high glucose levels were detected in some patients at baseline, the results cannot be applied to the population with diabetes. In the present study, a lower plasma DKK-1 level was observed in patients with a HOMA-IR index ≥ 2 than in those with a HOMA-R index < 2. In line with our results, Qiu et al. [36] reported that plasma DKK-1 was significantly lower in Han Chinese individuals with diabetic retinopathy than in healthy controls. In contrast, Lattanzio et al. [29] reported that plasma DKK-1 was significantly higher in patients with type 2 diabetes than in healthy controls. Ali et al. [37] reported no significant association of serum DKK-1 with fasting glucose or the HOMA-IR index in men of African ancestry aged ≥ 40 years. The long-term effects of plasma DKK-1 on the population with diabetes should be further investigated.
5. Conclusions
Plasma DKK-1 levels in patients with angina and significant coronary obstruction were not significantly different from those in patients with angina but no significant coronary obstruction. However, higher plasma DKK-1 levels and obstructive CAD had a synergistic effect on predictions of a higher risk of MACEs in patients with angina during a median follow-up of 4.8 years.
Author Contributions
Conceptualization, Y.-H.L., M.-H.W., W.-J.L. and I.-T.L.; data curation, I.-T.L.; formal analysis, Y.-H.L. and I.-T.L.; funding acquisition, I.-T.L.; investigation, Y.-H.L., M.-H.W., W.-J.L. and I.-T.L.; methodology, M.-H.W., W.-J.L. and I.-T.L.; project administration, I.-T.L.; resources, I.-T.L.; software, Y.-H.L.; supervision, I.-T.L.; validation, I.-T.L.; visualization, Y.-H.L.; writing—original draft, Y.-H.L.; writing—review and editing, I.-T.L. I.-T.L., the guarantor of this work, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology, Taiwan (grant number MOST 110-2314-B-075A-004 -MY3) and Taichung Veterans General Hospital, Taiwan (grant number TCVGH-1113501C).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Institutional Review Board of Taichung Veterans General Hospital (protocol code: C08215B and date of approval: 3 February 2009).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the Cardiovascular Center of Taichung Veterans General Hospital for their support. Statistical analysis was performed by the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e9349. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Much, A.A.; Maor, E.; Asher, E.; Younis, A.; Xu, Y.; Lu, Y.; Liu, X.; Shu, J.; Bragazzi, N.L. Global, regional, and national burden of ischemic heart disease and its attributable risk factors, 1990–2017: Results from the global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 50–60. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Traghella, I.; Mastorci, F.; Pepe, A.; Pingitore, A.; Vassalle, C. Nontraditional Cardiovascular Biomarkers and Risk Factors: Rationale and Future Perspectives. Biomolecules 2018, 8, 40. [Google Scholar] [CrossRef]
- Glinka, A.; Wu, W.; Delius, H.; Monaghan, A.P.; Blumenstock, C.; Niehrs, C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 1998, 391, 357–362. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2018, 70, 68–141. [Google Scholar] [CrossRef]
- Lee, D.K.; Nathan Grantham, R.; Trachte, A.L.; Mannion, J.D.; Wilson, C.L. Activation of the canonical Wnt/beta-catenin pathway enhances monocyte adhesion to endothelial cells. Biochem. Biophys. Res. Commun. 2006, 347, 109–116. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Zhang, Y.; Di, M.; Wang, H.; Wang, L.; Chen, Y.; Liu, X.; Cao, X.; Zeng, R.; et al. Upregulation of Dickkopf1 by oscillatory shear stress accelerates atherogenesis. J. Mol. Med. 2016, 94, 431–441. [Google Scholar] [CrossRef]
- Ueland, T.; Akerblom, A.; Ghukasyan, T.; Michelsen, A.E.; Becker, R.C.; Bertilsson, M.; Himmelmann, A.; James, S.K.; Siegbahn, A.; Storey, R.F.; et al. Admission Levels of DKK1 (Dickkopf-1) Are Associated with Future Cardiovascular Death in Patients with Acute Coronary Syndromes. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Otterdal, K.; Lekva, T.; Halvorsen, B.; Gabrielsen, A.; Sandberg, W.J.; Paulsson-Berne, G.; Pedersen, T.M.; Folkersen, L.; Gullestad, L.; et al. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Kurella Tamura, M.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Uno, H.; Cai, T.; Pencina, M.J.; D’Agostino, R.B.; Wei, L.J. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat. Med. 2011, 30, 1105–1117. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B., Sr.; Steyerberg, E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med. 2011, 30, 11–21. [Google Scholar] [CrossRef]
- Klingenschmid, G.; Tschiderer, L.; Himmler, G.; Rungger, G.; Brugger, S.; Santer, P.; Willeit, J.; Kiechl, S.; Willeit, P. Associations of Serum Dickkopf-1 and Sclerostin with Cardiovascular Events: Results from the Prospective Bruneck Study. J. Am. Heart Assoc. 2020, 9, e014816. [Google Scholar] [CrossRef]
- Seifert-Held, T.; Pekar, T.; Gattringer, T.; Simmet, N.E.; Scharnagl, H.; Stojakovic, T.; Fazekas, F.; Storch, M.K. Circulating Dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis 2011, 218, 233–237. [Google Scholar] [CrossRef]
- Register, T.C.; Hruska, K.A.; Divers, J.; Bowden, D.W.; Palmer, N.D.; Carr, J.J.; Wagenknecht, L.E.; Hightower, R.C.; Xu, J.; Smith, S.C.; et al. Plasma Dickkopf1 (DKK1) concentrations negatively associate with atherosclerotic calcified plaque in African-Americans with type 2 diabetes. J. Clin. Endocrinol. Metab. 2013, 98, E60–E65. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, X.B.; Zhang, W.; Wu, L.D.; Liu, Y.S.; Hu, B.; Bi, C.L.; Chen, Y.F.; Liu, X.X.; Ge, C.; et al. Dickkopf-1 as a novel predictor is associated with risk stratification by GRACE risk scores for predictive value in patients with acute coronary syndrome: A retrospective research. PLoS ONE 2013, 8, e54731. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.D.; Tian, Y.; Morrisey, E.E. Wnt signaling: An essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development 2008, 135, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Neogi, A.; Mani, A. The role of Wnt signalling in development of coronary artery disease and its risk factors. Open Biol. 2020, 10, 200128. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, X.; Xie, J.; Xu, C.; Xu, W.; Jiang, H. Exogenous high-mobility group box 1 protein injection improves cardiac function after myocardial infarction: Involvement of Wnt signaling activation. J. Biomed. Biotechnol. 2012, 2012, 743879. [Google Scholar] [CrossRef]
- Baetta, R.; Banfi, C. Dkk (Dickkopf) Proteins. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1330–1342. [Google Scholar] [CrossRef]
- Di, M.; Wang, L.; Li, M.; Zhang, Y.; Liu, X.; Zeng, R.; Wang, H.; Chen, Y.; Chen, W.; Zhang, Y.; et al. Dickkopf1 destabilizes atherosclerotic plaques and promotes plaque formation by inducing apoptosis of endothelial cells through activation of ER stress. Cell Death Dis. 2017, 8, e2917. [Google Scholar] [CrossRef]
- Guo, Y.; Mishra, A.; Howland, E.; Zhao, C.; Shukla, D.; Weng, T.; Liu, L. Platelet-derived Wnt antagonist Dickkopf-1 is implicated in ICAM-1/VCAM-1-mediated neutrophilic acute lung inflammation. Blood 2015, 126, 2220–2229. [Google Scholar] [CrossRef]
- Lattanzio, S.; Santilli, F.; Liani, R.; Vazzana, N.; Ueland, T.; Di Fulvio, P.; Formoso, G.; Consoli, A.; Aukrust, P.; Davi, G. Circulating dickkopf-1 in diabetes mellitus: Association with platelet activation and effects of improved metabolic control and low-dose aspirin. J. Am. Heart Assoc. 2014, 3, e001000. [Google Scholar] [CrossRef]
- Ford, T.J.; Berry, C. How to Diagnose and Manage Angina without Obstructive Coronary Artery Disease: Lessons from the British Heart Foundation CorMicA Trial. Interv. Cardiol. 2019, 14, 76–82. [Google Scholar] [CrossRef]
- Marzilli, M.; Crea, F.; Morrone, D.; Bonow, R.O.; Brown, D.L.; Camici, P.G.; Chilian, W.M.; DeMaria, A.; Guarini, G.; Huqi, A.; et al. Myocardial ischemia: From disease to syndrome. Int. J. Cardiol. 2020, 314, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Aribas, E.; van Lennep, J.E.R.; Elias-Smale, S.E.; Piek, J.J.; Roos, M.; Ahmadizar, F.; Arshi, B.; Duncker, D.J.; Appelman, Y.; Kavousi, M. Prevalence of microvascular angina among patients with stable symptoms in the absence of obstructive coronary artery disease: A systematic review. Cardiovasc. Res. 2022, 118, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Hvelplund, A.; Abildstrom, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jorgensen, E.; Kelbaek, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef]
- Rahman, H.; Corcoran, D.; Aetesam-Ur-Rahman, M.; Hoole, S.P.; Berry, C.; Perera, D. Diagnosis of patients with angina and non-obstructive coronary disease in the catheter laboratory. Heart 2019, 105, 1536–1542. [Google Scholar] [CrossRef]
- Qiu, F.; He, J.; Zhou, Y.; Bai, X.; Wu, G.; Wang, X.; Liu, Z.; Chen, Y.; Ma, J.X.; Liu, Z. Plasma and vitreous fluid levels of Dickkopf-1 in patients with diabetic retinopathy. Eye 2014, 28, 402–409. [Google Scholar] [CrossRef]
- Ali, H.; Zmuda, J.M.; Cvejkus, R.K.; Kershaw, E.E.; Kuipers, A.L.; Oczypok, E.A.; Wheeler, V.; Bunker, C.H.; Miljkovic, I. Wnt Pathway Inhibitor DKK1: A Potential Novel Biomarker for Adiposity. J. Endocr. Soc. 2019, 3, 488–495. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).